Controlled Release of Doxorubicin for Targeted Chemo-Photothermal Therapy in Breast Cancer HS578T Cells Using Albumin Modified Hybrid Nanocarriers
Abstract
:1. Introduction
2. Results and Discussion
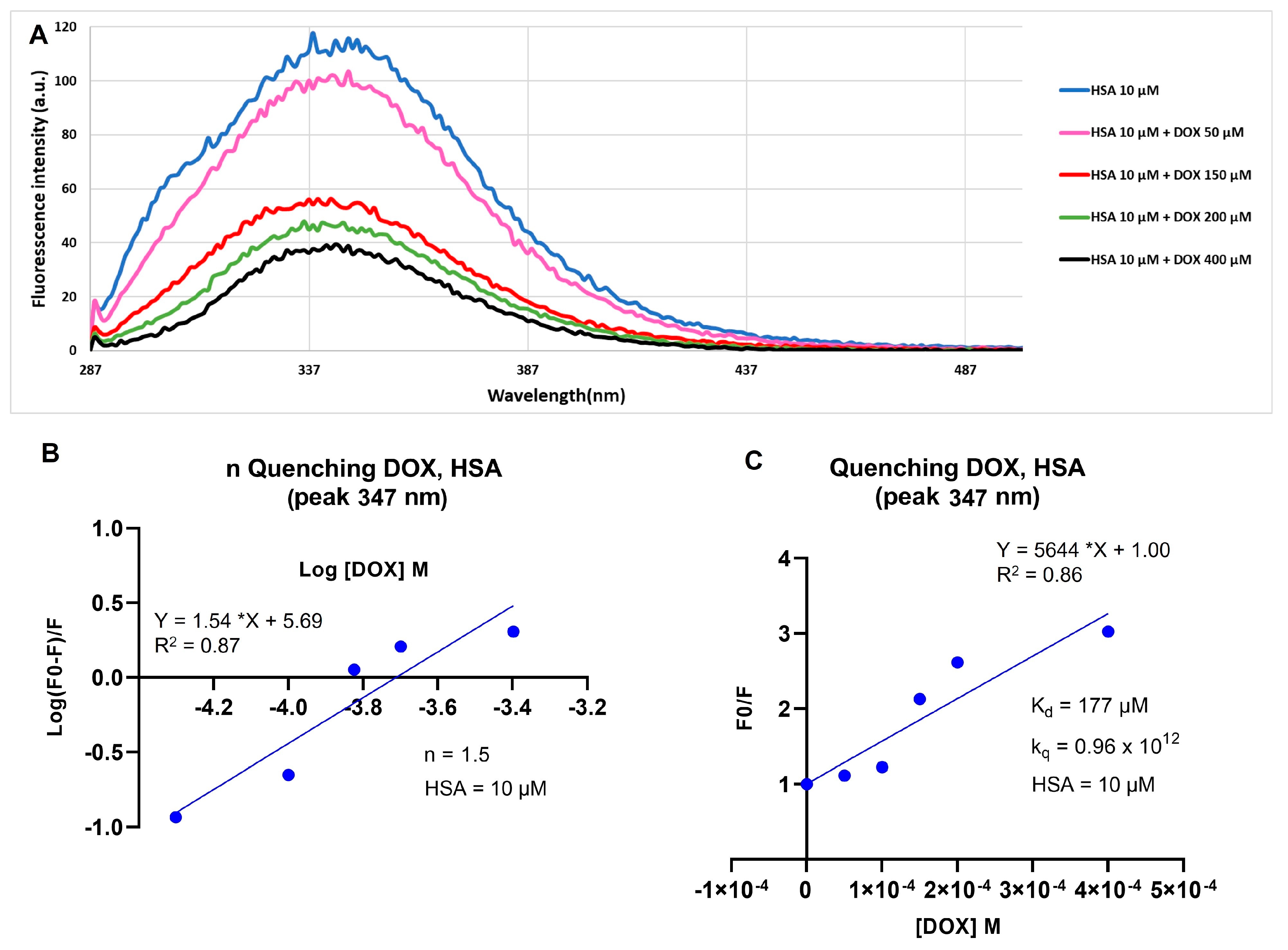
2.1. Doxorubicin and Human Serum Albumin Interaction
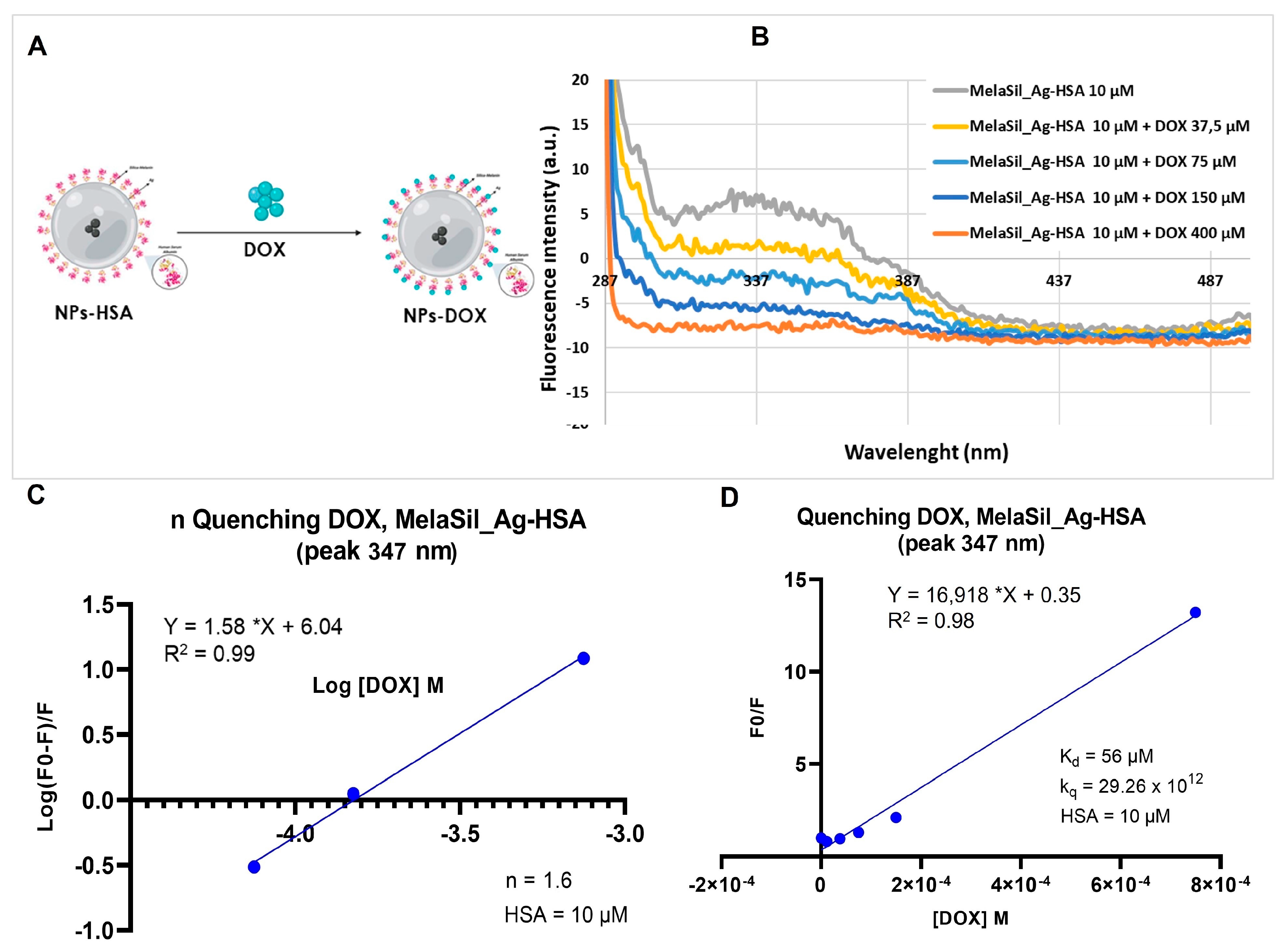
2.2. DOX and MelaSil_Ag-HSA Nanoparticles Interaction
2.3. DOX Loading and pH Dependent Release
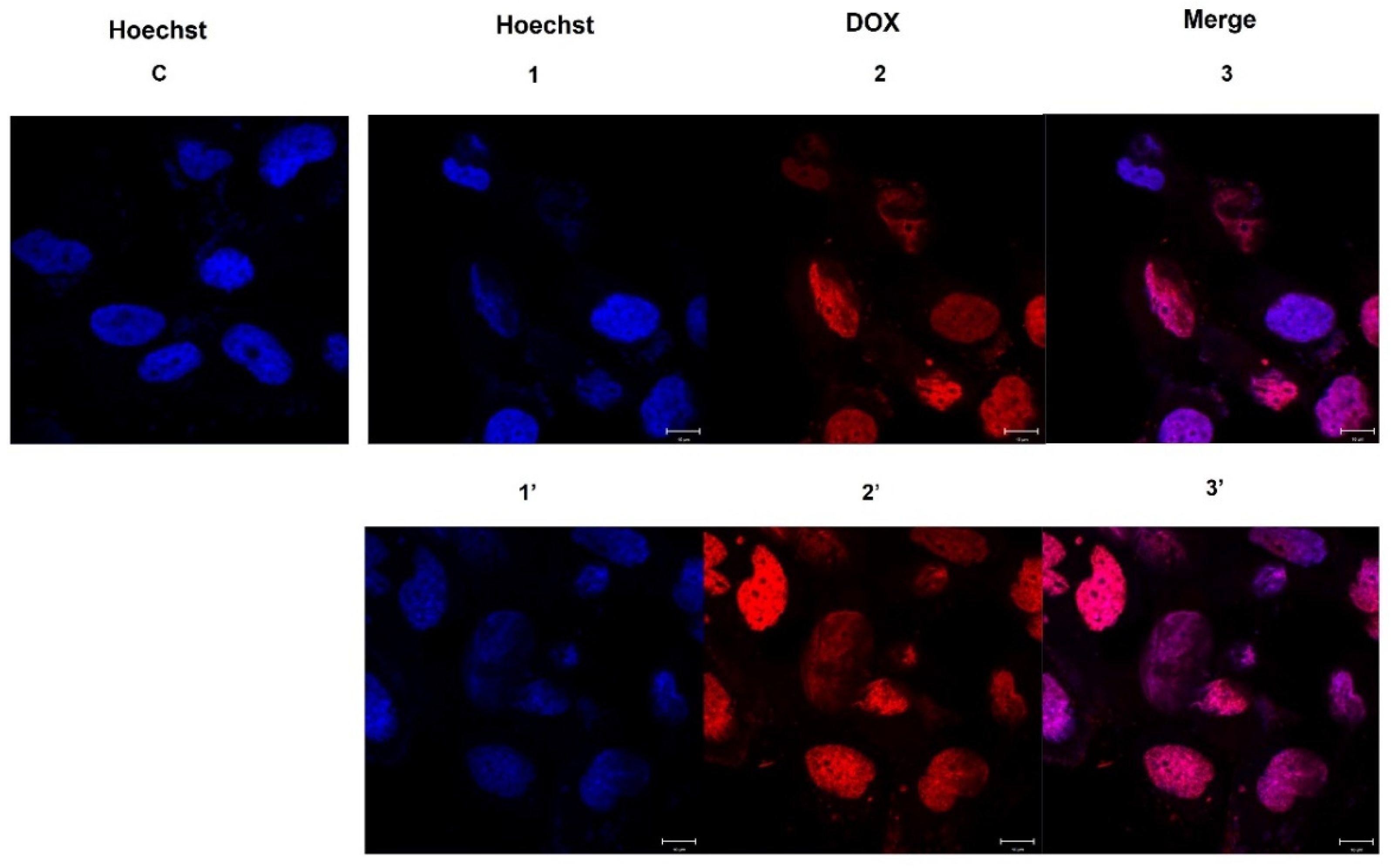
2.4. DOX Delivery in HS578T Cells
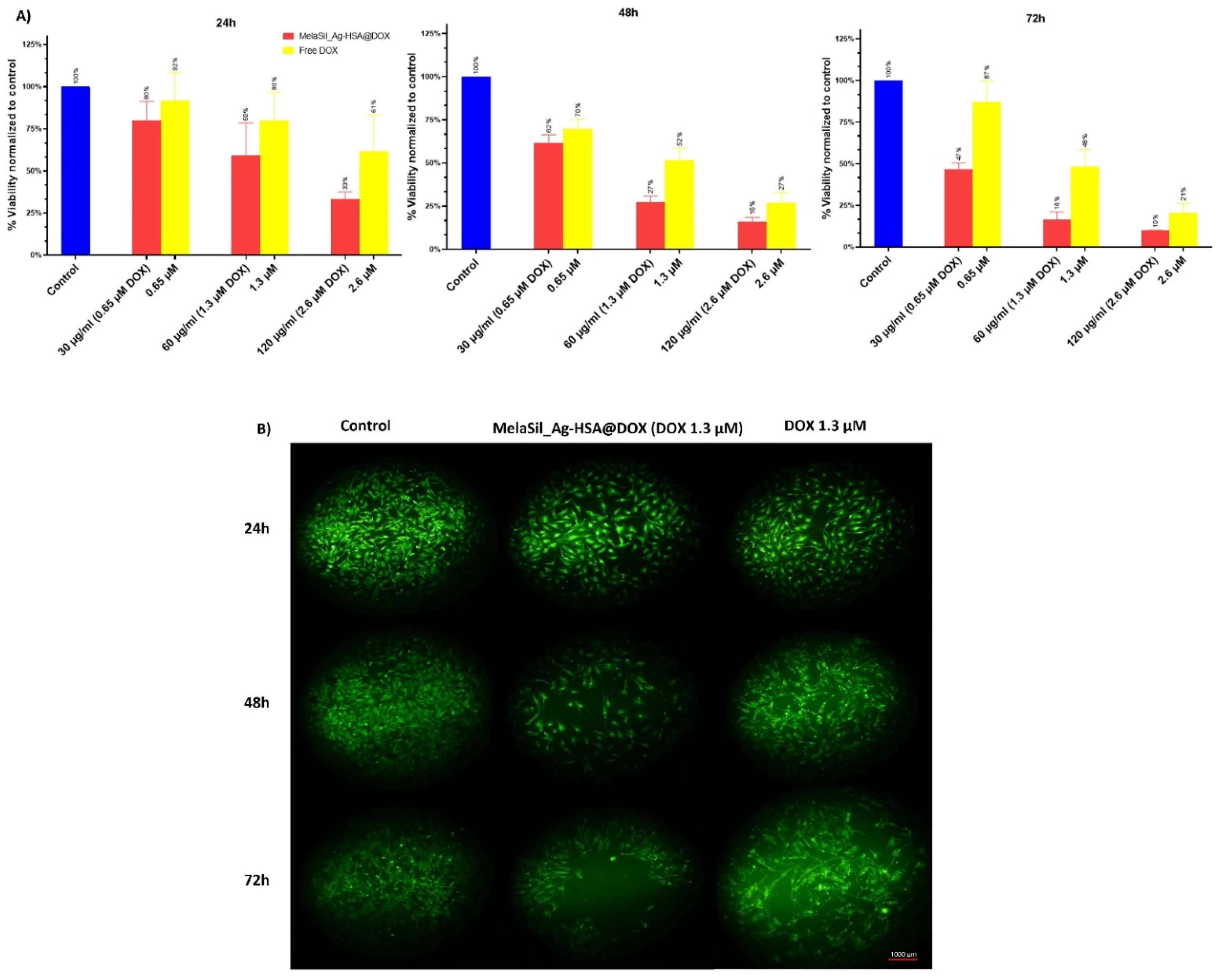
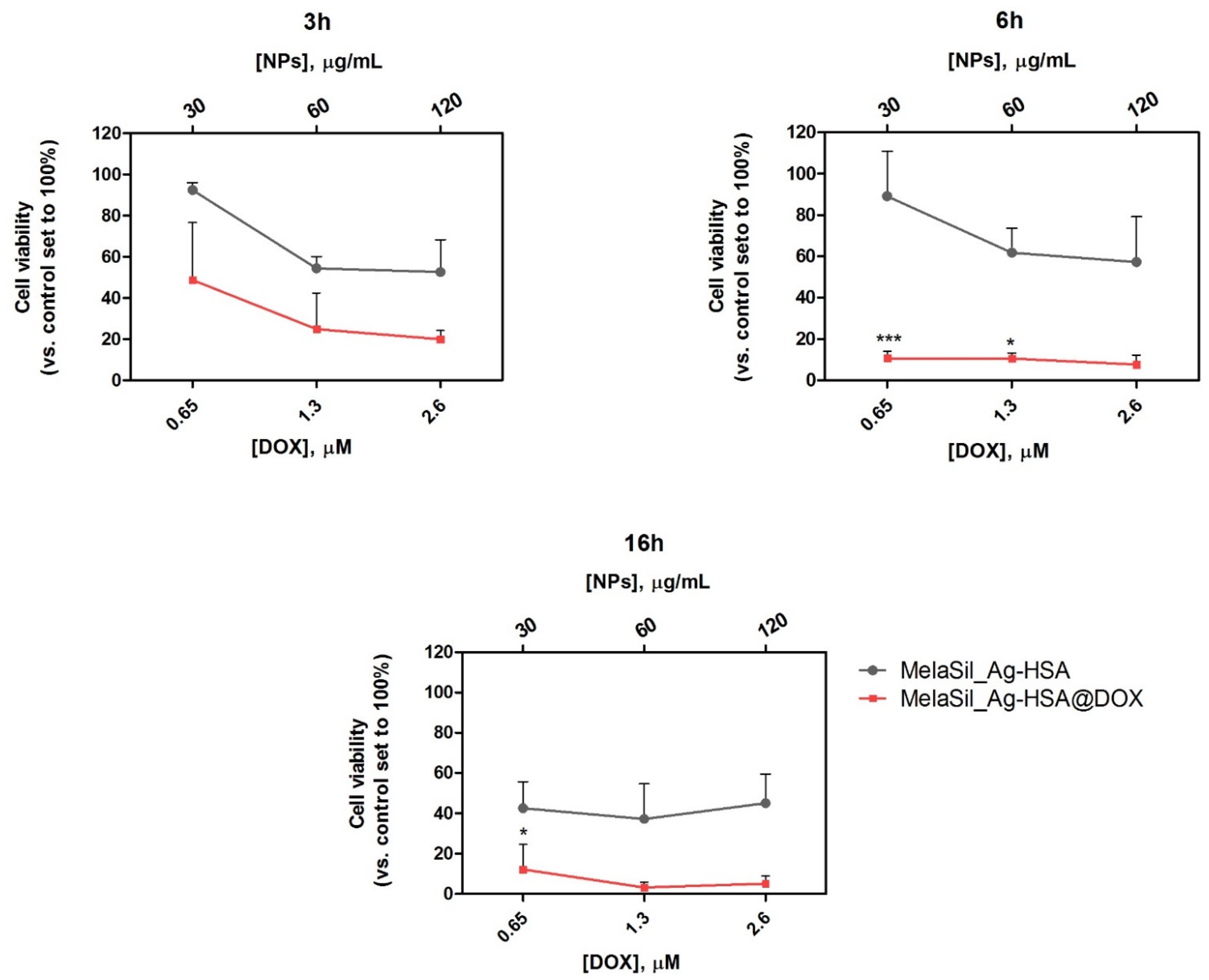
2.5. Citotoxicity of MelaSil_Ag-HSA@DOX vs. Free DOX
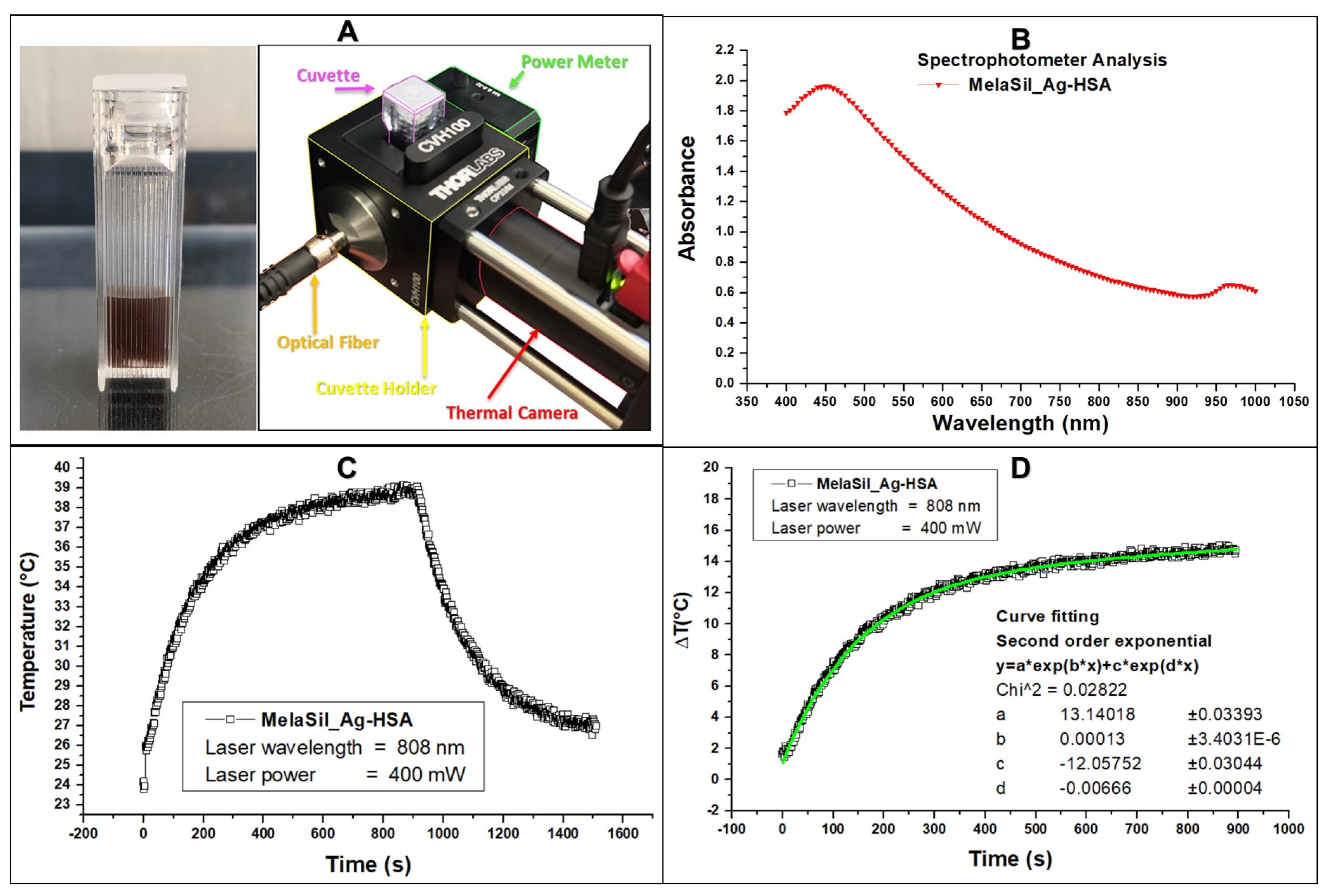
2.6. Thermal Properties of MelaSil_Ag-HSA NPs
2.7. Citotoxicity of MelaSilAg-HSA@DOX upon Laser Irradiation
3. Materials and Methods
3.1. Reagents
3.2. Cell Culture
3.3. HSA-DOX Quenching Effect
3.4. Stern–Volmer Plots
3.5. Evaluation of HSA Amount Bonded to MelaSil_Ag NPs
3.6. Loading of DOX to MelaSil_Ag-HSA NPs
3.7. Dynamic Light Scattering (DLS) and ζ-Potential Characterization
3.8. DOX Release from MelaSil_Ag-HSA@DOX
