Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Molecules: From Tissue Regeneration to Infection Control
Abstract
1. Introduction
Outline of the Review
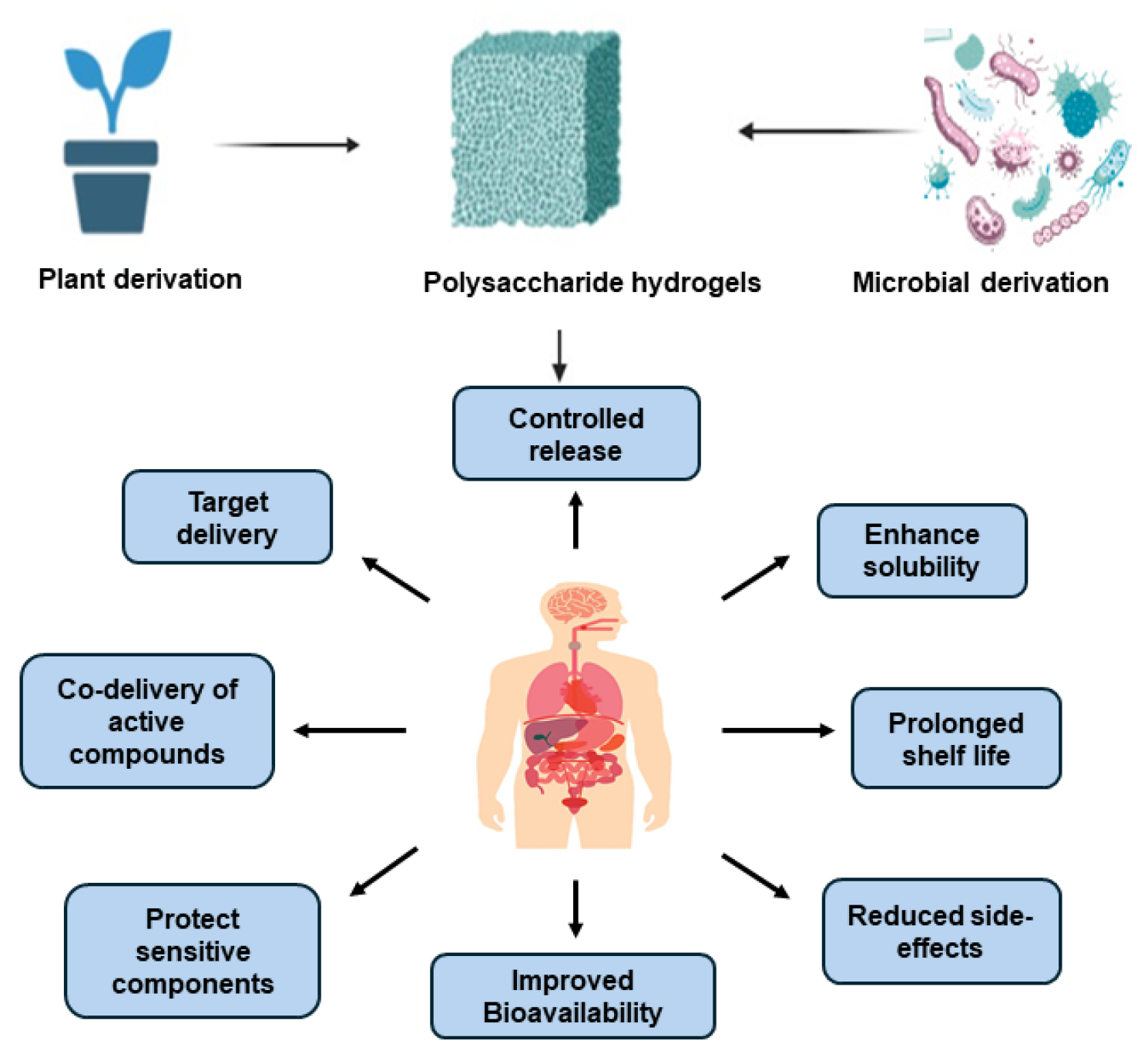
2. Polysaccharide Hydrogel System Characteristics
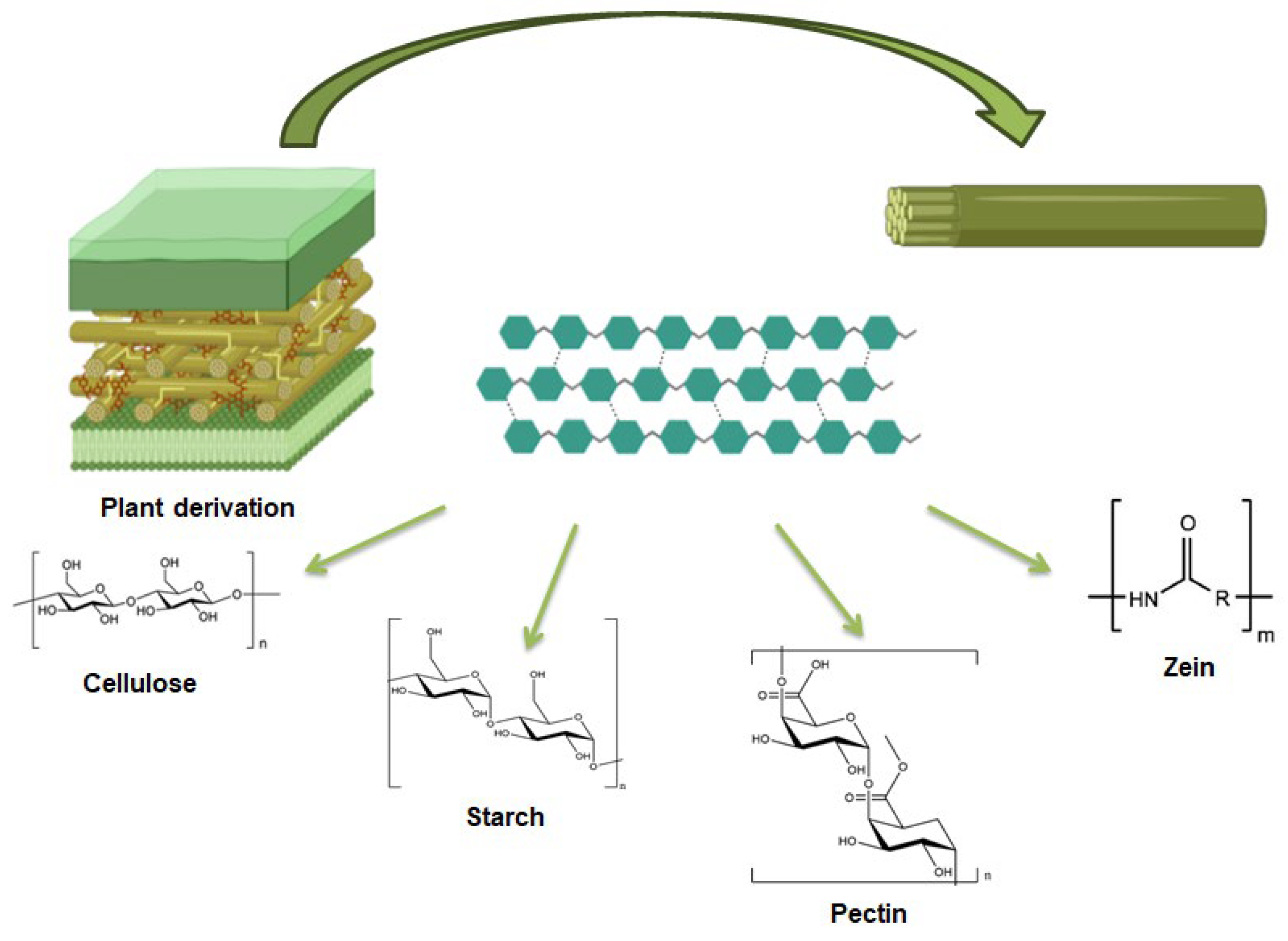
3. Plant Derived Polysaccharides
3.1. Starch
3.1.1. Starch: Origin and Property
3.1.2. Starch-Based Hydrogels Applications
3.2. Pectin
3.2.1. Pectin: Origin and Property
3.2.2. Pectin-Based Hydrogels Applications
3.3. Cellulose
3.3.1. Cellulose: Origin and Property
3.3.2. Cellulose-Based Hydrogels Applications
3.4. Zein
3.4.1. Zein: Origin and Property
3.4.2. Zein-Based Hydrogels Applications
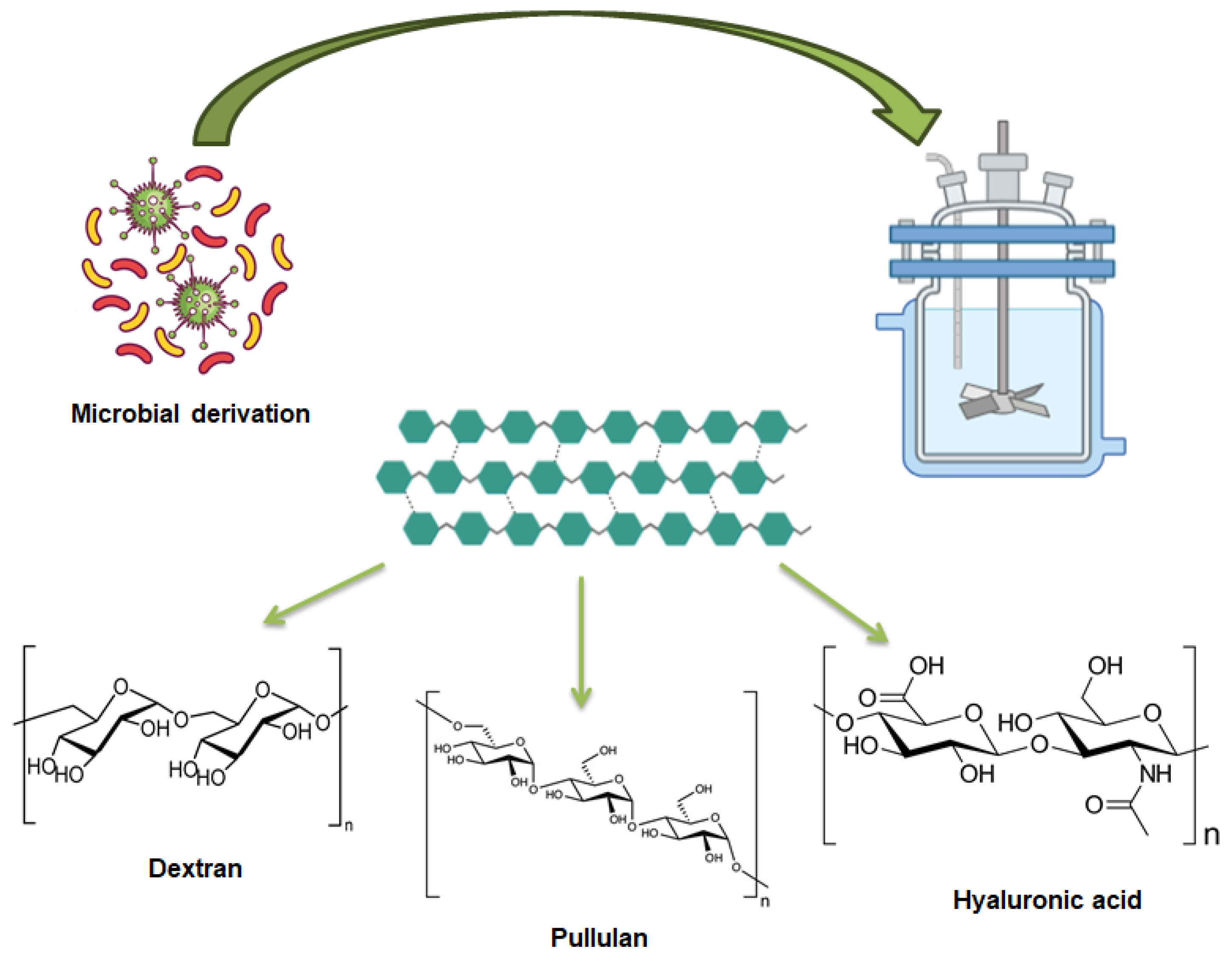
4. Microbial-Derived Polysaccharide
4.1. Dextran
4.1.1. Dextran: Origin and Property
4.1.2. Dextran-Based Hydrogels Applications
4.2. Pullulan
4.2.1. Pullulan: Origin and Property
4.2.2. Pullulan Based Hydrogels Applications
4.3. Hyaluronic Acid
4.3.1. Hyaluronic Acid: Origin and Property
4.3.2. Hyaluronan-Based Hydrogel Applications
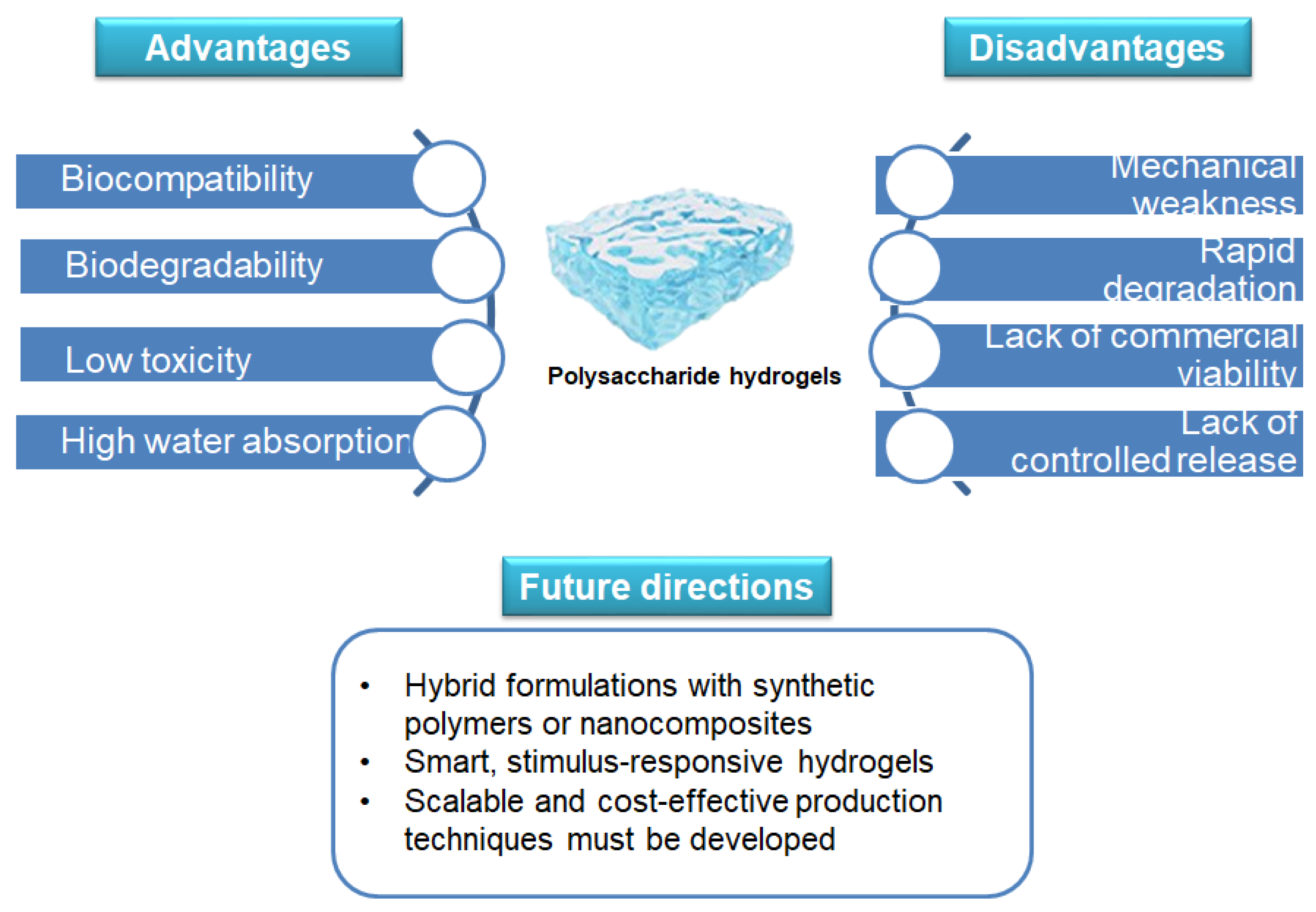
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumon, M.M.H.; Akib, A.A.; Sarkar, S.D.; Khan, M.A.R.; Uddin, M.M.; Nasrin, D.; Roy, C.K. Polysaccharide-Based Hydrogels for Advanced Biomedical Engineering Applications. ACS Polym. Au 2024, 4, 463–486. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Asthana, N.; Pal, K.; Khan, A.A.; Malik, A. Novel biopolymeric materials potential utilization for environmental practices. J. Mol. Struct. 2024, 1311, 138390. [Google Scholar] [CrossRef]
