Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy
Abstract
:1. Introduction
2. Diabetes Mellitus: General Overview
3. Plant-Derived Small Molecules as Antidiabetic Agents
4. Nanocarrier-Based Drug Delivery: A Contemporary Promise
5. Nanoformulations in Diabetes Treatment
5.1. Curcumin
5.2. Resveratrol
5.3. Naringenin
5.4. Quercetin
5.5. Apigenin
5.6. Myricitrin
5.7. Baicalin
5.8. Luteolin
5.9. Mangiferin
5.10. Gymnemic Acid
5.11. Emodin
5.12. Rosmarinic Acid
5.13. Berberine
5.14. Stevia Glycosides
5.15. Asiatic Acid
5.16. Glycyrrhizin
5.17. α-Eleostearic Acid
5.18. Scutellarin
5.19. Silybum Flavonolignans
5.20. Gallic Acid
5.21. Catechins
5.22. Pelargonidin
5.23. Thymoquinone
5.24. Ferulic Acid
5.25. Other Plant-Derived Antidiabetic Nanoformulations
5.26. Green-Synthesized Nanoformulations as Antidiabetic Phytotherapeuticals
6. Present Scenario and Future Perspective
7. Interpretation and Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kesharwani, P.; Gorain, B.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; Lee, C.M.; Ooi, C.H.; et al. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran. 2017, 31, 134. [Google Scholar] [CrossRef] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.K.; Gaspar, B.L.; Welsby, G.; Katare, O.P.; Singh, K.K.; Singh, B. Improving the biopharmaceutical attributes of mangiferin using vitamin E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv. Transl. Res. 2018, 8, 617–632. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Sharma, R.; Jain, D.K. Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Scientifica 2016, 2016, 8525679. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B. Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care 2011, 34, 1249–1257. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, J.; Gu, J.; Chen, S.; Wang, C.; Jia, W. Biosynthesis of polyphenol-stabilised nanoparticles and assessment of anti-diabetic activity. J. Photochem. Photobiol. B 2017, 169, 96–100. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R.; Joardar, S.; Nandy, A.; Saha, A.; De Feo, V.; Dewanjee, S. Protocatechuic acid, a phenolic from Sansevieria roxburghiana leaves, suppresses diabetic cardiomyopathy via stimulating glucose metabolism, ameliorating oxidative stress, and inhibiting inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef]
- Gothai, S.; Ganesan, P.; Park, S.Y.; Fakurazi, S.; Choi, D.K.; Arulselvan, P. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: Inflammation as a target. Nutrients 2016, 8, 461. [Google Scholar] [CrossRef]
- Qaseem, A.; Humphrey, L.L.; Sweet, D.E.; Starkey, M.; Shekelle, P. Oral pharmacologic treatment of type 2 diabetes mellitus: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2012, 156, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Bhattacharjee, N. MicroRNA: A new generation therapeutic target in diabetic nephropathy. Biochem. Pharmacol. 2018, 155, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Barma, S.; Konwar, N.; Dewanjee, S.; Manna, P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: An update. Eur. J. Pharmacol. 2016, 791, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Khanra, R.; Bhattacharjee, N.; Dua, T.K.; Nandy, A.; Saha, A.; Kalita, J.; Manna, P.; Dewanjee, S. Taraxerol, a pentacyclic triterpenoid, from Abroma augusta leaf attenuates diabetic nephropathy in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 726–741. [Google Scholar] [CrossRef]
- Xavier, G.D.S. The cells of the islets of langerhans. J. Clin. Med. 2018, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Berenson, D.F.; Weiss, A.R.; Wan, Z.L.; Weiss, M.A. Insulin analogs for the treatment of diabetes mellitus: Therapeutic applications of protein engineering. Ann. N. Y. Acad. Sci. 2011, 1243, E40–E54. [Google Scholar] [CrossRef] [Green Version]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Kamiyama, O.; Sanae, F.; Ikeda, K.; Higashi, Y.; Minami, Y.; Asano, N.; Adachi, I.; Kato, A. In vitro inhibition of a-glucosidases and glycogen phosphorylase by catechin gallates in green tea. Food Chem. 2010, 122, 1061–1066. [Google Scholar] [CrossRef]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Potential antihyperglycaemic effect of myricetin derivatives from Syzygium malaccense. J. Funct. Foods 2016, 22, 325–336. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure-activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, V.M. Resveratrol in Management of Diabetes and Obesity: Clinical Applications, Bioavailability, and Nanotherapy. In Resveratrol-Adding Life to Years, Not Adding Years to Life; IntechOpen: Rijeka, Croatia, 2019; pp. 139–156. [Google Scholar]
- Oh, Y.S. Plant-derived compounds targeting pancreatic beta cells for the treatment of diabetes. Evid. Based Complement. Altern. Med. 2015, 2015, 629863. [Google Scholar] [CrossRef] [Green Version]
- Rouse, M.; Younès, A.; Egan, J.M. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphodiesterase activity. J. Endocrinol. 2014, 223, 107–117. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, L.; Lu, Y.; Zhang, J.; Jian, F.; Liu, Y.; Li, F.; Li, W.; Wang, X.; Li, G. Activation of SIRT1 protects pancreatic β-cells against palmitate-induced dysfunction. Biochim. Biophys. Acta 2012, 1822, 1815–1825. [Google Scholar] [CrossRef] [Green Version]
- Suman, R.K.; Mohanty, I.R.; Maheshwari, U.; Borde, M.K.; Deshmukh, Y.A. Natural dipeptidyl peptidase-IV inhibitor mangiferin mitigates diabetes- and metabolic syndrome-induced changes in experimental rats. Diabetes Metab. Syndr. Obes. 2016, 9, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, R.S.; Prashanth, K.V.H.; Manonmani, H.K. In Vitro antidiabetic effects of isolated triterpene glycoside fraction from gymnema sylvestre. Evid. Based Complement. Altern. Med. 2018, 2018, 7154702. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, P.B.; Gregersen, S.; Poulsen, C.R.; Hermansen, K. Stevioside acts directly on pancreatic beta cells to secrete insulin: Actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K+-channel activity. Metabolism 2000, 49, 208–214. [Google Scholar] [CrossRef]
- Philippaert, K.; Pironet, A.; Mesuere, M.; Sones, W.; Vermeiren, L.; Kerselaers, S.; Pinto, S.; Segal, A.; Antoine, N.; Gysemans, C.; et al. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat. Commun. 2017, 8, 14733. [Google Scholar] [CrossRef]
- Liu, J.; He, T.; Lu, Q.; Shang, J.; Sun, H.; Zhang, L. Asiatic acid preserves beta cell mass and mitigates hyperglycaemia in streptozocin-induced diabetic rats. Diabetes Metab. Res. Rev. 2010, 26, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Rashidi, R.; Shafiee-Nick, R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomed. Pharmacother. 2019, 111, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, C.; Raya, L.; Pérez, J.; González, I.; Pérez, S. Silymarin induces expression of pancreatic Nkx6.1 transcription factor and β-cells neogenesis in a pancreatectomy model. Molecules 2014, 19, 4654–4668. [Google Scholar] [CrossRef] [Green Version]
- Sameermahmood, Z.; Raji, L.; Saravanan, T.; Vaidya, A.; Mohan, V.; Balasubramanyam, M. Gallic acid protects RINm5F beta-cells from glucolipotoxicity by its antiapoptotic and insulin-secretagogue actions. Phytother. Res. 2010, 24, S83–S94. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [Green Version]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D.; et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef]
- Eid, S.; Boutary, S.; Braych, K.; Sabra, R.; Massaad, C.; Hamdy, A.; Rashid, A.; Moodad, S.; Block, K.; Gorin, Y.; et al. mTORC2 signaling regulates Nox4-induced podocyte depletion in diabetes. Antioxid. Redox Signal. 2016, 25, 703–719. [Google Scholar] [CrossRef] [Green Version]
- Sayem, A.S.M.; Arya, A.; Karimian, H.; Krishnasamy, N.; Ashok Hasamnis, A.; Hossain, C.F. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules 2018, 23, 258. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.H.X.; Howe, P.R.C. Resveratrol counteracts insulin resistance-potential role of the circulation. Nutrients 2018, 10, 1160. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.W.; Fu, M.; Gao, S.H.; Liu, J.L. Curcumin and diabetes: A systematic review. Evid. Based Complement. Altern. Med. 2013, 2013, 636053. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Ye, J.; Jia, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. 2012, 2, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, B.; Zambonin, L.; Angeloni, C.; Leoncini, E.; Dalla Sega, F.V.; Prata, C.; Fiorentini, D.; Hrelia, S. Steviol glycosides modulate glucose transport in different cell types. Oxid. Med. Cell. Longev. 2013, 2013, 348169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, V.; Saravanan, R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum. Exp. Toxicol. 2015, 34, 884–893. [Google Scholar] [CrossRef]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin ameliorates insulin resistance, hyperglycaemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Indian J. Exp. Biol. 2013, 51, 129–138. [Google Scholar]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic potential of gallic acid from Emblica officinalis: Improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine 2019, 152906. [Google Scholar] [CrossRef]
- Badr, G.; Mahmoud, M.H.; Farhat, K.; Waly, H.; Al-Abdin, O.Z.; Rabah, D.M. Maternal supplementation of diabetic mice with thymoquinone protects their offspring from abnormal obesity and diabetes by modulating their lipid profile and free radical production and restoring lymphocyte proliferation via PI3K/AKT signaling. Lipids Health Dis. 2013, 12, 37. [Google Scholar] [CrossRef] [Green Version]
