Sugar Transport, Metabolism and Signaling in Fruit Development of Litchi chinensis Sonn: A Review
Abstract
:1. Introduction
2. Sugar Accumulation in Litchi Fruit
2.1. Litchi Fruit Development
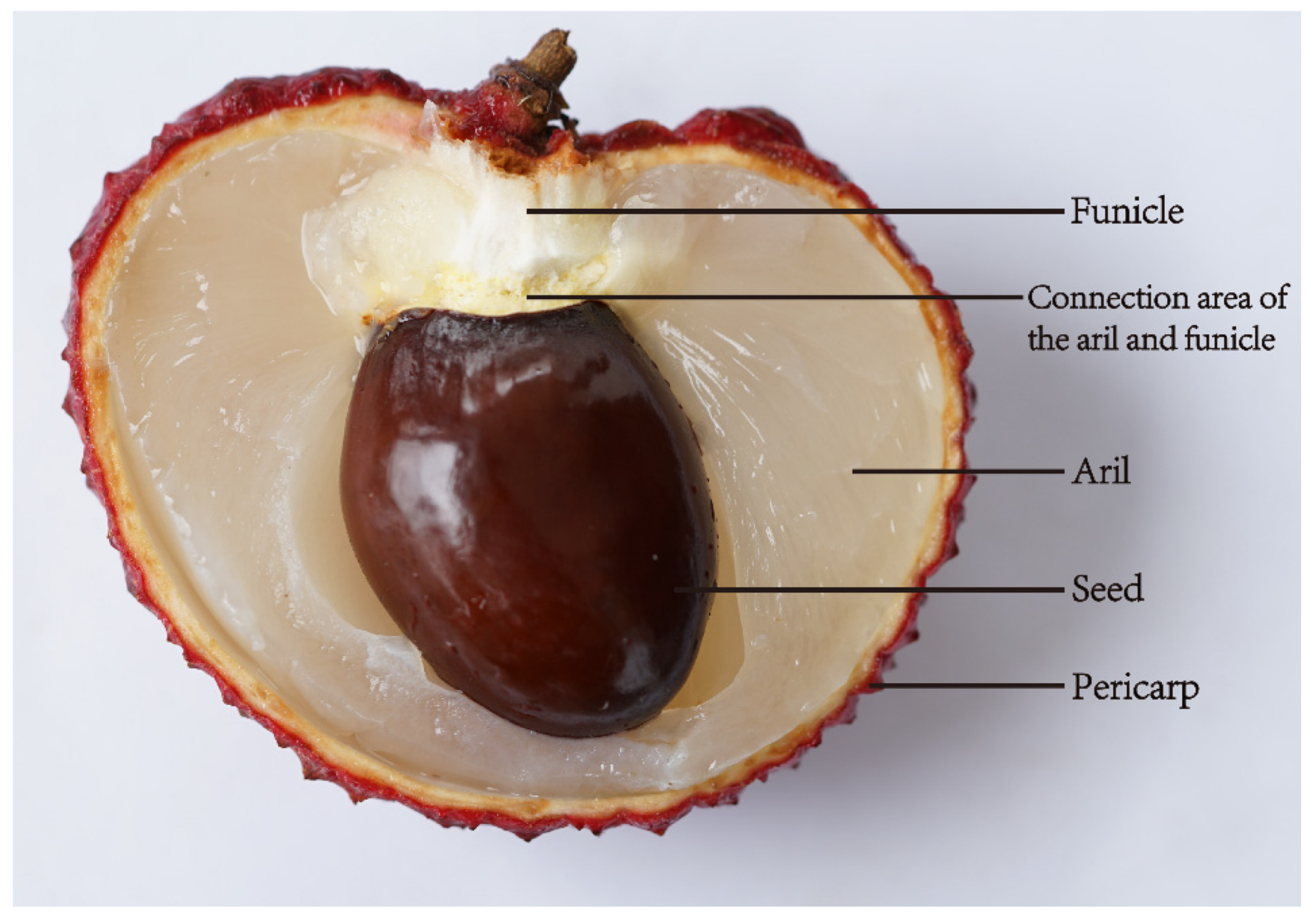
2.2. Sugar Accumulation during Aril Development
2.3. Sugar Transport and Sugar Transporters of Litchi Fruit
2.4. Biosynthesis of Quebrachitol, a Transportable Photosynthate
3. Sugar Metabolism in Litchi Fruit
3.1. SPS
3.2. SuSy
3.3. INV
4. Sugar Signaling and Fruit Development
4.1. Sugar Regulation of Genes Involved in Fruit Abscission
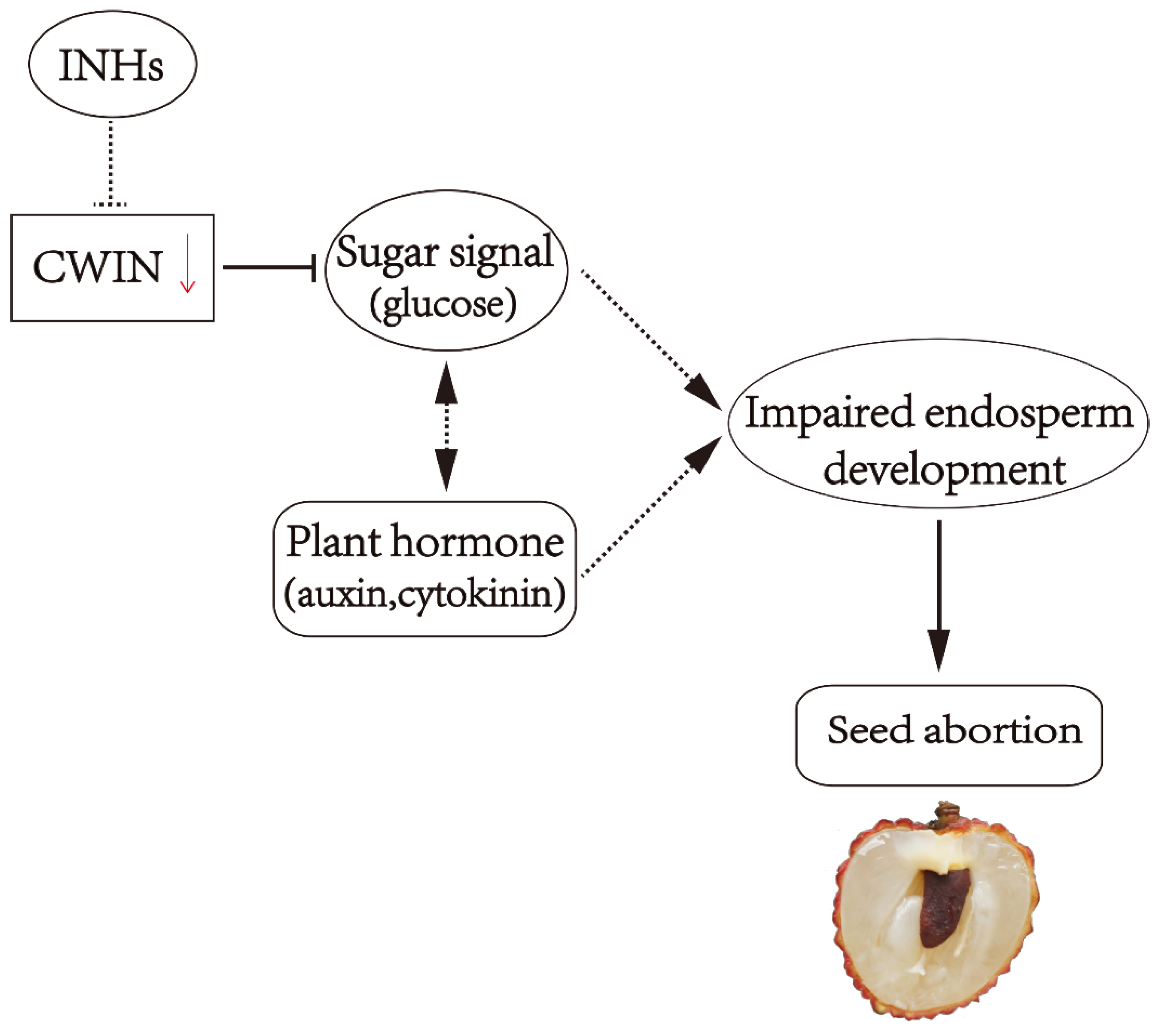
4.2. Sugar and Seed Development
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source-sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Yu, S.M.; Lo, S.F.; Ho, T.D. Source-sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Forney, C.; Bondada, B.; Leng, F.; Xie, Z.S. The molecular regulation of carbon sink strength in grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 11, 606918. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, Y. Sugar metabolism and fruit development in the tomato. Horticult. J. 2017, 86, 417–425. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Litchi (Litchi chinensis): Morphology, taxonomy, composition and health benefits. In Fruits Grown in Highland Regions of the Himalayas; Springer: Cham, Switzerland, 2021; pp. 181–191. [Google Scholar]