- Beena Unni, A.; Muringayil Joseph, T. Enhancing Polymer Sustainability: Eco-Conscious Strategies. Polymers 2024, 16, 1769. [Google Scholar] [CrossRef]
- Salehi, M.; Rashidinejad, A. Multifaceted roles of plant-derived bioactive polysaccharides: A review of their biological functions, delivery, bioavailability, and applications within the food and pharmaceutical sectors. Int. J. Biol. Macromol. 2025, 290, 138855. [Google Scholar] [CrossRef]
- Rial-Hermida, M.I.; Rey-Rico, A.; Blanco-Fernandez, B.; Carballo-Pedrares, N.; Byrne, E.M.; Mano, J.F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater. Sci. Eng. 2021, 7, 4102–4127. [Google Scholar] [CrossRef]
- Valentino, A.; Yazdanpanah, S.; Conte, R.; Calarco, A.; Peluso, G. Smart Nanocomposite Hydrogels as Next-Generation Therapeutic and Diagnostic Solutions. Gels 2024, 10, 689. [Google Scholar] [CrossRef]
- Conte, R.; Valentino, A.; Romano, S.; Margarucci, S.; Petillo, O.; Calarco, A. Stimuli-Responsive Nanocomposite Hydrogels for Oral Diseases. Gels 2024, 10, 478. [Google Scholar] [CrossRef]