- Jay, M.A.; Ren, J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr. Diabetes Rev. 2007, 3, 33–39. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, D.; Yu, N.; An, T.; Miao, J.; Mo, F.; Gu, Y.; Zhang, D.; Gao, S.; Jiang, G. Curcumin improves glycolipid metabolism through regulating peroxisome proliferator activated receptor γ signalling pathway in high-fat diet-induced obese mice and 3T3-L1 adipocytes. R. Soc. Open Sci. 2017, 4, 170917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Xiao, X.; Feng, K.; Wang, T.; Li, W.; Yuan, T.; Sun, X.; Sun, Q.; Xiang, H.; Wang, H. Berberine moderates glucose and lipid metabolism through multipathway mechanism. Evid. Based Complement. Altern. Med. 2011, 2011, 924851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eu, C.H.; Lim, W.Y.; Ton, S.H.; bin Abdul Kadir, K. Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis. 2010, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.; Han, M.; Ting, H.L.; Liu, Z.; Zhang, D. Scutellarin from Scutellaria baicalensis suppresses adipogenesis by upregulating PPARα in 3T3-L1 cells. J. Nat. Prod. 2013, 76, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Kim, Y.O.; Andrade, J.E.; Burgess, J.R.; Kim, Y.C. Dietary naringenin increases hepatic peroxisome proliferators-activated receptor α protein expression and decreases plasma triglyceride and adiposity in rats. Eur. J. Nutr. 2011, 50, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Tzeng, T.F.; Liou, S.S.; Chang, Y.S.; Liu, I.M. Myricetin increases hepatic peroxisome proliferator-activated receptor α protein expression and decreases plasma lipids and adiposity in rats. Evid. Based Complement. Altern. Med. 2012, 2012, 787152. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.I.; Xiao, F.L.; Mao, Y.J.; Ying, L.L.; Zhou, B.; Li, Y. Quercetin decreases the triglyceride content through the PPAR signalling pathway in primary hepatocytes of broiler chickens. Biotechnol. Biotechnol. Equip. 2019, 33, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Fomenko, E.V.; Chi, Y. Mangiferin modulation of metabolism and metabolic syndrome. Biofactors 2016, 42, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef]
- Goto, T.; Takahashi, N.; Kato, S.; Kim, Y.I.; Kusudo, T.; Taimatsu, A.; Egawa, K.; Kang, M.S.; Hiramatsu, T.; Sakamoto, T.; et al. Bixin activates PPARα and improves obesity-induced abnormalities of carbohydrate and lipid metabolism in mice. J. Agric. Food Chem. 2012, 60, 11952–11958. [Google Scholar] [CrossRef]
- Gu, M.; Zhao, P.; Huang, J.; Zhao, Y.; Wang, Y.; Li, Y.; Li, Y.; Fan, S.; Ma, Y.M.; Tong, Q.; et al. Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Front. Pharmacol. 2016, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Yan, Y.; Shen, X. Diabetic macular morphology changes may occur in the early stage of diabetes. BMC Ophthalmol. 2016, 16, 12. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Sun, W.; Guo, X.; Wu, L.; Hou, Y.; Ma, X.; Qin, L.; Gao, M.; Liu, T. Asiatic acid promotes liver fatty acid metabolism in diabetic models. Int. J. Clin. Exp. Med. 2018, 11, 11837–11845. [Google Scholar]
- Li, Y.; Sun, M.; Liu, Y.; Liang, J.; Wang, T.; Zhang, Z. Gymnemic acid alleviates type 2 diabetes mellitus and suppresses endoplasmic reticulum stress in vivo and in vitro. J. Agric. Food Chem. 2019, 67, 3662–3669. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Avila, J.A.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; de la Rosa, L.A. Modulation of PPAR expression and activity in response to polyphenolic compounds in high fat diets. Int. J. Mol. Sci. 2016, 17, 1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floyd, Z.E.; Wang, Z.Q.; Kilroy, G.; Cefalu, W.T. Modulation of peroxisome proliferator-activated receptor gamma stability and transcriptional activity in adipocytes by resveratrol. Metabolism 2008, 57, S32–S38. [Google Scholar] [CrossRef] [Green Version]
- Bing, S.; Haoqiang, Z.; Chunyu, M.; Yali, Y.; Jing, W.; Yumeng, G.; Hongwei, Z.; Xuezheng, L. The effects of emodin on insulin resistance in KKAy mice with diabetes mellitus. Pharmacogn. Mag. 2018, 14, 344–350. [Google Scholar]
- Pang, B.; Zhao, L.H.; Zhou, Q.; Zhao, T.Y.; Wang, H.; Gu, C.J.; Tong, X.L. Application of berberine on treating type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef] [Green Version]
- Chae, B.S. Protective effect of baicalin on the TNF-α-mediated development of insulin resistance in differentiated 3T3-L1 cells. Nat. Prod. Sci. 2013, 19, 316–323. [Google Scholar]
- Cho, S.Y.; Park, P.J.; Shin, H.J.; Kim, Y.K.; Shin, D.W.; Shin, E.S.; Lee, H.H.; Lee, B.G.; Baik, J.H.; Lee, T.R. (-)-Catechin suppresses expression of Kruppel-like factor 7 and increases expression and secretion of adiponectin protein in 3T3-L1 cells. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1166–E1172. [Google Scholar] [CrossRef] [Green Version]
- Dodda, D.; Ciddi, V. Plants used in the management of diabetic complications. Indian J. Pharm Sci. 2014, 76, 97–106. [Google Scholar] [PubMed]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Butler, A.E.; Sahebkar, A. Therapeutic potential of curcumin in diabetic complications. Pharmacol. Res. 2018, 136, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Oyenihi, O.R.; Oyenihi, A.B.; Adeyanju, A.A.; Oguntibeju, O.O. Antidiabetic effects of resveratrol: The way forward in its clinical utility. J. Diabetes Res. 2016, 2016, 9737483. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.J.; Li, Y.; Cao, Q.H.; Wu, H.X.; Tang, X.Y.; Gao, X.H.; Yu, J.Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Yu, J.; Liu, Z.Q.; Zhou, H.H. Apigenin attenuates diabetes-associated cognitive decline in rats via suppressing oxidative stress and nitric oxide synthase pathway. Int. J. Clin. Exp. Med. 2015, 8, 15506–15513. [Google Scholar] [PubMed]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am. J. Physiol. Ren. Physiol. 2017, 313, F414–F422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Shen, Q.; Chen, Y.; Pan, R.; Kuang, S.; Liu, G.; Sun, G.; Sun, X. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci. Rep. 2017, 7, 44239. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Solid lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid. Med. Cell. Longev. 2018, 2018, 7496936. [Google Scholar] [CrossRef] [Green Version]
- Ku, S.K.; Bae, J.S. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. 2015, 48, 519–524. [Google Scholar] [CrossRef]
- Wang, G.; Liang, J.; Gao, L.R.; Si, Z.P.; Zhang, X.T.; Liang, G.; Yan, Y.; Li, K.; Cheng, X.; Bao, Y.; et al. Baicalin administration attenuates hyperglycaemia-induced malformation of cardiovascular system. Cell Death Dis. 2018, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.G.; Lu, X.H.; Li, W.; Zhao, X.; Zhang, C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid. Based Complement. Altern. Med. 2011, 2011, 323171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Li, W.; Lu, X.; Bao, P.; Zhao, X. Luteolin ameliorates cardiac failure in type I diabetic cardiomyopathy. J. Diabetes Complicat. 2012, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.B.; Sinha, K.; Sil, P.C. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFα related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS ONE 2014, 9, e107220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Tong, R.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders (Review). Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Chen, Q.; Ke, D.; Li, G.; Deng, W. Emodin protects against diabetic cardiomyopathy by regulating the AKT/GSK-3β signaling pathway in the rat model. Molecules 2014, 19, 14782–14793. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, F.; Wang, W.; Su, Z.; Guo, C.; Cao, S. Emodin suppresses hyperglycaemia-induced proliferation and fibronectin expression in mesangial cells via inhibiting cFLIP. PLoS ONE 2014, 9, e93588. [Google Scholar]
- Hasanein, P.; Mohammad Zaheri, L. Effects of rosmarinic acid on an experimental model of painful diabetic neuropathy in rats. Pharm. Biol. 2014, 52, 1398–1402. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef]
- Prata, C.; Zambonin, L.; Rizzo, B.; Maraldi, T.; Angeloni, C.; Vieceli Dalla Sega, F.; Fiorentini, D.; Hrelia, S. Glycosides from Stevia rebaudiana Bertoni possess insulin-mimetic and antioxidant activities in rat cardiac fibroblasts. Oxid. Med. Cell. Longev. 2017, 2017, 3724545. [Google Scholar] [CrossRef] [Green Version]
- Rotimi, S.O.; Rotimi, O.A.; Adelani, I.B.; Onuzulu, C.; Obi, P.; Okungbaye, R. Stevioside modulates oxidative damage in the liver and kidney of high fat/low streptozocin diabetic rats. Heliyon 2018, 4, e00640. [Google Scholar] [CrossRef] [Green Version]
- Nagoor Meeran, M.F.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: A pentacyclic triterpenoid of therapeutic promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, Z.; Quan, M.; Lv, Y.; Li, Y.; Xin, H.B.; Qian, Y. Asiatic acid protests against myocardial ischemia/reperfusion injury via modulation of glycometabolism in rat cardiomyocyte. Drug Des. Dev. Ther. 2018, 12, 3573–3582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.N.; Wu, C.G.; Shi, B.M.; Qian, K.; Ding, Y. The protective effect of asiatic acid on podocytes in the kidney of diabetic rats. Am. J. Transl. Res. 2018, 10, 3733–3741. [Google Scholar] [PubMed]
- Kim, A.; Lee, W.; Yun, J.M. Luteolin and fisetin suppress oxidative stress by modulating sirtuins and forkhead box O3a expression under in vitro diabetic conditions. Nutr. Res. Pract. 2017, 11, 430–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, V.; Nargis, S.; Gonzalez, M.; Pradhan, S.; Terreros, D.; Chattopadhyay, M. Role of glycyrrhizin in the reduction of inflammation in diabetic kidney disease. Nephron 2017, 137, 137–147. [Google Scholar] [CrossRef]
- Akutagawa, K.; Fujita, T.; Ouhara, K.; Takemura, T.; Tari, M.; Kajiya, M.; Matsuda, S.; Kuramitsu, S.; Mizuno, N.; Shiba, H.; et al. Glycyrrhizic acid suppresses inflammation and reduces the increased glucose levels induced by the combination of Porphyromonas gulae and ligature placement in diabetic model mice. Int. Immunopharmacol. 2019, 68, 30–38. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Steinle, J.J. Glycyrrhizin protects the diabetic retina against permeability, neuronal, and vascular damage through anti-inflammatory mechanisms. J. Clin. Med. 2019, 8, 957. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Wang, J.; Lu, X.; Xu, Y.; Zheng, S.; Luo, C.; Li, Y. Protective effects of scutellarin on type II diabetes mellitus-induced testicular damages related to reactive oxygen species/Bcl-2/Bax and reactive oxygen species/microcirculation/staving pathway in diabetic rat. J. Diabetes Res. 2015, 2015, 252530. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, X.; Wang, L.; Hao, T.; Cheng, Y.; Wang, D. Scutellarin exerts hypoglycaemic and renal protective effects in db/db mice via the Nrf2/HO-1 signaling pathway. Oxid. Med. Cell. Longev. 2019, 2019, 1354345. [Google Scholar]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Res. 2016, 2016, 5147468. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.S.; Goyal, R.K. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacogn. Res. 2011, 3, 239–245. [Google Scholar]