- Hu, B.; Lai, B.; Wang, D.; Li, J.; Chen, L.; Qin, Y.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. Three LcABFs are involved in the regulation of chlorophyll degradation and anthocyanin biosynthesis during fruit ripening in Litchi chinensis. Plant Cell Physiol. 2019, 60, 448–461. [Google Scholar] [CrossRef]
- Wang, H.C.; Huang, H.B.; Huang, X.M.; Hu, Z.Q. Sugar and acid compositions in the arils of Litchi chinensis Sonn.: Cultivar differences and evidence for the absence of succinic acid. J. Hortic. Sci. Biotechnol. 2006, 81, 57–62. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, J.; Hu, B.; Li, J.; Qin, Y.; Chen, L.; Qin, Y.; Hu, G. Identification and expression profile analysis of the sucrose phosphate synthase gene family in Litchi chinensis sonn. PeerJ 2018, 6, e4379. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Wang, H.; Huang, X.; Qin, Y.; Hu, G. Patterns of enzyme activities and gene expressions in sucrose metabolism in relation to sugar accumulation and composition in the aril of Litchi chinensis Sonn. J. Plant Physiol. 2013, 170, 731–740. [Google Scholar] [CrossRef]
- Wu, Z.C.; Yang, Z.Y.; Li, J.G.; Chen, H.B.; Huang, X.M.; Wang, H.C. Methyl-inositol, γ -aminobutyric acid and other health benefit compounds in the aril of litchi. Int. J. Food Sci. Nutr. 2016, 67, 762–772. [Google Scholar]
- Stern, R.A.; Gazit, S. The reproductive biology of the lychee. In Horticultural Reviews; Janick, J., Ed.; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 393–453. [Google Scholar]
- Li, J.G.; Huang, H.; Huang, X. A revised division of the developmental stages in litchi fruit. Yuan Yi Xue Bao 2003, 30, 307–310. [Google Scholar]