- Olteanu, G.; Neacșu, S.M.; Joița, F.A.; Musuc, A.M.; Lupu, E.C.; Ioniță-Mîndrican, C.B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3849. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol 2022, 213, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Kamel, S.; El-Sayed, N.S. Cross-linking Strategies for the Design of Smart Injectable Hydrogels. In Injectable Smart Hydrogels for Biomedical Applications; Dodda, J.M., Ashammakhi, N., Sadiku, E.R., Eds.; Royal Society of Chemistry: Edinburgh, UK, 2024; Volume 17, pp. 128–149. [Google Scholar]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Kim, K.M.; D’Elia, A.M.; Rodell, C.B. Hydrogel-based approaches to target hypersensitivity mechanisms underlying autoimmune disease. Adv. Drug Deliv. Rev. 2024, 212, 115395. [Google Scholar] [CrossRef] [PubMed]
- Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Conte, R.; Margarucci, S.; Peluso, G.; Calarco, A. Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. Int. J. Mol. Sci. 2025, 26, 764. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Knipe, J.; Chen, F.; Peppas, N. Enzymatic Biodegradation of Hydrogels for Protein Delivery Targeted to the Small Intestine. Biomacromolecules 2015, 16, 962–972. [Google Scholar] [CrossRef]
- Vulic, K.; Shoichet, M.S. Affinity-Based Drug Delivery Systems for Tissue Repair and Regeneration. Biomacromolecules 2014, 15, 3867–3880. [Google Scholar] [CrossRef]
- Ali, F.; Khan, I.; Chen, J.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Emerging Fabrication Strategies of Hydrogels and Its Applications. Gels 2022, 8, 205. [Google Scholar] [CrossRef]