3.9. Confocal Microscopy
3.10. Cell Viability of MelaSil_Ag-HSA@DOX versus Free DOX
3.11. Photothermal Response of MelaSil_Ag NPs under CW Laser Irradiation
3.12. Cell Viability Following Laser Irradiation
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, M.; Chen, F.; Shao, D.; Weis, P.; Wei, Z.; Sun, W. Photoresponsive Metallopolymer Nanoparticles for Cancer Theranostics. Biomaterials 2021, 275, 120915. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, T.; Yang, Z.; Zhou, Z.; Tang, W.; Fan, W.; Liu, Y.; Mu, J.; Li, L.; Bregadze, V.I.; et al. Small-Sized Gadolinium Oxide Based Nanoparticles for High-Efficiency Theranostics of Orthotopic Glioblastoma. Biomaterials 2020, 235, 119783. [Google Scholar] [CrossRef]
- Tanziela, T.; Shaikh, S.; Jiang, H.; Lu, Z.; Wang, X. Efficient Encapsulation of Biocompatible Nanoparticles in Exosomes for Cancer Theranostics. Nano Today 2020, 35, 100964. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional Magnetic Iron Oxide Nanoparticles: An Advanced Platform for Cancer Theranostics. Theranostics 2020, 10, 6278. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Indoria, S.; Singh, V.; Hsieh, M.F. Recent Advances in Theranostic Polymeric Nanoparticles for Cancer Treatment: A Review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef]
- Gao, H.; Bai, Y.; Chen, L.; Ei Fakhri, G.; Wang, M. Self-Assembly Nanoparticles for Overcoming Multidrug Resistance and Imaging-Guided Chemo-Photothermal Synergistic Cancer Therapy. Int. J. Nanomed. 2020, 15, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, H.; Chen, B. Application of Nanoparticles to Reverse Multi-Drug Resistance in Cancer. Nanotechnol. Rev. 2016, 5, 489–496. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Tanner, M.; Rantanen, V.; Bärlund, M.; Borg, Å.; Grénman, S.; Isola, J. Amplification and Deletion of Topoisomerase II Associate with ErbB-2 Amplification and Affect Sensitivity to Topoisomerase II Inhibitor Doxorubicin in Breast Cancer. Am. J. Pathol. 2000, 156, 839–847. [Google Scholar] [CrossRef]
- Swift, L.P.; Rephaeli, A.; Nudelman, A.; Phillips, D.R.; Cutts, S.M. Doxorubicin-DNA Adducts Induce a Non-Topoisomerase II-Mediated Form of Cell Death. Cancer Res. 2006, 66, 4863–4871. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.H.; Saadatpour, Z.; Salmaninejad, A.; Momeni, F.; Mokhtari, M.; Nahand, J.S.; Rahmati, M.; Mirzaei, H.; Kianmehr, M. Breast Cancer Diagnosis: Imaging Techniques and Biochemical Markers. J. Cell. Physiol. 2018, 233, 5200–5213. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Deng, Z.S.; Liu, J. A Review of Hyperthermia Combined with Radiotherapy/Chemotherapy on Malignant Tumors. Crit. Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Hou, Z.; Wang, M.; Li, C.; Lin, J. Recent Advances in Hyperthermia Therapy-Based Synergistic Immunotherapy. Adv. Mater. 2021, 33, 2004788. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Crezee, J.; Franken, N.A.P.; Krawczyk, P.M. Effects of Hyperthermia on DNA Repair Pathways: One Treatment to Inhibit Them All. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunne, M.; Evans, J.C.; Dewhirst, M.W.; Allen, C. The Integration of Hyperthermia and Drug Delivery. Adv. Drug Deliv. Rev. 2020, 163–164, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef]
- Sanità, G.; Armanetti, P.; Silvestri, B.; Carrese, B.; Calì, G.; Pota, G.; Pezzella, A.; D’Ischia, M.; Luciani, G.; Menichetti, L.; et al. Albumin-Modified Melanin-Silica Hybrid Nanoparticles Target Breast Cancer Cells via a SPARC-Dependent Mechanism. Front. Bioeng. Biotechnol. 2020, 8, 765. [Google Scholar] [CrossRef]
- Silvestri, B.; Armanetti, P.; Sanità, G.; Vitiello, G.; Lamberti, A.; Calì, G.; Pezzella, A.; Luciani, G.; Menichetti, L.; Luin, S.; et al. Silver-Nanoparticles as Plasmon-Resonant Enhancers for Eumelanin’s Photoacoustic Signal in a Self-Structured Hybrid Nanoprobe. Mater. Sci. Eng. C 2019, 102, 788–797. [Google Scholar] [CrossRef]
- Agudelo, D.; Bourassa, P.; Bruneau, J.; Bérubé, G.; Asselin, É.; Tajmir-Riahi, H.A. Probing the Binding Sites of Antibiotic Drugs Doxorubicin and N-(Trifluoroacetyl) Doxorubicin with Human and Bovine Serum Albumins. PLoS ONE 2012, 7, e43814. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Fu, W.; Liu, Y.; Yao, H.; Zheng, K.; Liu, L.; Xue, J.; Xu, P.; Chen, Y.; Huang, M.; et al. Unveiling the Molecular Mechanism of PH-Dependent Interactions of Human Serum Albumin with Chemotherapeutic Agent Doxorubicin: A Combined Spectroscopic and Constant-PH Molecular Dynamics Study. J. Mol. Liq. 2021, 333, 115949. [Google Scholar] [CrossRef]