- Huang, H.; Xu, J. The developmental patterns of fruit tissue and their correlative relationships in Litchi chinensis Sonn. Sci. Hortic. 1983, 19, 335–342. [Google Scholar] [CrossRef]
- Cronje, R.B. Effect of fruit development, maturity and harvesting of litchi (Litchi chinensis Sonn.) on postharvest fruit quality. Stewart Postharvest Rev. 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Wang, H.C.; Lai, B.; Huang, X.M. Litchi fruit set, development, and maturation. In The Lychee Biotechnology; Kumar, M., Kumar, V., Prasad, R., Varma, A., Eds.; Springer: Singapore, 2017; pp. 1–30. [Google Scholar]
- Huang, H. Towards a better insight into the development of the arillate fruit of litchi, and longan. Acta Hort. 2001, 558, 185–192. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Wu, Z.C.; Huang, X.M.; Hu, G.B.; Chen, H.B. Determination of quebrachitol in Litchi chinensis and Dimocarpus longan in Sapindacea family. J. South China Agr. Univ. 2013, 34, 316–319. [Google Scholar]
- Wu, Z.C.; Zhang, J.Q.; Zhao, J.T.; Li, J.G.; Huang, X.M.; Wang, H.C. The biosynthesis of quebrachitol, a transportable photosynthate, in Litchi chinensis. J. Exp. Bot. 2018, 69, 1649–1661. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, H.; Shi, J.; Neethirajan, S.; Lin, Y.; Chen, Y.; Wang, H.; Lin, Y. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 2018, 252, 134–141. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, J.; Qin, Y.; Qin, Y.; Hu, G. Molecular cloning, characterization and expression profile of the sucrose synthase gene family in Litchi chinensis. Hortic. Plant J. In press.