- Bahú, J.O.; de Andrade, L.R.M.; de Melo Barbosa, R.; Crivellin, S.; da Silva, A.P.; Souza, S.D.A.; Cárdenas Concha, V.O.; Severino, P.; Souto, E.B. Plant Polysaccharides in Engineered Pharmaceutical Gels. Bioengineering 2022, 9, 376. [Google Scholar] [CrossRef]
- Shang, L.; Wang, S.; Mao, Y. Recent advances in plant-derived polysaccharide scaffolds in tissue engineering: A review. Int. J. Biol. Macromol. 2024, 277, 133830. [Google Scholar] [CrossRef]
- Navasingh, R.J.H.; Gurunathan, M.K.; Nikolova, M.P.; Królczyk, J.B. Sustainable Bioplastics for Food Packaging Produced from Renewable Natural Sources. Polymers 2023, 15, 3760. [Google Scholar] [CrossRef]
- Fakhri, V.; Jafari, A.; Layaei Vahed, F.; Su, C.-H.; Pirouzfar, V. Polysaccharides as eco-friendly bio-adsorbents for wastewater remediation: Current state and future perspective. J. Water Process Eng. 2023, 54, 103980. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer—Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research advances in chemical modifications of starch for hydrophobicity and its applications: A review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Xiao, S.; Yao, Z.; Deng, H.; Chen, X.; Yang, T. Factors and modification techniques enhancing starch gel structure and their applications in foods:A review. Food Chem. 2024, 24, 102045. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ghasemzadeh, M.; Khorsandi, Z.; Sheibani, R.; Nasrollahzadeh, M. Starch-based hydrogels for environmental applications: A review. Int. J. Biol. Macromol. 2024, 269, 131956. [Google Scholar] [CrossRef]