- Ramkumar, K.M.; Vijayakumar, R.S.; Vanitha, P.; Suganya, N.; Manjula, C.; Rajaguru, P.; Sivasubramanian, S.; Gunasekaran, P. Protective effect of gallic acid on alloxan-induced oxidative stress and osmotic fragility in rats. Hum. Exp. Toxicol. 2014, 33, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; El-Twab, S.M.A.; Ashour, M.B.; Yousef, A.I. Hepato-renal protective effects of gallic acid and p-coumaric acid in nicotinamide/streptozotocin-induced diabetic rats. Int. J. Bioassays 2016, 5, 4641–4649. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wang, L.; Zhou, Q.; Yan, S.; Li, Z.; Sheng, J.; Zhang, W. (+)-Catechin ameliorates diabetic nephropathy by trapping methylglyoxal in type 2 diabetic mice. Mol. Nutr. Food Res. 2014, 58, 2249–2260. [Google Scholar] [CrossRef]
- Addepalli, V.; Suryavanshi, S.V. Catechin attenuates diabetic autonomic neuropathy in streptozotocin induced diabetic rats. Biomed. Pharmacother. 2018, 108, 1517–1523. [Google Scholar] [CrossRef]
- Sankaranarayanan, C. Thymoquinone, a panacea for diabetic complications-an overview. EC Diabetes Metab. Res. 2019, 3, 120–126. [Google Scholar]
- Choi, R.; Kim, B.H.; Naowaboot, J.; Lee, M.Y.; Hyun, M.R.; Cho, E.J.; Lee, E.S.; Lee, E.Y.; Yang, Y.C.; Chung, C.H. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med. 2011, 43, 676–683. [Google Scholar] [CrossRef]
- Song, Y.; Wen, L.; Sun, J.; Bai, W.; Jiao, R.; Hu, Y.; Peng, X.; He, Y.; Ou, S. Cytoprotective mechanism of ferulic acid against high glucose-induced oxidative stress in cardiomyocytes and hepatocytes. Food Nutr. Res. 2016, 60, 30323. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic acid protects hyperglycaemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed. 2019, 14, 5303–5321. [Google Scholar] [CrossRef]
- Suresh, K.; Nangia, A. Curcumin: Pharmaceutical solids as a platform to improve solubility and bioavailability. CrystEngComm 2018, 24, 3277–3296. [Google Scholar] [CrossRef]
- Ernest, U.; Chen, H.Y.; Xu, M.J.; Taghipour, Y.; Asad, M.; Rahimi, R.; Murtaza, G. Anti-cancerous potential of polyphenol-loaded polymeric nanotherapeutics. Molecules 2018, 23, 2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. Biofactors 2013, 39, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J.M. Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [Green Version]
- Gera, S.; Talluri, S.; Rangaraj, N.; Sampathi, S. Formulation and evaluation of naringenin nanosuspensions for bioavailability enhancement. AAPS PharmSciTech 2017, 18, 3151–3162. [Google Scholar] [CrossRef]
- Song, I.; Cha, J.; Choi, M. Enhanced oral bioavailability of naringenin administered in a mixed micelle formulation with Pluronic F127 and Tween 80 in rats. J. Pharm. Investig. 2015, 45, 633–640. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kang, M.J.; Choi, H.N.; Kim, J.H.; Kim, J.I. Quercetin ameliorates hyperglycaemia and dyslipidaemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, P.; Prajapati, A.K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers—A review. RSC Adv. 2015, 5, 97547–97562. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Shakeel, F.; Ibrahim, M.A.; Elzayat, E.M.; Altamimi, M.; Mohsin, K.; Almeanazel, O.T.; Alkholief, M.; Alshetaili, A.; Alsulays, B.; et al. Dissolution and bioavailability improvement of bioactive apigenin using solid dispersions prepared by different techniques. Saudi Pharm. J. 2019, 27, 264–273. [Google Scholar] [CrossRef]
- Fernandez, S.P.; Nguyen, M.; Yow, T.T.; Chu, C.; Johnston, G.A.R.; Hanrahan, J.R.; Chebib, M. The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochem. Res. 2009, 34, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Man, N.; Wang, Q.; Li, H.; Adu-Frimpong, M.; Sun, C.; Zhang, K.; Yang, Q.; Wei, Q.; Ji, H.; Toreniyazov, E.; et al. Improved oral bioavailability of myricitrin by liquid self-microemulsifying drug delivery systems. J. Drug Deliv. Sci. Technol. 2019, 52, 597–606. [Google Scholar] [CrossRef]
- Cui, L.; Sune, E.; Song, J.; Wang, J.; Jia, X.B.; Zhang, Z.H. Characterization and bioavailability study of baicalin-mesoporous carbon nanopowder solid dispersion. Pharmacogn. Mag. 2016, 12, 326–332. [Google Scholar] [PubMed]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and bioavailability enhancement of baicalin: A Review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef]
- Sarawek, S.; Derendorf, H.; Butterweck, V. Pharmacokinetics of luteolin and metabolites in rats. Nat. Prod. Commun. 2008, 3, 2019–2036. [Google Scholar] [CrossRef] [Green Version]
- Khurana, R.K.; Bansal, A.K.; Beg, S.; Burrow, A.J.; Katare, O.P.; Singh, K.K.; Singh, B. Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: Systematic development, characterization and evaluation. Int. J. Pharm. 2017, 518, 289–306. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. Biomed. Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, R. Studies on gymnemic acids nanoparticulate formulations against diabetes mellitus. In Nanotechnology: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2014; pp. 1276–1288. [Google Scholar]
- Janković-Tomanić, M.; Todorović, D.; Stanivuković, Z.; Perić Mataruga, V.; Wessjohann, L.A.; Kaluđerović, G.N. Mesoporous silica nanoparticles SBA-15 loaded with emodin upregulate the antioxidative defense of Euproctis chrysorrhoea (L.) larvae. Turk. J. Biol. 2017, 41, 935–942. [Google Scholar] [CrossRef]
- Ban, E.; Park, M.; Jeong, S.; Kwon, T.; Kim, E.H.; Jung, K.; Kim, A. Poloxamer-based thermoreversible gel for topical delivery of emodin: Influence of P407 and P188 on solubility of emodin and its application in cellular activity screening. Molecules 2017, 22, 246. [Google Scholar] [CrossRef] [Green Version]
- Madureira, A.R.; Nunes, S.; Campos, D.A.; Fernandes, J.C.; Marques, C.; Zuzarte, M.; Gullón, B.; Rodríguez-Alcalá, L.M.; Calhau, C.; Sarmento, B.; et al. Safety profile of solid lipid nanoparticles loaded with rosmarinic acid for oral use: In vitro and animal approaches. Int. J. Nanomed. 2016, 11, 3621–3640. [Google Scholar]
- Wang, J.; Li, G.; Rui, T.; Kang, A.; Li, G.; Fu, T.; Li, J.; Di, L.; Cai, B. Pharmacokinetics of rosmarinic acid in rats by LC-MS/MS: Absolute bioavailability and dose proportionality. RSC Adv. 2017, 7, 9057–9063. [Google Scholar] [CrossRef] [Green Version]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Somagoni, J.; Singh, M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS ONE 2014, 9, e89919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Wang, T.; Chen, K.; Wang, H.; Jia, Q.; Li, Y. Enhancement of berberine hypoglycaemic activity by oligomeric proanthocyanidins. Molecules 2018, 23, 3318. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Liu, F.; Li, Z.; Lin, Y.; Liu, H.; Hu, P.; Chen, M.; Sun, Z.; Xu, Z.; Zhang, Y.; et al. Enhanced anti-diabetic effect of berberine combined with timosaponin b2 in goto-kakizaki rats, associated with increased variety and exposure of effective substances through intestinal absorption. Front. Pharmacol. 2019, 10, 19. [Google Scholar] [CrossRef]
- Barwal, I.; Sood, A.; Sharma, M.; Singh, B.; Yadav, S.C. Development of stevioside Pluronic-F-68 copolymer based PLA-nanoparticles as an antidiabetic nanomedicine. Colloids Surf. B Biointerfaces 2013, 101, 510–516. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources (ANS). Scientific opinion on safety of steviol glycosides for the proposed uses as a food additive. EFSA J. 2010, 8, 1537. [Google Scholar] [CrossRef]
- Bundgaard Anker, C.C.; Rafiq, S.; Jeppesen, P.B. Effect of steviol glycosides on human health with emphasis on type 2 diabetic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2019, 11, 1965. [Google Scholar] [CrossRef] [Green Version]
- Lingling, G.; Yuan, Z.; Weigen, L. Preparation, optimization, characterization and in vivo pharmacokinetic study of asiatic acid tromethamine salt-loaded solid lipid nanoparticles. Drug Dev. Ind. Pharm. 2016, 42, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Fu, S.; Han, J.; Jin, S.; Lv, Q.; Lu, Y.; Qi, J.; Wu, W.; Yuan, H. Improvement of oral bioavailability of glycyrrhizin by sodium deoxycholate/phospholipid-mixed nanomicelles. J. Drug Target. 2012, 20, 615–622. [Google Scholar] [CrossRef]
- Yuan, G.F.; Sinclair, A.J.; Zhou, C.Q.; Li, D. α-eleostearic acid is more effectively metabolized into conjugated linoleic acid than punicic acid in mice. J. Sci. Food Agric. 2009, 89, 1006–1011. [Google Scholar] [CrossRef]
- Paul, D.; Manna, K.; Sengupta, A.; Mukherjee, S.; Dey, S.; Bag, P.K.; Dhar, P. A novel nanoformulation of α-eleostearic acid restores molecular pathogenesis of hypersensitivity. Nanomedicine (Lond.) 2019, 14, 529–552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wan, J.; Wu, L.; Yi, T.; Liu, W.; Xu, H.; Yang, X. A new strategy for enhancing the oral bioavailability of drugs with poor water-solubility and low liposolubility based on phospholipid complex and supersaturated SEDDS. PLoS ONE 2013, 8, e84530. [Google Scholar] [CrossRef]
- Liu, S.; Ho, P.C. Formulation optimization of scutellarin-loaded HP-β-CD/chitosan nanoparticles using response surface methodology with Box-Behnken design. Asian J. Pharm. Sci. 2017, 12, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; Angelico, R. Formulation strategies for enhancing the bioavailability of silymarin: The state of the art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Far, Y.M.; Zakaria, M.M.; Gabr, M.M.; El Gayar, A.M.; El-Sherbiny, I.M.; Eissa, L.A. A newly developed silymarin nanoformulation as a potential antidiabetic agent in experimental diabetes. Nanomedicine (Lond.) 2016, 11, 2581–2602. [Google Scholar] [CrossRef] [Green Version]
- de Cristo Soares Alves, A.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 126–134. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of tea catechins and its improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [Green Version]
- El Mohsen, M.A.; Marks, J.; Kuhnle, G.; Moore, K.; Debnam, E.; Kaila Srai, S.; Rice-Evans, C.; Spencer, J.P. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br. J. Nutr. 2006, 95, 51–58. [Google Scholar] [CrossRef]