- Ma, S.; Li, Y.; Li, X.; Sui, X.; Zhang, Z. Phloem unloading strategies and mechanisms in crop fruits. J. Plant Growth Regul. 2019, 38, 494–500. [Google Scholar] [CrossRef]
- Falchi, R.; Bonghi, C.; Drincovich, M.F.; Famiani, F.; Lara, M.V.; Walker, R.P.; Vizzotto, G. Sugar metabolism in stone fruit: Source-sink relationships and environmental and agronomical effects. Front. Plant Sci. 2020, 11, 573982. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.D.; Zhang, H.F.; Wu, Z.C.; Li, J.G.; Huang, X.M.; Wang, H.C. Sugar uptake in the aril of litchi fruit depends on the apoplasmic post-phloem transport and the activity of proton pumps and the putative transporter LcSUT4. Plant Cell Physiol. 2015, 56, 377–387. [Google Scholar] [CrossRef]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef]
- Xie, H.; Wang, D.; Qin, Y.; Ma, A.; Fu, J.; Qin, Y.; Hu, G.; Zhao, J. Genome-wide identification and expression analysis of SWEET gene family in Litchi chinensis reveal the involvement of LcSWEET2a/3b in early seed development. BMC Plant Biol. 2019, 19, 499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, L.; Ren, C.; Ren, F.; Wang, Y.; Fan, P.; Li, S.; Liang, Z. VvSWEET10 mediates sugar accumulation in grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Anur, R.M.; Mufithah, N.; Sawitri, W.D.; Sakakibara, H.; Sugiharto, B. Overexpression of sucrose phosphate synthase enhanced sucrose content and biomass production in transgenic sugarcane. Plants 2020, 9, 200. [Google Scholar] [CrossRef]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in peach fruit: A breeding perspective. Hortic. Res. 2016, 3, 15067. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant. 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Shen, S.; Ma, S.; Liu, Y.; Liao, S.; Li, J.; Wu, L.; Kartika, D.; Mock, H.P.; Ruan, Y.L. Cell wall invertase and sugar transporters are differentially activated in tomato styles and ovaries during pollination and fertilization. Front. Plant Sci. 2019, 10, 506. [Google Scholar] [CrossRef]
- Ru, L.; He, Y.; Zhu, Z.; Patrick, J.W.; Ruan, Y.L. Integrating sugar metabolism with transport: Elevation of endogenous cell wall invertase activity up-regulates SlHT2 and SlSWEET12c expression for early fruit development in tomato. Front. Genet. 2020, 11, 592596. [Google Scholar] [CrossRef]
- Ma, M.; Wang, L.; Zhang, S.; Guo, L.; Zhang, Z.; Li, J.; Sun, L.; Zhang, S. Acid vacuolar invertase 1 (PbrAc-Inv1) and invertase inhibitor 5 (PbrII5) were involved in sucrose hydrolysis during postharvest pear storage. Food Chem. 2020, 320, 126635. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y.L. Evolution of sucrose metabolism: The dichotomy of invertases and beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Klann, E.M.; Hall, B.; Bennett, A.B. Antisense acid invertase (TIV1) gene alters soluble sugar composition and size in transgenic tomato fruit. Plant Physiol. 1996, 112, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.D.; Ogé, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef]
- Li, L.; Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Meitzel, T.; Radchuk, R.; McAdam, E.L.; Thormählen, I.; Feil, R.; Munz, E.; Hilo, A.; Geigenberger, P.; Ross, J.J.; Lunn, J.E.; et al. Trehalose 6-phosphate promotes seed filling by activating auxin biosynthesis. New Phytol. 2021, 229, 1553–1565. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Huang, X.; Li, J.; Wang, H.; Li, J. An improved fruit transcriptome and the identification of the candidate genes involved in fruit abscission induced by carbohydrate stress in litchi. Front. Plant Sci. 2015, 6, 439. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J. Molecular events involved in fruitlet abscission in litchi. Plants 2020, 9, 151. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Huang, X.; Li, J.; Wang, H.; Li, J. De novo assembly and characterization of fruit transcriptome in Litchi chinensis. Sonn and analysis of differentially regulated genes in fruit in response to shading. BMC Genom. 2013, 14, 552. [Google Scholar] [CrossRef]