- Mohanta, V.; Madras, G.; Patil, S. Layer-by-Layer Assembled Thin Film of Albumin Nanoparticles for Delivery of Doxorubicin. J. Phys. Chem. C 2012, 116, 5333–5341. [Google Scholar] [CrossRef]
- Jiang, Q.; Luo, Z.; Men, Y.; Yang, P.; Peng, H.; Guo, R.; Tian, Y.; Pang, Z.; Yang, W. Red Blood Cell Membrane-Camouflaged Melanin Nanoparticles for Enhanced Photothermal Therapy. Biomaterials 2017, 143, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-Responsive Photothermal-Chemotherapy with Drug-Loaded Melanin-like Nanoparticles for Synergetic Tumor Ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, V.; Prakash, J.; Malekzadeh-Najafabadi, J.; Stiel, A.; Klemm, U.; Mettenleiter, G.; Aichler, M.; Walch, A.; Ntziachristos, V. Bioengineered Bacterial Vesicles as Biological Nano-Heaters for Optoacoustic Imaging. Nat. Commun. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Zhang, S.; Zhang, N.; Wang, Y.; Zhong, J.; Sun, X.; Qi, Y.; Chen, X.; Li, Z.; Li, Y. Tailoring Synthetic Melanin Nanoparticles for Enhanced Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 42671–42679. [Google Scholar] [CrossRef]