- Mohammadabadi, M.R.; Mozafari, M.R. Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.H.; Kumar, S. Improvement of antihyperglycaemic activity of nano-thymoquinone in rat model of type-2 diabetes. Chem. Biol. Interact. 2018, 295, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Anson, N.M.; den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, W.; Zu, Y.; Zhang, Y.; Li, Y.; Sun, W.; Shan, C.; Ge, Y. Preparation and characterization of betulin nanoparticles for oral hypoglycaemic drug by antisolvent precipitation. Drug Deliv. 2014, 21, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Myz, S.A.; Shakhtshneider, T.P.; Mikhailenko, M.A.; Ogienko, A.G.; Bogdanova, E.G.; Ogienko, A.A.; Kuznetsova, S.A.; Boldyreva, E.V.; Boldyrev, V.V. Ultrafine betulin formulation with biocompatible carriers exhibiting improved dissolution rate. Nat. Prod. Commun. 2015, 10, 1345–1347. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ma, Y.; Ma, W. Pharmacokinetics and bioavailability of cinnamic acid after oral administration of Ramulus Cinnamomi in rats. Eur. J. Drug Metab. Pharmacokinet. 2009, 34, 51–56. [Google Scholar] [CrossRef]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A review on biosynthesis, analytical techniques, and pharmacological activities of trigonelline as a plant alkaloid. J. Diet. Suppl. 2018, 15, 207–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, F.; Zhen, L.; Zhu, X.; Wang, J.; Li, S.; Geng, J.; Sun, R.; Yu, X.; Chen, T.; et al. Sensitive analysis and simultaneous assessment of pharmacokinetic properties of crocin and crocetin after oral administration in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1044–1045, 1–7. [Google Scholar] [CrossRef]
- Yang, X. Design and optimization of crocetin loaded PLGA nanoparticles against diabetic nephropathy via suppression of inflammatory biomarkers: A formulation approach to preclinical study. Drug Deliv. 2019, 26, 849–859. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, Y.; Fu, Z.; Li, D. Preparation, characterization, and in vivo study of rhein solid lipid nanoparticles for oral delivery. Chem. Biol. Drug Des. 2017, 90, 867–872. [Google Scholar] [CrossRef]
- Luo, J.; Sun, J.; Luo, X.; Wei, Y.; Zheng, H.; Mu, C.; Yao, W. Low molecular weight chitosan-based conjugates for efficient Rhein oral delivery: Synthesis, characterization, and pharmacokinetics. Drug Dev. Ind. Pharm. 2019, 45, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.C.; Liu, Y.T.; Lin, Y.J.; Yang, Y.C.; Chen, C.C.; Yao, H.T.; Chen, H.W.; Lii, C.K. Bioavailability of the diterpenoid 14-deoxy-11,12-didehydroandrographolide in rats and up-regulation of hepatic drug-metabolizing enzyme and drug transporter expression. Phytomedicine 2019, 61, 152841. [Google Scholar] [CrossRef] [PubMed]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.; Sistla, R. Enhanced oral bioavailability and anticancer efficacy of fisetin by encapsulating as inclusion complex with HPβCD in polymeric nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Grynkiewicz, G.; Demchuk, O.M. New perspectives for fisetin. Front. Chem. 2019, 7, 697. [Google Scholar] [CrossRef] [PubMed]
- Mercke Odeberg, J.; Lignell, A.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, V.; Salatti-Dorado, J.Á.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Bastias Venegas, J.; Pauthe, E.; et al. Astaxanthin-loaded nanostructured lipid carriers for preservation of antioxidant activity. Molecules 2018, 23, 2601. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.B.; Vuong le, T.; Ruckle, J.; Synal, H.A.; Schulze-König, T.; Wertz, K.; Rümbeli, R.; Liberman, R.G.; Skipper, P.L.; Tannenbaum, S.R.; et al. Lycopene bioavailability and metabolism in humans: An accelerator mass spectrometry study. Am. J. Clin. Nutr. 2011, 93, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- de Sousa Lobato, K.B.; Paese, K.; Forgearini, J.C.; Guterres, S.S.; Jablonski, A.; de Oliveira Rios, A. Evaluation of stability of bixin in nanocapsules in model systems of photosensitization and heating. LWT Food Sci. Technol. 2015, 60, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Amar, I.; Aserin, A.; Garti, N. Solubilization patterns of lutein and lutein esters in food grade nonionic microemulsions. J. Agric. Food Chem. 2003, 51, 4775–4781. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Xu, X.-D.; Shao, B.; Shen, Q.; Zhou, H.; Hong, Y.-M.; Yu, L-M. Physicochemical properties and bioavailability of lutein microencapsulation (LM). Food Sci. Technol. Res. 2015, 21, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RSC Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.K.; Lin, S.X.; Tsai, M.J.; Leong, M.K.; Lin, S.R.; Kankala, R.K.; Lee, C.H.; Weng, C.F. Encapsulation of 16-hydroxycleroda-3,13-dine-16,15-olide in mesoporous silica nanoparticles as a natural dipeptidyl peptidase-4 inhibitor potentiated hypoglycaemia in diabetic mice. Nanomaterials 2017, 7, 112. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.M.; Chiang. P.Y. Preparation and evaluation of release formulation of γ-oryzanol/algae oil self-emulsified with alginate beads. Mar. Drugs 2019, 17, 156. [Google Scholar] [CrossRef] [Green Version]
- Patlolla, J.M.R.; Rao, C.V. Anti-inflammatory and anti-cancer properties of β-escin, a triterpene saponin. Curr. Pharmacol. Rep. 2015, 1, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.S.; Lei, L.; Deng, X.; Zheng, Y.; Li, Y.; Li, J.; Mei, Z.N. Novel application of neural network modelling for multicomponent herbal medicine optimization. Sci. Rep. 2019, 9, 15442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libinaki, R.; Gavin, P.D. Changes in bioavailability of omega-3 (DHA) through Alpha-Tocopheryl Phosphate Mixture (TPM) after oral administration in rats. Nutrients 2017, 9, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petyaev, I.M.; Chalyk, N.E.; Klochkov, V.A.; Pristensky, D.V.; Chernyshova, M.P.; Kyle, N.H.; Bashmakov, Y.K. Pharmacokinetics and oxidation parameters in volunteers supplemented with microencapsulated docosahexaenoic acid. Int. J. Appl. Basic Med. Res. 2018, 8, 148–154. [Google Scholar] [PubMed]
- LaVan, D.A.; Lynn, D.M.; Langer, R. Moving smaller in drug discovery and delivery. Nat. Rev. Drug Discov. 2002, 1, 77. [Google Scholar] [CrossRef]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef] [Green Version]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Kapoor, B.; Gulati, M.; Kumar, R.; Ramanunny, A.K.; Awasthi, A.; Dua, K. Treatment strategies against diabetes: Success so far and challenges ahead. Eur. J. Pharmacol. 2019, 862, 172625. [Google Scholar] [CrossRef]
- Hu, C.; Jia, W. Therapeutic medications against diabetes: What we have and what we expect. Adv. Drug Deliv. Rev. 2019, 139, 3–15. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.H.; Bansal, V.; Kumar. S.; Dilbaghi, N.; Kim, Y.H. Modern progress and future challenges in nanocarriers for probe applications. TrAC Trend. Anal. Chem. 2017, 86, 235–250. [Google Scholar] [CrossRef]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2015, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dile, Z.; Chakraborty, S.; Roy, S.; Mukherjee, B.; Besra, S.E.; Dewanjee, S.; Mukherjee, A.; Ojha, P.K.; Kumar, V.; et al. Aptamer-functionalized drug-nanocarrier improves hepatocellular carcinoma towards normal by targeting neoplastic hepatocytes. Mol. Ther. Nucleic Acids 2020. [Google Scholar] [CrossRef]
- Allam, A.N.; Komeil, I.A.; Fouda, M.A.; Abdallah, O.Y. Preparation, characterization and in vivo evaluation of curcumin self-nano phospholipid dispersion as an approach to enhance oral bioavailability. Int. J. Pharm. 2015, 489, 117–123. [Google Scholar] [CrossRef]
- Gouda, W.; Hafiz, N.A.; Mageed, L.; Alazzouni, A.S.; Khalil, W.K.; Afify, M.; Abdelmaksoud, M.D. Effects of nano-curcumin on gene expression of insulin and insulin receptor. Bull. Natl. Res. Cent. 2019, 43, 128. [Google Scholar] [CrossRef] [Green Version]
- Raslan, M.M.; Mohamed, S.; Abd El Maksoud, M.D.E.; El Nesr, K. Role of curcumin-zinc oxide composite nanoparticles on streptozotocin-induced diabetic rats. J. Biotechnol. Biomater. 2018, 8, 55. [Google Scholar]
- Tong, F.; Chai, R.; Jiang, H.; Dong, B. In vitro/vivo drug release and anti-diabetic cardiomyopathy properties of curcumin/PBLG-PEG-PBLG nanoparticles. Int. J. Nanomed. 2018, 13, 1945. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Joshi, R.P.; Negi, G.; Kumar, A.; Pawar, Y.B.; Munjal, B.; Bansal, A.K.; Sharma, S.S. SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: An insight into its mechanism for neuroprotection. Nanomedicine 2013, 9, 776–785. [Google Scholar] [CrossRef]
- Grama, C.N.; Suryanarayana, P.; Patil, M.A.; Raghu, G.; Balakrishna, N.; Kumar, M.R.; Reddy, G.B. Efficacy of biodegradable curcumin nanoparticles in delaying cataract in diabetic rat model. PLoS ONE 2013, 8, e78217. [Google Scholar] [CrossRef] [Green Version]
- El-Far, Y.M.; Zakaria, M.M.; Gabr, M.M.; El Gayar, A.M.; Eissa, L.A.; El-Sherbiny, I.M. Nanoformulated natural therapeutics for management of streptozotocin-induced diabetes: Potential use of curcumin nanoformulation. Nanomedicine 2017, 12, 1689–1711. [Google Scholar] [CrossRef]
- Devadasu, V.R.; Wadsworth, R.M.; Kumar, M.R. Protective effects of nanoparticulate coenzyme Q 10 and curcumin on inflammatory markers and lipid metabolism in streptozotocin-induced diabetic rats: A possible remedy to diabetic complications. Drug Deliv. Transl. Res. 2011, 1, 448–455. [Google Scholar] [CrossRef]
- Jia, T.; Rao, J.; Zou, L.; Zhao, S.; Yi, Z.; Wu, B.; Li, L.; Yuan, H.; Shi, L.; Zhang, C.; et al. Nanoparticle-encapsulated curcumin inhibits diabetic neuropathic pain involving the P2Y12 receptor in the dorsal root ganglia. Front. Neurosci. 2018, 11, 755. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.E.; Al-Joufi, F.; Anwar, M.; Attia, M.F.; El-Bana, M.A. Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf. B Biointerfaces 2019, 177, 389–398. [Google Scholar] [CrossRef]
- Chauhan, P.; Tamrakar, A.K.; Mahajan, S.; Prasad, G.B.K.S. Chitosan encapsulated nanocurcumin induces GLUT-4 translocation and exhibits enhanced anti-hyperglycaemic function. Life Sci. 2018, 213, 226–235. [Google Scholar] [CrossRef]