- Kuang, J.F.; Wu, J.Y.; Zhong, H.Y.; Li, C.Q.; Chen, J.Y.; Lu, W.J.; Li, J.G. Carbohydrate stress affecting fruitlet abscission and expression of genes related to auxin signal transduction pathway in litchi. Int. J. Mol. Sci. 2012, 13, 16084–16103. [Google Scholar] [CrossRef]
- Denisov, Y.; Glick, S.; Zviran, T.; Ish-Shalom, M.; Levin, A.; Faigenboim, A.; Cohen, Y.; Irihimovitch, V. Distinct organ-specific and temporal expression profiles of auxin-related genes during mango fruitlet drop. Plant Physiol. Biochem. 2017, 115, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.R.; Ma, X.S.; Rahman, M.Z.; Yang, M.C.; Huang, X.M.; Li, J.G.; Wang, H.C. Thermo-sensitive sterility and self-sterility underlie the partial seed abortion phenotype of Litchi chinensis. Sci. Hortic. 2019, 247, 156–164. [Google Scholar] [CrossRef]
- Orozco-Arroyo, G.; Paolo, D.; Ezquer, I.; Colombo, L. Networks controlling seed size in Arabidopsis. Plant Reprod. 2015, 28, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Z.; Hu, F.; Liu, L.; Huang, X.; Zhao, J.; Wang, H. Aberrant seed development in Litchi chinensis is associated with the impaired expression of cell wall invertase genes. Hortic. Res. 2018, 5, 39. [Google Scholar] [CrossRef]
- Lafon-Placette, C.; Köhler, C. Embryo and endosperm, partners in seed development. Curr. Opin. Plant Biol. 2014, 17, 64–69. [Google Scholar] [CrossRef]
- Sekhon, R.S.; Hirsch, C.N.; Childs, K.L.; Breitzman, M.W.; Kell, P.; Duvick, S.; Spalding, E.P.; Buell, C.R.; de Leon, N.; Kaeppler, S.M. Phenotypic and transcriptional analysis of divergently selected maize populations reveals the role of developmental timing in seed size determination. Plant Physiol. 2014, 165, 658–669. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Zhang, J.Q.; Wang, T.D.; Huang, X.M.; Hu, G.B.; Wang, H.C. Does acid invertase regulate the seed development of Litchi Chinensis? Acta Hortic. 2014, 1029, 301–307. [Google Scholar] [CrossRef]
- Cheng, W.H.; Taliercio, E.W.; Chourey, P.S. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 1996, 8, 971–983. [Google Scholar] [CrossRef]
- Wang, E.T.; Wang, J.; Zhu, X.; Hao, W.; Wang, L.; Li, Q.; Zhang, L.; He, W.; Lu, B.; Lin, H.; et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008, 40, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.A.; Ruan, Y.L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef]
- Weschke, W.; Panitz, R.; Gubatz, S.; Wang, Q.; Radchuk, R.; Weber, H.; Wobus, U. The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. Plant J. 2003, 33, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ruan, Y.L. New insights into roles of cell wall invertase in early seed development revealed by comprehensive spatial and temporal expression patterns of GhCWIN1 in cotton. Plant Physiol. 2012, 160, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Lin, I.W.; Qu, X.Q.; Sosso, D.; McFarlane, H.E.; Londoño, A.; Samuels, A.L.; Frommer, W.B. A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef]
- Sosso, D.; Luo, D.; Li, Q.B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1496. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Wang, D.; Xie, H.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. Sugar Transport, Metabolism and Signaling in Fruit Development of Litchi chinensis Sonn: A Review. Int. J. Mol. Sci. 2021, 22, 11231. https://doi.org/10.3390/ijms222011231
Fan S, Wang D, Xie H, Wang H, Qin Y, Hu G, Zhao J. Sugar Transport, Metabolism and Signaling in Fruit Development of Litchi chinensis Sonn: A Review. International Journal of Molecular Sciences. 2021; 22(20):11231. https://doi.org/10.3390/ijms222011231
Chicago/Turabian StyleFan, Shuying, Dan Wang, Hanhan Xie, Huicong Wang, Yonghua Qin, Guibing Hu, and Jietang Zhao. 2021. "Sugar Transport, Metabolism and Signaling in Fruit Development of Litchi chinensis Sonn: A Review" International Journal of Molecular Sciences 22, no. 20: 11231. https://doi.org/10.3390/ijms222011231