- Awan, U.A.; Raza, A.; Ali, S.; Saeed, R.F.; Akhtar, N. Doxorubicin-Loaded Gold Nanorods: A Multifunctional Chemo-Photothermal Nanoplatform for Cancer Management. Beilstein J. Nanotechnol. 2021, 12, 295–303. [Google Scholar] [CrossRef] [PubMed]

| ζ-Average (nm) | PDI | ζ-Potential (mV) | |
|---|---|---|---|
| MelaSil_Ag-HSA | 394 ± 32 | 0.26 ± 0.9 | −27.2 ± 1.65 |
| MelaSil_Ag-HSA@DOX | 407 ± 29 | 0.45 ± 0.09 | −17 ± 2.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrese, B.; Cavallini, C.; Sanità, G.; Armanetti, P.; Silvestri, B.; Calì, G.; Pota, G.; Luciani, G.; Menichetti, L.; Lamberti, A. Controlled Release of Doxorubicin for Targeted Chemo-Photothermal Therapy in Breast Cancer HS578T Cells Using Albumin Modified Hybrid Nanocarriers. Int. J. Mol. Sci. 2021, 22, 11228. https://doi.org/10.3390/ijms222011228
Carrese B, Cavallini C, Sanità G, Armanetti P, Silvestri B, Calì G, Pota G, Luciani G, Menichetti L, Lamberti A. Controlled Release of Doxorubicin for Targeted Chemo-Photothermal Therapy in Breast Cancer HS578T Cells Using Albumin Modified Hybrid Nanocarriers. International Journal of Molecular Sciences. 2021; 22(20):11228. https://doi.org/10.3390/ijms222011228
Chicago/Turabian StyleCarrese, Barbara, Chiara Cavallini, Gennaro Sanità, Paolo Armanetti, Brigida Silvestri, Gaetano Calì, Giulio Pota, Giuseppina Luciani, Luca Menichetti, and Annalisa Lamberti. 2021. "Controlled Release of Doxorubicin for Targeted Chemo-Photothermal Therapy in Breast Cancer HS578T Cells Using Albumin Modified Hybrid Nanocarriers" International Journal of Molecular Sciences 22, no. 20: 11228. https://doi.org/10.3390/ijms222011228
APA StyleCarrese, B., Cavallini, C., Sanità, G., Armanetti, P., Silvestri, B., Calì, G., Pota, G., Luciani, G., Menichetti, L., & Lamberti, A. (2021). Controlled Release of Doxorubicin for Targeted Chemo-Photothermal Therapy in Breast Cancer HS578T Cells Using Albumin Modified Hybrid Nanocarriers. International Journal of Molecular Sciences, 22(20), 11228. https://doi.org/10.3390/ijms222011228