- Kamar, S.S.; Abdel-Kader, D.H.; Rashed, L.A. Beneficial effect of Curcumin Nanoparticles-Hydrogel on excisional skin wound healing in type-I diabetic rat: Histological and immunohistochemical studies. Ann. Anat. 2019, 222, 94–102. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of curcumin nanoparticles with MMP9-responsive and thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef]
- Katas, H.; Wen, C.Y.; Siddique, M.I.; Hussain, Z.; Mohd Fadhil, F.H. Thermoresponsive curcumin/DsiRNA nanoparticle gels for the treatment of diabetic wounds: Synthesis and drug release. Ther. Deliv. 2017, 8, 137–150. [Google Scholar] [CrossRef]
- Karri, V.V.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.; Malayandi, R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Nagesh, P.K.; Jaggi, M.; Chauhan, S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Mobarhan, M.G.; Oskuee, R.K. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 567–577. [Google Scholar]
- Na, L.X.; Li, Y.; Pan, H.Z.; Zhou, X.L.; Sun, D.J.; Meng, M.; Li, X.X.; Sun, C.H. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Santos, A.C.; Veiga, F.J.; Sequeira, J.A.D.; Fortuna, A.; Falcão, A.; Pereira, I.; Pattekari, P.; Fontes-Ribeiro, C.; Ribeiro, A.J. First-time oral administration of resveratrol-loaded layer-by-layer nanoparticles to rats-a pharmacokinetics study. Analyst 2019, 144, 2062–2079. [Google Scholar] [CrossRef]
- Yücel, Ç.; Karatoprak, G.Ş.; Aktaş, Y. Nanoliposomal resveratrol as a novel approach to treatment of diabetes mellitus. J. Nanosci. Nanotechnol. 2018, 18, 3856–3864. [Google Scholar] [CrossRef]
- Yücel, Ç.; Karatoprak, G.Ş.; Atmar, A. Novel resveratrol-loaded nanocochleates and effectiveness in the treatment of diabetes. Fabad J. Pharm. Sci. 2018, 43, 35–44. [Google Scholar]
- Al-Bishri, W.M. Attenuating impacts of chromium and nano resveratrol against hyperglycaemia induced oxidative stress in diabetic rats. Int. J. Pharm. Res. Allied Sci. 2017, 6, 61–69. [Google Scholar]
- Mohseni, R.; ArabSadeghabadi, Z.; Ziamajidi, N.; Abbasalipourkabir, R.; RezaeiFarimani, A. Oral administration of resveratrol-loaded solid lipid nanoparticle improves insulin resistance through targeting expression of SNARE proteins in adipose and muscle tissue in rats with type 2 diabetes. Nanoscale Res. Lett. 2019, 14, 227. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, L.; Quan, Y.; Wei, K. Resveratrol-loaded PLGA nanoparticles: Enhanced stability, solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R. Soc. Open Sci. 2018, 5, 181457. [Google Scholar] [CrossRef] [Green Version]
- Siu, F.Y.; Ye, S.; Lin, H.; Li, S. Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: Enhanced bioavailability and in vitro anti-inflammatory activity. Int. J. Nanomed. 2018, 13, 4133–4144. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef]
- Chaurasia, S.; Patel, R.R.; Vure, P.; Mishra, B. Oral naringenin nanocarriers: Fabrication, optimization, pharmacokinetic and chemotherapeutic efficacy assessments. Nanomedicine (Lond.) 2017, 12, 1243–1260. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Firempong, C.K.; Zhang, H.; Wang, M.; Zhang, Y.; Zhu, Y.; Yu, J.; Xu, X. Enhanced solubility and bioavailability of naringenin via liposomal nanoformulation: Preparation and in vitro and in vivo evaluations. AAPS PharmSciTech 2017, 18, 586–594. [Google Scholar] [CrossRef]
- Ganesan, P.; Arulselvan, P.; Choi, D.K. Phytobioactive compound-based nanodelivery systems for the treatment of type 2 diabetes mellitus-current status. Int. J. Nanomed. 2017, 12, 1097–1111. [Google Scholar] [CrossRef] [Green Version]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals-An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Chitkara, D.; Nikalaje, S.K.; Mittal, A.; Chand, M.; Kumar, N. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Deliv. Transl. Res. 2012, 2, 112–123. [Google Scholar] [CrossRef]
- Alam, M.M.; Abdullah, K.M.; Singh, B.R.; Naqvi, A.H.; Naseem, I. Ameliorative effect of quercetin nanorods on diabetic mice: Mechanistic and therapeutic strategies. RSC Adv. 2016, 6, 55092–55103. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Maity, S.; Mandal, S.; Chakraborti, A.S.; Prajapati, A.K.; Kundu, P.P. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym. 2018, 182, 42–51. [Google Scholar] [CrossRef]
- Singh, J.; Mittal, P.; Vasant Bonde, G.; Ajmal, G.; Mishra, B. Design, optimization, characterization and in-vivo evaluation of Quercetin enveloped Soluplus®/P407 micelles in diabetes treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, S546–S555. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Esmaeili, A.; Beheshti, S. Effect of quercetin-conjugated superparamagnetic iron oxide nanoparticles on diabetes-induced learning and memory impairment in rats. Int. J. Nanomed. 2018, 13, 6311–6324. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Cui, C.; Wei, F.; Lv, H. Improved solubility and oral bioavailability of apigenin via soluplus/pluronic F127 binary mixed micelles system. Drug Dev. Ind. Pharm. 2017, 43, 1276–1282. [Google Scholar] [CrossRef]
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X.B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327–2333. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. Effect of apigenin-loaded nanoliposomes on myocardial cells apoptosis induced by diabetic cardiomyopathy. Her. Med. 2019, 38, 555–559. [Google Scholar]
- Chen, H.; Gao, Y.; Wu, J.; Chen, Y.; Chen, B.; Hu, J.; Zhou, J. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014, 354, 5–11. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, J.; Zheng, X.; Wu, J.; Zhou, Y.; Yu, Y.; Ye, Y.; Zhang, L.; Zhao, L. Preparation, pharmacokinetics and biodistribution of baicalin-loaded liposomes. Int. J. Nanomed. 2014, 9, 3623–3630. [Google Scholar]
- Shi, F.; Wei, Z.; Zhao, Y.; Xu, X. Nanostructured lipid carriers loaded with baicalin: An efficient carrier for enhanced antidiabetic effects. Pharmacogn. Mag. 2016, 12, 198–202. [Google Scholar]
- Puhl, A.C.; Fagundes, M.; dos Santos, K.C.; Polikarpov, I.; das Graças, M.F.; da Silva, F.; Fernandes, J.B.; Vieira, P.C.; Forim, M.R. Preparation and characterization of polymeric nanoparticles loaded with the flavonoid luteolin, by using factorial design. Int. J. Drug Deliv. 2011, 3, 683–698. [Google Scholar]
- Majumdar, D.; Jung, K.H.; Zhang, H.; Nannapaneni, S.; Wang, X.; Amin, A.R.; Chen, Z.; Chen, Z.G.; Shin, D.M. Luteolin nanoparticle in chemoprevention: In vitro and in vivo anticancer activity. Cancer Prev. Res. (Phila.) 2014, 7, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Dang, H.; Meng, M.H.W.; Zhao, H.; Iqbal, J.; Dai, R.; Deng, Y.; Lv, F. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J. Nanopart. Res. 2014, 16, 2347. [Google Scholar]
- Samadarsi, R.; Dutta, D. Design and characterization of mangiferin nanoparticles for oral delivery. J. Food. Eng. 2019, 247, 80–94. [Google Scholar] [CrossRef]
- Ravichandran, R. Physico-chemical evaluation of gymnemic acids nanocrystals. Int. J. Nanopart. 2010, 3, 280–296. [Google Scholar] [CrossRef]
- Ravichandran, R. Formulation of nanosuspensions of gymnemic acids for oral administration. Int. J. Nanopart. 2010, 3, 309–324. [Google Scholar] [CrossRef]
- Rajarajeshwari, T.; Shivashria, C.; Rajasekar, P. Synthesis and characterization of biocompatible gymnemic acid-gold nanoparticles: A study on glucose uptake stimulatory effect in 3T3-L1 adipocytes. RSC Adv. 2014, 4, 63285–63295. [Google Scholar] [CrossRef]
- Senthilnathan, B.; Vivekanandan, K.; Bhavya, E.; Masilamani; Swarna, P. B. Impact of nanoparticulate drug delivery system of herbal drug in control of diabetes mellitus. Res. J. Pharm. Technol. 2019, 12, 1688–1694. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Ren, Y.; Wang, H.; Cong, Z.; Wu, G.; Du, L.; Li, H.; Zhang, X. Pharmacokinetics and tissue distribution of emodin loaded nanoemulsion in rats. J. Drug Deliv. Sci. Technol. 2015, 30, 242–249. [Google Scholar] [CrossRef]
- Li, L.; Sheng, X.; Zhao, S.; Zou, L.; Han, X.; Gong, Y.; Yuan, H.; Shi, L.; Guo, L.; Jia, T.; et al. Nanoparticle-encapsulated emodin decreases diabetic neuropathic pain probably via a mechanism involving P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2017, 13, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.-C.; Tsai, H-C. Rosmarinic acid and curcumin-loaded polyacrylamide-cardiolipin-poly(lactide-co-glycolide) nanoparticles with conjugated 83-14 monoclonal antibody to protect β-amyloid-insulted neurons. J. Alzheimers Dis. Parkinsonism. 2018, 8, 41. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Rajesh, R. Targeted delivery of rosmarinic acid across the blood-brain barrier for neuronal rescue using polyacrylamide-chitosan-poly(lactide-co-glycolide) nanoparticles with surface cross-reacting material 197 and apolipoprotein E. Int. J. Pharm. 2017, 528, 228–241. [Google Scholar] [CrossRef]
- Grote, C.W.; Wright, D.E. A role for insulin in diabetic neuropathy. Front. Neurosci. 2016, 10, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, D.A.; Madureira, A.R.; Gomes, A.M.; Sarmento, B.; Pintado, M.M. Optimization of the production of solid Witepsol nanoparticles loaded with rosmarinic acid. Colloids Surf. B Biointerfaces 2014, 115, 109–117. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery-in vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef]
- Wani, T.U.; Raza, S.N.; Khan, N.A. Rosmarinic acid loaded chitosan nanoparticles for wound healing in rats. Int. J. Pharm. Sci. Res. 2019, 10, 1126–1135. [Google Scholar]
- Li, Z.; Zheng, J.; Zhang, N.; Li, C. Berberine improves airway inflammation and inhibits NF-κB signaling pathway in an ovalbumin-induced rat model of asthma. J. Asthma 2016, 53, 999–1005. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Liu, L.; Abdalla, A.M.E.; Gauthier, M.; Yang, G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef]
- Gupta, L.; Sharma, A.K.; Gothwal, A.; Khan, M.S.; Khinchi, M.P.; Qayum, A.; Singh, S.K.; Gupta, U. Dendrimer encapsulated and conjugated delivery of berberine: A novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int. J. Pharm. 2017, 528, 88–99. [Google Scholar] [CrossRef]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, N.; Song, H.; Xi, X.; Wang, J.; Hao, A.; Li, T. Preparation of an anhydrous reverse micelle delivery system to enhance oral bioavailability and anti-diabetic efficacy of berberine. Eur. J. Pharm. Sci. 2011, 44, 127–135. [Google Scholar] [CrossRef]
- Xue, M.; Yang, M.X.; Zhang, W.; Li, X.M.; Gao, D.H.; Ou, Z.M.; Li, Z.P.; Liu, S.H.; Li, X.J.; Yang, S.Y. Characterization, pharmacokinetics, and hypoglycaemic effect of berberine loaded solid lipid nanoparticles. Int. J. Nanomed. 2013, 8, 4677–4687. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Zhang, L.; Yang, M.X.; Zhang, W.; Li, X.M.; Ou, Z.M.; Li, Z.P.; Liu, S.H.; Li, X.J.; Yang, S.Y. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int. J. Nanomed. 2015, 10, 5049–5057. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, R.; Singh, S.; Tripathi, M.; Bhatnagar, P.; Kakkar, P.; Gupta, K.C. O-hexadecyl-dextran entrapped berberine nanoparticles abrogate high glucose stress induced apoptosis in primary rat hepatocytes. PLoS ONE 2014, 9, e89124.2. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, J.; Zhou, Q.; Wang, Y.; Chen, T. Berberine nanosuspension enhances hypoglycaemic efficacy on streptozotocin induced diabetic C57BL/6 mice. Evid. Based Complement. Altern. Med. 2015, 2015, 239749. [Google Scholar]
- Yin, J.; Hou, Y.; Yin, Y.; Song, X. Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycaemic effect. Int. J. Nanomed. 2017, 12, 8671–8680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barwal, I.; Yadav, S.C. Rebaudioside A loaded PLA-nanoparticles as an anti-diabetic nanomedicine. J. Bionanosci. 2014, 8, 137–140. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, H.; Zhang, F.; Sun, F.; Xin, M.; Li, M.; Li, J.; Wu, X. Novel self-nanomicellizing solid dispersion based on rebaudioside A: A potential nanoplatform for oral delivery of curcumin. Int. J. Nanomed. 2019, 14, 557–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.W.; Yin, L.N.; Huang, X.Y.; Liang, Z.H.; Chen, X.X.; Wang, S.H. Oral absorption of asiatic acid nanoparticles modified with PEG. Zhongguo Zhong Yao Za Zhi 2017, 42, 2784–2788. [Google Scholar]
- Radwant, M.A.; Aboul-Enein, H.Y. The effect of oral absorption enhancers on the in vivo performance of insulin-loaded poly(ethylcyanoacrylate) nanospheres in diabetic rats. J. Microencapsul. 2002, 19, 225–235. [Google Scholar] [CrossRef]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.H.; Kumar, S. Evaluation of anti-diabetic activity of glycyrrhizin-loaded nanoparticles in nicotinamide-streptozotocin-induced diabetic rats. Eur. J. Pharm. Sci. 2017, 106, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kaushik, A.; Kim, K.H.; Kumar, S. Antidiabetic activity enhancement in streptozotocin + nicotinamide-induced diabetic rats through combinational polymeric nanoformulation. Int. J. Nanomed. 2019, 14, 4383–4395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, D.; Dey, T.K.; Mukherjee, S.; Ghosh, M.; Dhar, P. Comparative prophylactic effects of α-eleostearic acid rich nano and conventional emulsions in induced diabetic rats. J. Food Sci. Technol. 2014, 51, 1724–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, D.; Mukherjee, S.; Chakraborty, R.; Mallick, S.K.; Dhar, P. Comparative real-time study of cellular uptake of a formulated conjugated linolenic acid rich nano and conventional macro emulsions and their bioactivity in ex vivo models for parenteral applications. Colloids Surf. B Biointerfaces 2015, 126, 426–436. [Google Scholar] [CrossRef]
- Wei, Y.; Li, L.; Xi, Y.; Qian, S.; Gao, Y.; Zhang, J. Sustained release and enhanced bioavailability of injectable scutellarin-loaded bovine serum albumin nanoparticles. Int. J. Pharm. 2014, 476, 142–148. [Google Scholar] [CrossRef]
- Wang, J.; Tan, J.; Luo, J.; Huang, P.; Zhou, W.; Chen, L.; Long, L.; Zhang, L.M.; Zhu, B.; Yang, L.; et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J. Nanobiotechnol. 2017, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Roy, P.; Pal, R.; Auddy, R.G.; Chakraborti, A.S.; Mukherjee, A. Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS ONE 2014, 9, e101818. [Google Scholar] [CrossRef] [Green Version]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011, 16, 239–249. [Google Scholar]
- Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Landucci, E.; Bilia, A.R.; Bergonzi, M.C. Formulation of nanomicelles to improve the solubility and the oral absorption of silymarin. Molecules 2019, 24, 1688. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, M.; Qi, J.; Lu, Y.; Wu, W. Comparison of the oral bioavailability of silymarin-loaded lipid nanoparticles with their artificial lipolysate counterparts: Implications on the contribution of integral structure. Int. J. Pharm. 2015, 489, 195–202. [Google Scholar] [CrossRef]
- Xie, Y.; Yi, Y.; Hu, X.; Shangguan, M.; Wang, L.; Lu, Y.; Qi, J.; Wu, W. Synchronous microencapsulation of multiple components in silymarin into PLGA nanoparticles by an emulsification/solvent evaporation method. Pharm. Dev. Technol. 2016, 21, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Cinci, L.; D’Ambrosio, M.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Solid lipid nanoparticles and chitosan-coated solid lipid nanoparticles as promising tool for silybin delivery: Formulation, characterization, and in vitro evaluation. Curr. Drug Deliv. 2019, 16, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.M.; Karthivashan, G.; Arulselvan, P.; Fakuraz, S.; Hussein, M.Z. Characterization and in vitro sustained release of silibinin from pH responsive carbon nanotube-based drug delivery system. J. Nanomater. 2014, 2014, 439873. [Google Scholar]
- Ma, Y.; He, H.; Xia, F.; Li, Y.; Lu, Y.; Chen, D.; Qi, J.; Lu, Y.; Zhang, W.; Wu, W. In vivo fate of lipid-silybin conjugate nanoparticles: Implications on enhanced oral bioavailability. Nanomedicine 2017, 13, 2643–2654. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for protection against scopolamine-induced amnesia. Drug Deliv. 2013, 20, 112–119. [Google Scholar] [CrossRef]
- Martakov, I.S.; Shevchenko, O.G.; Torlopov, M.A.; Gerasimov, E.Y.; Sitnikov, P.A. Formation of gallic acid layer on γ-AlOOH nanoparticles surface and their antioxidant and membrane-protective activity. J. Inorg. Biochem. 2019, 199, 110782. [Google Scholar] [CrossRef]
- Purbowatiningrum; Ngadiwiyana; Ismiyarto; Fachriyah, E.; Eviana, I.; Eldiana, O.; Amaliyah, N.; Sektianingrum, A.N. Antidiabetic activity from gallic acid encapsulated nanochitosan. IOP Conf. Ser. Mater. Sci. Eng. 2017, 172, 012042. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, L.; Ban, Q.; Li, J.; Li, C.H.; Guan, Y.Q. Preparation and characterization of hydroxyapatite nanoparticles carrying insulin and gallic acid for insulin oral delivery. Nanomedicine 2018, 14, 353–364. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, D.; Kumari, A.; Yadav, S.K. Encapsulation of catechin and epicatechin on BSA NPS improved their stability and antioxidant potential. EXCLI J. 2014, 13, 331–346. [Google Scholar]
- Samanta, A.; Bandopadhyay, B.; Das, N. Formulation of catechin hydrate nanocapsule and study of its bioavailability. Med. Chem. 2016, 6, 6. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligunda, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic potential of epigallocatechin gallate nanodelivery systems. Biomed. Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef] [PubMed]
- Sistanipour, E.; Meshkini, A.; Oveisi, H. Catechin-conjugated mesoporous hydroxyapatite nanoparticle: A novel nano-antioxidant with enhanced osteogenic property. Colloids Surf. B Biointerfaces 2018, 169, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, J.F.; Kan, J.; Wen, X.Y.; Jin, C.H. Synthesis, characterization and in vitro anti-diabetic activity of catechin grafted inulin. Int. J. Biol. Macromol. 2014, 64, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, Z. Preparation and characterization of catechin-grafted chitosan with antioxidant and antidiabetic potential. Int. J. Biol. Macromol. 2014, 70, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Design, development, and characterization of lipid nanocarriers-based epigallocatechin gallate delivery system for preventive and therapeutic supplementation. Drug Des. Dev. Ther. 2016, 10, 3519–3528. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (-)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2013, 61, 875–881. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2016, 12, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Sen, S.; Chakraborti, A.S. Pelargonidin-PLGA nanoparticles: Fabrication, characterization, and their effect on streptozotocin induced diabetic rats. Ind. J. Exp. Biol. 2017, 55, 819–830. [Google Scholar]
- Samadder, A.; Abraham, S.K.; Khuda-Bukhsh, A.R. Nanopharmaceutical approach using pelargonidin towards enhancement of efficacy for prevention of alloxan-induced DNA damage in L6 cells via activation of PARP and p53. Environ. Toxicol. Pharmacol. 2016, 43, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Samadder, A.; Tarafdar, D.; Abraham, S.K.; Ghosh, K.; Khuda-Bukhsh, A.R. Nano-pelargonidin protects hyperglycaemic-induced L6 cells against mitochondrial dysfunction. Planta Med. 2017, 83, 468–475. [Google Scholar] [PubMed]
- Shaarani, S.; Hamid, S.S.; Mohd Kaus, N.H. The influence of pluronic F68 and F127 nanocarrier on physicochemical properties, in vitro release, and antiproliferative activity of thymoquinone drug. Pharmacogn. Res. 2017, 9, 12–20. [Google Scholar]
- Ahmad, R.; Kaus, N.H.M.; Hamid, S. Synthesis and characterization of PLGA-PEG thymoquinone nanoparticles and its cytotoxicity effects in tamoxifen-resistant breast cancer cells. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; pp. 1–18. [Google Scholar]
- Shahein, S.A.; Aboul-Enein, A.M.; Higazy, I.M.; Abou-Elella, F.; Lojkowski, W.; Ahmed, E.R.; Mousa, S.A.; AbouAitah, K. Targeted anticancer potential against glioma cells of thymoquinone delivered by mesoporous silica core-shell nanoformulations with pH-dependent release. Int. J. Nanomed. 2019, 14, 5503–5526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignesh Kumar, S.; Renuka Devi, P.; Hemananthan, E. In vitro studies to analyze the stability and bioavailability of thymoquinone encapsulated in the developed nanocarrier. J. Disper. Sci. Technol. 2020, 41, 243–256. [Google Scholar]
- Khan, M.A.; Aldebasi, Y.H.; Alsuhaibani, S.A.; AlSahli, M.A.; Alzohairy, M.A.; Khan, A.; Younus, H. Therapeutic potential of thymoquinone liposomes against the systemic infection of Candida albicans in diabetic mice. PLoS ONE 2018, 13, e0208951. [Google Scholar] [CrossRef]
- Nallamuthu, I.; Parthasarathi, A.; Khanum, F. Thymoquinone-loaded PLGA nanoparticles: Antioxidant and anti-microbial properties. Int. Curr. Pharm. J. 2013, 2, 202–207. [Google Scholar] [CrossRef]
- de Lima, I.A.; Khalil, N.M.; Tominaga, T.T.; Lechanteur, A.; Sarmento, B.; Mainardes, R.M. Mucoadhesive chitosan-coated PLGA nanoparticles for oral delivery of ferulic acid. Artif. Cell Nanomed. B 2018, 46, S993–S1002. [Google Scholar] [CrossRef] [Green Version]
- Heep, G.; Almeida, A.; Marcano, R.; Vieira, D.; Mainardes, R.M.; Khalil, N.M.; Sarmento, B. Zein-casein-lysine multicomposite nanoparticles are effective in modulate the intestinal permeability of ferulic acid. Int. J. Biol. Macromol. 2019, 138, 244–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zhang, K.; Yang, G.; Wang, Z.; Zhao, J.; Hu, R.; Feng, N. Ethyl oleate-containing nanostructured lipid carriers improve oral bioavailability of trans-ferulic acid ascompared with conventional solid lipid nanoparticles. Int. J. Pharm. 2016, 511, 57–64. [Google Scholar] [CrossRef]
- Bairagi, U.; Mittal, P.; Singh, J.; Mishra, B. Preparation, characterization, and in vivo evaluation of nano formulations of ferulic acid in diabetic wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Q.; Deng, W.; Omari-Siaw, E.; Wang, Q.; Wang, S.; Wang, S.; Cao, X.; Xu, X.; Yu, J. Self-nanoemulsifying drug delivery system of trans-cinnamic acid: Formulation development and pharmacodynamic evaluation in alloxan-induced type 2 diabetic rat model. Drug Dev. Res. 2015, 76, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Daunde, J.A.; Desai, S.S.; Desai, P.J.; Kamble, P.S.; Bhoi, A.V.; Gaiwad, P.R.; Walvekar, M.V. Nano-scaling of trigonelline improves antioxidative status of hfd-stz induced diabetic mice. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 2547–2552. [Google Scholar]
- Farkhondeh, T.; Samarghandian, S. The effect of saffron (Crocus sativus L.) and its ingredients on the management of diabetes mellitus and dyslipidaemia. Afr. J. Pharm. Pharmacol. 2014, 8, 541–549. [Google Scholar]
- Jia, Z.; Guo, X.; Wang, J.; Liu, Z. Beneficial effect of rhein on the treatment of diabetic nephropathy in nonobese diabetic (NOD) mice. J. Nephrol. Ther. 2012, 2, 112. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, Z.; Zhang, Y.; Zhang, M.; Zhu, X.; Fan, Y.; Shi, S.; Zen, K.; Liu, Z. Rhein protects pancreatic β-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycaemia. Diabetes 2013, 62, 3927–3935. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, Q.; Chen, D.; Wu, B.; Wu, Y.; Tong, W.; Huang, P. Kidney-targeted rhein-loaded liponanoparticles for diabetic nephropathy therapy via size control and enhancement of renal cellular uptake. Theranostics 2019, 9, 6191–6208. [Google Scholar] [CrossRef]
- Kamaraj, N.; Rajaguru, P.Y.; Issac, P.K.; Sundaresan, S. Fabrication, characterization, in vitro drug release and glucose uptake activity of 14-deoxy, 11, 12-didehydroandrographolide loaded polycaprolactone nanoparticles. Asian J. Pharm. Sci. 2017, 12, 353–362. [Google Scholar] [CrossRef]
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem. Toxicol. 2014, 69, 55–62. [Google Scholar] [CrossRef]
- Ku, S.-K.; Bae, J-S. Vicenin-2 and scolymoside inhibit high-glucose-induced vascular inflammation in vitro and in vivo. Can. J. Physiol. Pharm. 2016, 94, 287–295. [Google Scholar] [CrossRef]
- Chockalingam, S.; Thada, R.; Dhandapani, R.K.; Panchamoorthy, R. Biogenesis, characterization, and the effect of vicenin-gold nanoparticles on glucose utilization in 3T3-L1 adipocytes: A bioinformatic approach to illuminate its interaction with PTP 1B and AMPK. Biotechnol. Prog. 2015, 31, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int. J. Pharm. 2012, 427, 452–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Maity, A.; Purkayastha, P. Effect of environment pH on the photophysics of fisetin in solid lipid nanoparticles. J. Photochem. Photobiol. B 2015, 153, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Sechi, M.; Syed, D.N.; Pala, N.; Mariani, A.; Marceddu, S.; Brunetti, A.; Mukhtar, H.; Sanna, V. Nanoencapsulation of dietary flavonoid fisetin: Formulation and in vitro antioxidant and α-glucosidase inhibition activities. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 594–602. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Anuradha, C.V. Astaxanthin prevents loss of insulin signaling and improves glucose metabolism in liver of insulin resistant mice. Can. J. Physiol. Pharmacol. 2012, 90, 1544–1552. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of astaxanthin-loaded core-shell nanoparticles consisting of chitosan oligosaccharides and poly(lactic- co-glycolic acid): Enhancement of water solubility, stability, and bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef]
- Liu, B.; Liu, W.S.; Han, B.Q.; Sun, Y.Y. Antidiabetic effects of chitooligosaccharides on pancreatic islet cells in streptozotocin-induced diabetic rats. World J. Gastroenterol. 2007, 13, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. A new alternative insight of nanoemulsion conjugated with κ-carrageenan for wound healing study in diabetic mice: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2019, 133, 236–250. [Google Scholar] [CrossRef]
- Sharma, P.K.; Saxena, P.; Jaswanth, A.; Chalamaiah, M.; Balasubramaniam, A. Anti-diabetic activity of lycopene niosomes: Experimental observation. J. Pharm. Drug Dev. 2017, 4, 103. [Google Scholar]
- Rao, M.P.; Manjunath, K.; Bhagawati, S.T.; Thippeswamy, B.S. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection—Preparation, characterisation and in vivo evaluation. Int. J. Pharm. 2014, 473, 485–492. [Google Scholar] [CrossRef]
- Pinzón-García, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; de Matos Jensen, C.E.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient cutaneous wound healing using bixin-loaded PCL nanofibers in diabetic mice. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Bosch-Morell, F.; Arnal, E.; Alexander, G.; Blomhoff, R.; Romero, F.J. Lutein prevents the effect of high glucose levels on immune system cells in vivo and in vitro. J. Physiol. Biochem. 2008, 64, 149–157. [Google Scholar] [CrossRef]
- Arnal, E.; Miranda, M.; Almansa, I.; Muriach, M.; Barcia, J.M.; Romero, F.J.; Diaz-Llopis, M.; Bosch-Morell, F. Lutein prevents cataract development and progression in diabetic rats. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Shanmugam, S.; Thapa, P.; Lee, E.S.; Balakrishnan, P.; Baskaran, R.; Yoon, S.K.; Choi, H.G.; Yong, C.S.; Yoo, B.K.; et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch. Pharm. Res. 2010, 33, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shen, X.; Guo, M. Stability of lutein encapsulated whey protein nano-emulsion during storage. PLoS ONE 2018, 13, e0192511. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.B.; Malaviya, J.; Mukerjee, A. Attenuation of oxidative stress and glucose toxicity by lutein loaded nanoparticles from Spinacia oleracea leaves. J. Pharm. Sci. Pharmacol. 2015, 2, 242–249. [Google Scholar] [CrossRef]
- Nishikawa, S.; Hosokawa, M.; Miyashita, K. Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-A(y) mice. Phytomedicine 2012, 19, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Koo, S.Y.; Mok, I.K.; Pan, C.H.; Kim, S.M. Preparation of fucoxanthin-loaded nanoparticles composed of casein and chitosan with improved fucoxanthin bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef]
- Ravi, H.; Arunkumar, R.; Baskaran, V. Chitosan-glycolipid nanogels loaded with anti-obese marine carotenoid fucoxanthin: Acute and sub-acute toxicity evaluation in rodent model. J. Biomater. Appl. 2015, 30, 420–434. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.; Wang, J.; Zhu, J.; Wang, D.; Zhao, L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, L.; Zhu, X.; Hu, J.; Hailong, P.; Zeng, Z.; Xiong, H. Stability and bioaccessibility of fucoxanthin in nanoemulsions prepared from pinolenic acid-contained structured lipid. Int. J. Food Eng. 2017, 13, 20160273. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Lee, D.H.; Ahn, J.; Lee, H.; Choi, W.H.; Jang, Y.J.; Ha, T.Y. γ-oryzanol enhances adipocyte differentiation and glucose uptake. Nutrients 2015, 7, 4851–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozuka, C.; Shimizu-Okabe, C.; Takayama, C.; Nakano, K.; Morinaga, H.; Kinjo, A.; Fukuda, K.; Kamei, A.; Yasuoka, A.; Kondo, T.; et al. Marked augmentation of PLGA nanoparticle-induced metabolically beneficial impact of γ-oryzanol on fuel dyshomeostasis in genetically obese-diabetic ob/ob mice. Drug Deliv. 2017, 24, 558–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilie, I.; Ilie, R.; Mocan, T.; Tabaran, F.; Iancu, C.; Mocan, L. Nicotinamide-functionalized multiwalled carbon nanotubes increase insulin production in pancreatic beta cells via MIF pathway. Int. J. Nanomed. 2013, 8, 3345–3353. [Google Scholar]
- Venkatachalam, M.; Govindaraju, K.; Mohamed Sadiq, A.; Tamilselvan, S.; Ganesh Kumar, V.; Singaravelu, G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Uma Suganya, K.S.; Govindaraju, K.; Veena Vani, C.; Premanathan, M.; Ganesh Kumar, V.K. In vitro biological evaluation of anti-diabetic activity of organic-inorganic hybrid gold nanoparticles. IET Nanobiotechnol. 2019, 13, 226–229. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Matsuda, H. Chemical and Pharmacological Studies on Triterpene Saponins, Escins, from Horse Chestnut Seeds. In Saponins in Food, Feedstuffs and Medicinal Plants; Oleszek, W., Marston, A., Eds.; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Wang, H.; Zhang, L.; Jiang, N.; Wang, Z.; Chong, Y.; Fu, F. Anti-inflammatory effects of escin are correlated with the glucocorticoid receptor/NF-κB signaling pathway, but not the COX/PGF2α signaling pathway. Exp. Ther. Med. 2013, 6, 19–422. [Google Scholar] [CrossRef] [Green Version]
- Shamprasad, B.R.; Keerthana, S.; Megarajan, S.; Lotha, R.; Aravind, S.; Veerappan, A. Photosynthesized escin stabilized gold nanoparticles exhibit antidiabetic activity in L6 rat skeletal muscle cells. Mater. Lett. 2019, 241, 198–201. [Google Scholar] [CrossRef]
- Khaleel Basha, S.; Govindaraju, K.; Manikandan, R.; Ahn, J.S.; Bae, E.Y.; Singaravelu, G. Phytochemical mediated gold nanoparticles and their PTP 1B inhibitory activity. Colloids Surf. B Biointerfaces 2010, 75, 405–409. [Google Scholar] [CrossRef]
- Li, P.; Zhang, L.; Tian, X.; Xing, J. Docosahexaenoic acid has an anti-diabetic effect in streptozotocin-induced diabetic mice. Int. J. Clin. Exp. Med. 2014, 7, 3021–3029. [Google Scholar]
- Hussein, J.; Attia, M.F.; El Bana, M.; El-Daly, S.M.; Mohamed, N.; El-Khayat, Z.; El-Naggar, M.E. Solid state synthesis of docosahexaenoic acid-loaded zinc oxide nanoparticles as a potential antidiabetic agent in rats. Int. J. Biol. Macromol. 2019, 140, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Hussein, J.S.; Rasheed, W.; Ramzy, T.; Nabeeh, M.; Harvy, M.; El-Toukhy, S.; Ali, O.; Raafat, J.; El-Naggar, M. Synthesis of docosahexaenoic acid-loaded silver nanoparticles for improving endothelial dysfunctions in experimental diabetes. Hum. Exp. Toxicol. 2019, 38, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Tilburt, J.C.; Kaptchuk, T.J. Herbal medicine research and global health: An ethical analysis. Bull. World Health Organ. 2008, 86, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2013, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Khan, V.; Najmi, A.K.; Akhtar, M.; Aqil, M.; Mujeeb, M.; Pillai, K.K. A pharmacological appraisal of medicinal plants with antidiabetic potential. J. Pharm. Bioallied Sci. 2012, 4, 27–42. [Google Scholar]
- Alamoudi, E.F.; Khalil, W.K.B.; Ghaly, I.S.; Hassan, N.H.A.; Ahmed, E.S. Nanoparticles from of Costus speciosus extract improves the antidiabetic and antilipidaemic effects against STZ-induced diabetes mellitus in albino rats. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 279–288. [Google Scholar]
- Al Rashid, H. Preparation and characterization of plga loaded nanoparticles obtained from D. melanoxylon Roxb. leaves for their antiproliferative and antidiabetic activity. Int. J. Green Pharm. 2017, 11, S438–S447. [Google Scholar]
- Anand, K.; Tiloke, C.; Naidoo, P.; Chuturgoon, A.A. Phytonanotherapy for management of diabetes using green synthesis nanoparticles. J. Photochem. Photobiol. B 2017, 173, 626–639. [Google Scholar] [CrossRef]
- Deng, W.; Wang, H.; Wu, B.; Zhang, X. Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycaemic activity to synergistically potentiate the antidiabetic effect. Acta Pharm. Sin. B 2019, 9, 74–86. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccam, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Khan, T.; Gurav, P. PhytoNanotechnology: Enhancing delivery of plant based anti-cancer drugs. Front. Pharmacol. 2018, 8, 1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and controlled delivery for bioactive compounds: Outlining challenges for new “smart-foods” for health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on nature: The role of nanomedicine in the development of clinical natural drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Patra, N.; Kar, D.; Pal, A.; Behera, A. Antibacterial, anticancer, anti-diabetic and catalytic activity of bio-conjugated metal nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035001. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
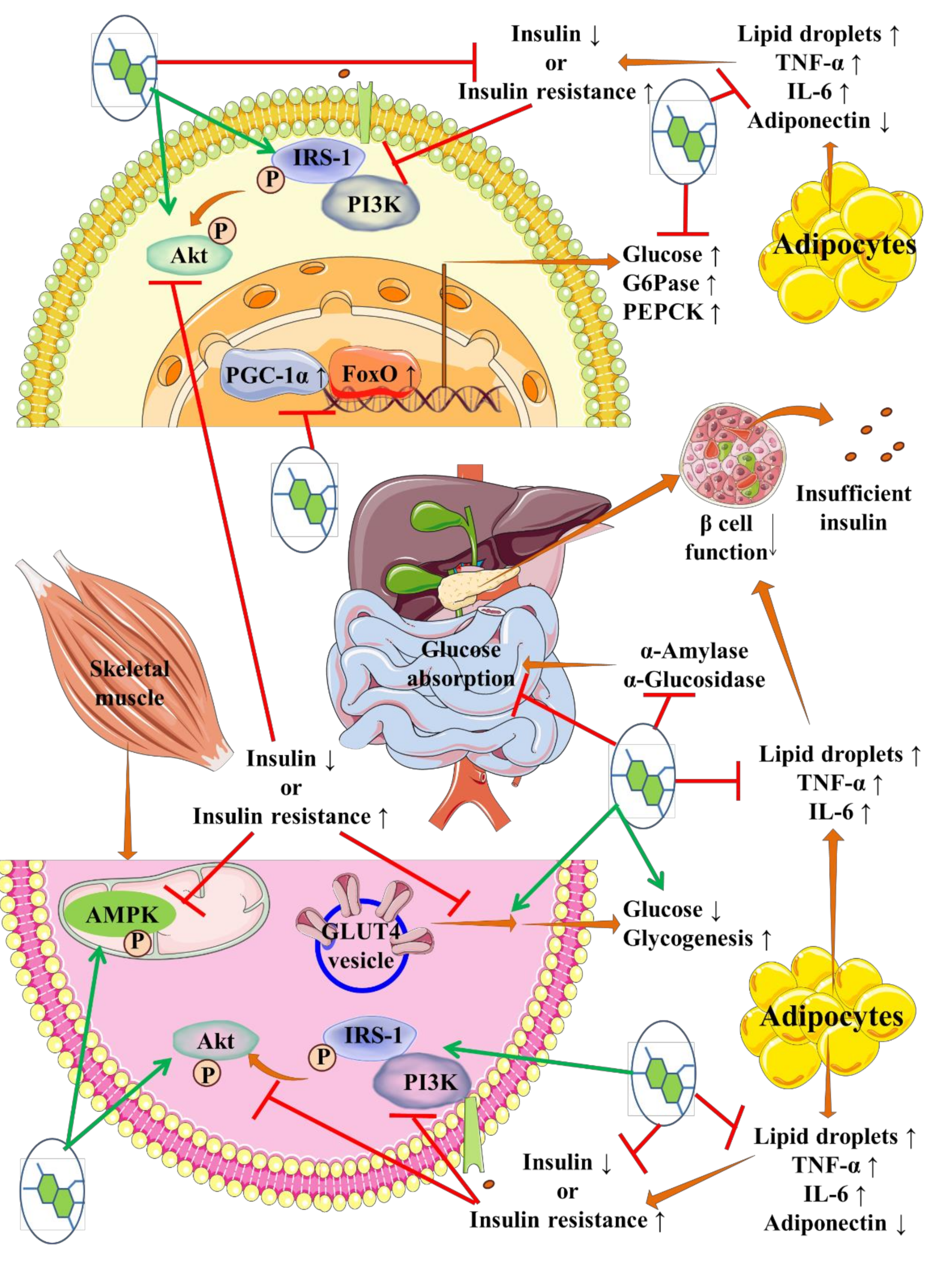
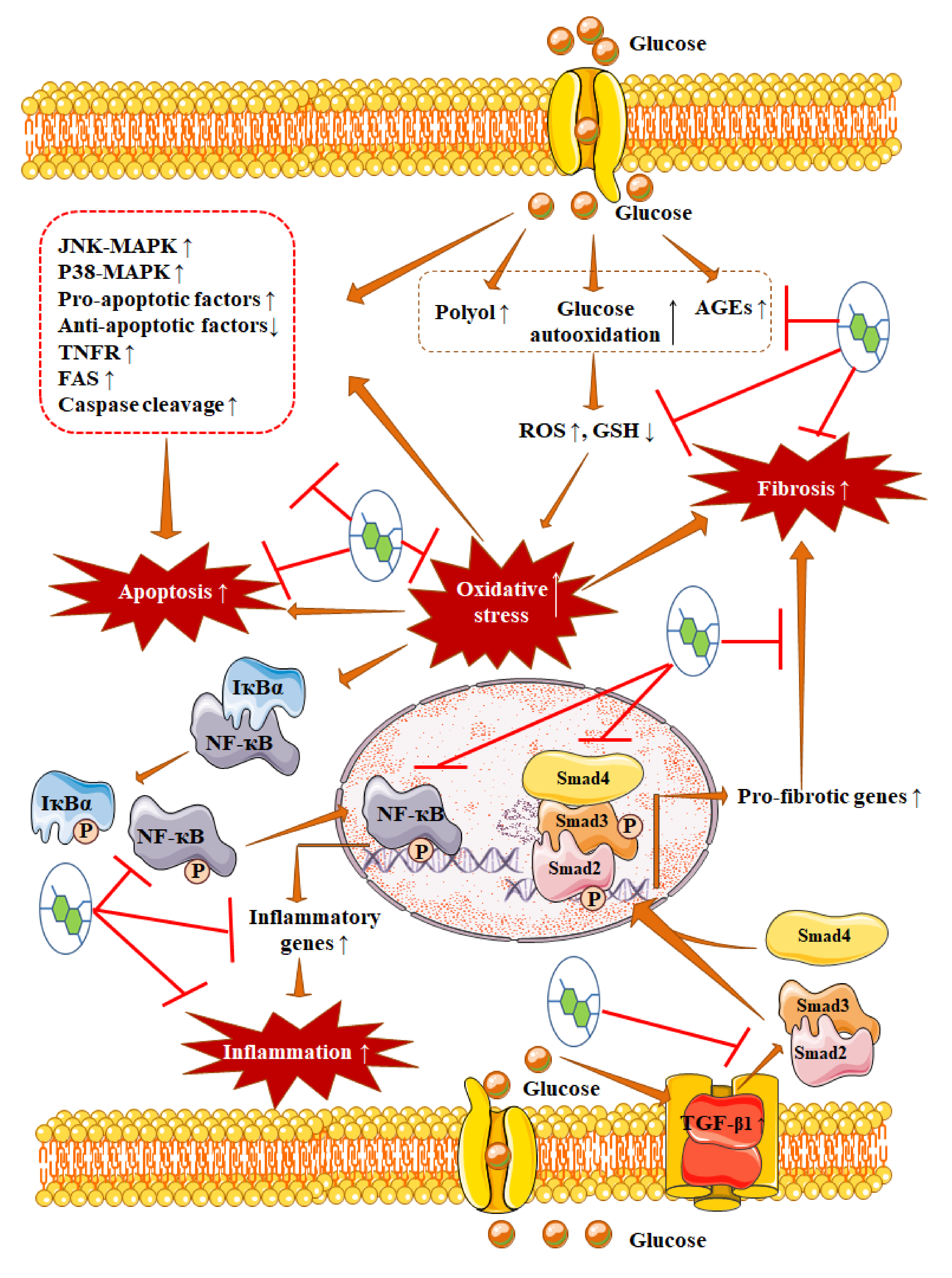
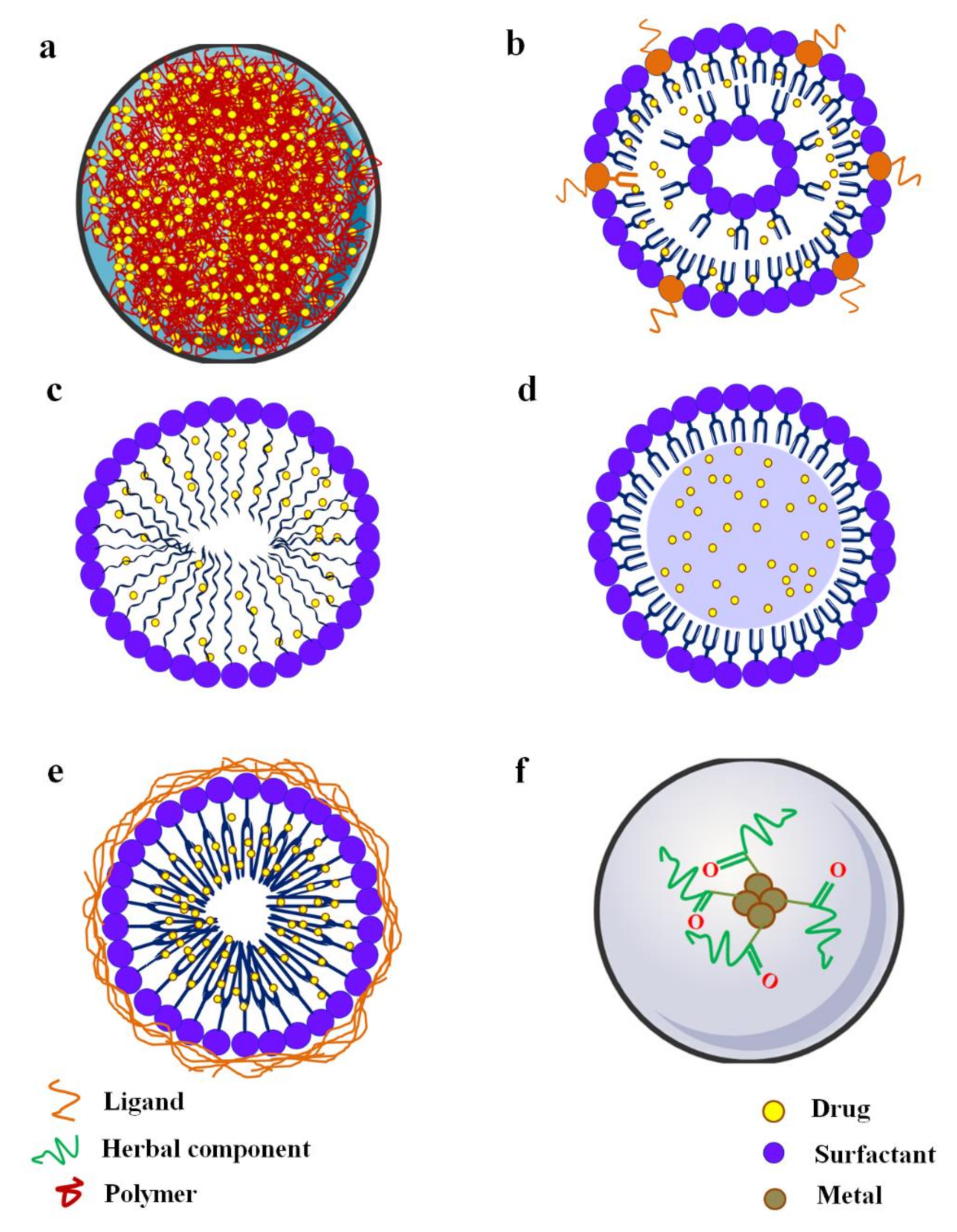
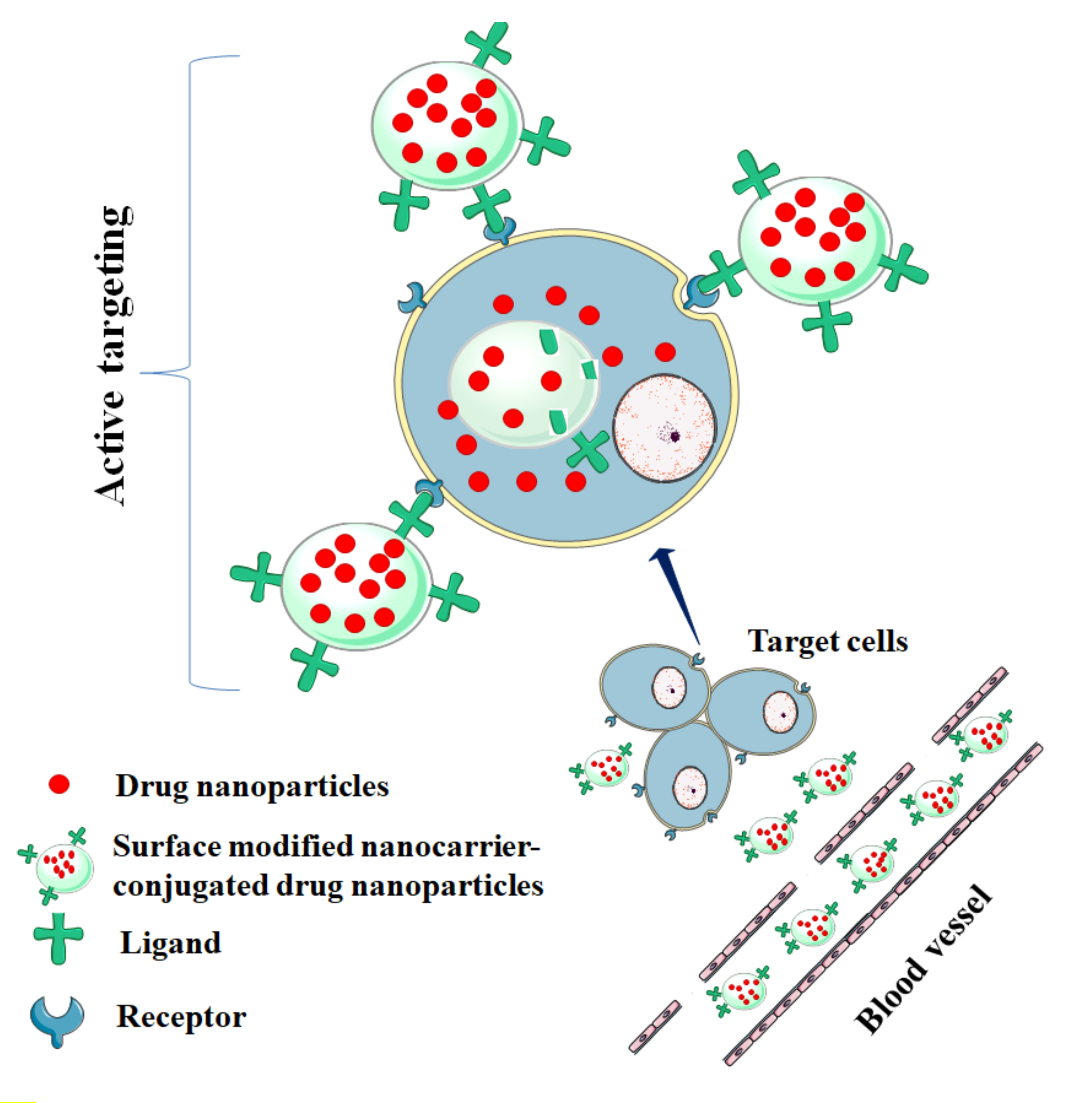
| S No. | Compounds | Pharmaceutical Limitations | References |
|---|---|---|---|
| 1 | Curcumin | Water solubility ~ 8 mg/L, poor chemical stability, low penetrability, poor absorption, rapid metabolism, high faecal excretion, elimination half-life ~ 2 h. | [112,113,114] |
| 2 | Resveratrol | Water solubility ~ 30 mg/L, rapid metabolism, rapid elimination, low plasma concentration, limited systemic distribution, oral bioavailability ~ 1–5%, poor physicochemical stability, rapid trans to cis (less active) isomerization. | [111,115,116] |
| 3 | Naringenin | Water solubility ~ 9.8 mg/L, low absorption, rapid metabolic transformation by the hepatic and gastric enzymes, oral bioavailability ~ 5%, high intestinal P-gp efflux. | [111,117,118] |
| 4 | Quercetin | Water solubility ~ 10 mg/L, poor chemicobiological stability, low absorption, fast metabolism, rapid elimination, poor oral bioavailability ~ 1%. | [119,120] |
| 5 | Apigenin | Water solubility ~ 16 mg/L, poor lipid solubility, high metabolic transformation, poor oral bioavailability, high inter-individual variability. | [121] |
| 6 | Myricitrin | Water solubility 300 mg/L, poor gastrointestinal stability, rapid conversion into poorer soluble myricetin (solubility ~ 17 mg/L) by colonic microflora, very low absorption, poor bioavailability. | [122,123] |
| 7 | Baicalin | Water solubility 91 mg/L, poor absorption, high biliary excretion, high metabolic conversion, poor bioavailability (~ 3 % in rats). | [124,125] |
| 8 | Luteolin | Water solubility 140 mg/L, low absorption, rapid first pass effect, bioavailability ~ 4 %. | [126] |
| 9 | Mangiferin | Water solubility ~ 300 mg/L, poor absorption, high first-pass property, rapid metabolism (by cytochrome P-450), high P-gp efflux, oral bioavailability ~ 1.5–5%. | [127] |
| 10 | Gymnemic acid | Poor water solubility, poor lipid solubility, very poor oral bioavailability. | [128,129] |
| 11 | Emodin | Water solubility ~ 222 mg/L, poor intestinal absorption, faster metabolism, rapid elimination, low bioavailability. | [130,131] |
| 12 | Rosmarinic acid | Poor biological stability, poor absorption, rapid metabolic transformation, poor bioavailability 0.9–1.7 %. | [132,133] |
| 13 | Berberine | Poor water solubility ~ 2.1 g/L, high P-gp efflux, low plasma concentration, rapid biotransformation, large intestinal and hepatic first-pass, poor oral bioavailability < 1%. | [134,135,136,137] |
| 14 | Stevioside | Poor intestinal absorption, low persistence, rapid metabolic degradation by human microflora, low bioavailability. | [138,139,140] |
| 15 | Asiatic acid | Poor water solubility ~ 158 mg/L (in saturated saline), rapid hepatic metabolism, poor oral bioavailability (~ 16% in rats) | [92,141] |
| 16 | Glycyrrhizin | Poor absorption, prosystemic hydrolysis by gastric fluid and by gastrointestinal flora, rapid hepatic metabolism, low oral bioavailability. | [142] |
| 17 | α-Eleostearic acid | Poor chemical stability, high metabolic conversion, low oral bioavailability. | [143,144] |
| 18 | Scutellarin | Water solubility ~ 15 mg/L, poor lipid solubility, poor membrane permeability, very low absorption, rapid metabolism, rapid faecal elimination, poor oral bioavailability (< 0.75% in dog). | [145,146] |
| 19 | Silymarin | Poor water solubility < 50 mg/L, poor intestinal permeability, rapid metabolism, rapid excretion, poor oral bioavailability. | [147,148] |
| 20 | Gallic Acid | Fast gastrointestinal absorption, fast systemic metabolism, rapid elimination, poor oral bioavailability. | [149] |
| 21 | Catechins | Poor stability, slow intestinal absorption, rapid P-gp efflux, fast metabolism, rapid clearance, poor oral bioavailability ~ 5%, poor cellular permeability | [150] |
| 22 | Pelargonidin | Low water solubility, poor stability, rapid metabolic degradation, poor bioavailability. | [151] |
| 23 | Thymoquinone | Poor aqueous solubility, high lipophilicity, slow absorption, fast metabolism, rapid elimination, low bioavailability, poor physicochemical stability. | [152,153] |
| 24 | Ferulic acid | Poor water solubility, poor gastrointestinal stability, rapid metabolism, low bioavailability ~ 3%. | [154,155] |
| 25 | Betulin | Low aqueous solubility, high permeability, low and variable bioavailability | [156,157] |
| 26 | Trans-cinnamic acid | Rapid absorption, rapid elimination, quick metabolism. | [158] |
| 27 | Trigonelline | Moderate absorption rate, fast elimination. | [159] |
| 28 | Crocetin | Water solubility ~ 1.2 mg/L, instability, rapid absorption, low oral bioavailability. | [160,161] |
| 29 | Rhein | Low hydrophilicity, aqueous solubility < 1 mg/L, low oral absorption, fast metabolic degradation, poor oral bioavailability t1/2 ~ 15 min. | [162,163] |
| 30 | 14-Deoxy 11, 12-didehydro andrographolide | Poor aqueous solubility, rapid absorption, fast metabolism, poor oral bioavailability. | [164] |
| 31 | Fisetin | Water solubility ~ 10.5 mg/L, pro-systemic metabolism, rapid first pass metabolism, high P-gp efflux, low oral bioavailability. | [165,166] |
| 32 | Astaxanthin | High lipophilicity, poor water solubility, poor stability, low oral bioavailability. | [167,168] |
| 33 | Lycopene | Extensively isomerized after dosing, chemical instability, rapidly metabolized into polar metabolites, rapid excretion. | [169] |
| 34 | Bixin | Poor water solubility, very poor chemical stability. | [170] |
| 35 | Lutein | High lipophilicity, poor water solubility, poor physic-chemical stability, low oral bioavailability. | [171,172] |
| 36 | Fucoxanthin | Poor aqueous solubility, poor physic-chemical stability, low oral bioavailability. | [173] |
| 37 | 16-Hydroxycleroda-3,13-dine-16,15-olide | Poor water solubility, low oral bioavailability. | [174] |
| 38 | γ-Oryzanol | Poor water solubility, rapid metabolism, poor oral bioavailability. | [175] |
| 39 | Escin isomers | Poor water solubility, Extensive metabolism in the gut, low bioavailability. | [176,177] |
| 40 | Docosahexaenoic acid | Poor water solubility, hydrophobic, low absorption, low bioavailability, redox instability, age-related differential responses. | [178,179] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217. https://doi.org/10.3390/ijms21062217
Dewanjee S, Chakraborty P, Mukherjee B, De Feo V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. International Journal of Molecular Sciences. 2020; 21(6):2217. https://doi.org/10.3390/ijms21062217
Chicago/Turabian StyleDewanjee, Saikat, Pratik Chakraborty, Biswajit Mukherjee, and Vincenzo De Feo. 2020. "Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy" International Journal of Molecular Sciences 21, no. 6: 2217. https://doi.org/10.3390/ijms21062217
APA StyleDewanjee, S., Chakraborty, P., Mukherjee, B., & De Feo, V. (2020). Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. International Journal of Molecular Sciences, 21(6), 2217. https://doi.org/10.3390/ijms21062217