- Koshenaj, K.; Ferrari, G. A Comprehensive Review on Starch-Based Hydrogels: From Tradition to Innovation, Opportunities, and Drawbacks. Polymers 2024, 16, 1991. [Google Scholar] [CrossRef]
- Abdel-Ghaffar, A.M. Radiation synthesis and modification of biopolymers and polymeric composites for biomedical applications. Poly. Compos. 2023, 31, 1–13. [Google Scholar] [CrossRef]
- D’Aniello, A.; Koshenaj, K.; Ferrari, G. A Preliminary Study on the Release of Bioactive Compounds from Rice Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels 2023, 9, 521. [Google Scholar] [CrossRef]
- García Tejeda, Y.; Salinas-Moreno, Y.; Hernandez-Martinez, A.; MartÍNez-Bustos, F. Encapsulation of Purple Maize Anthocyanins in Phosphorylated Starch by Spray Drying. Cereal Chem. 2015, 93, 130–137. [Google Scholar] [CrossRef]
- Peng, S.; Xue, L.; Leng, X.; Yang, R.; Zhang, G.; Hamaker, B.R. Slow digestion property of octenyl succinic anhydride modified waxy maize starch in the presence of tea polyphenols. J. Agric. Food Chem. 2015, 63, 2820–2829. [Google Scholar] [CrossRef]
- Roman-Benn, A.; Contador, C.; Li, M.W.; Lam, H.-M.; Ah-Hen, K.; Ulloa, P.; Ravanal, M. Pectin: An overview of sources, extraction and applications in food products and health. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Moutaharrik, S.; Palugan, L.; Cerea, M.; Meroni, G.; Casagni, E.; Roda, G.; Martino, P.A.; Gazzaniga, A.; Maroni, A.; Foppoli, A. Colon Drug Delivery Systems Based on Swellable and Microbially Degradable High-Methoxyl Pectin: Coating Process and In Vitro Performance. Pharmaceutics 2024, 16, 508. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.; Inzalaco, G.; Dapporto, F.; Maturi, M.; Sambri, L.; Vetri Buratti, V.; Chiariello, M.; Comes Franchini, M.; Locatelli, E. Biocompatible pectin-based hybrid hydrogels for tissue engineering applications. New J. Chem. 2021, 45, 22386–22395. [Google Scholar] [CrossRef]
- Mishra, R.; Banthia, A.; Majeed, A. Pectin based formulations for biomedical applications: A review. Asian J. Pharm. Biol. Res. 2012, 5, 1–7. [Google Scholar] [CrossRef]
- Mishra, R.; Ray, A. Synthesis and Characterization of Poly{N-[3-(dimethylamino) propyl] methacrylamide-co-itaconic acid} Hydrogels for Drug Delivery. J. Appl. Polym. Sci. 2011, 119, 3199–3206. [Google Scholar] [CrossRef]
- Gautam, M.; Santhiya, D. In-situ mineralization of calcium carbonate in pectin based edible hydrogel for the delivery of protein at colon. J. Drug Deliv. Sci. Technol. 2019, 53, 101137. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, S.; Zhao, C.; Cui, J.; Wang, Y.; Fang, X.; Zheng, J. Caseinate-reinforced pectin hydrogels: Efficient encapsulation, desirable release, and chemical stabilization of (−)-epigallocatechin. Int. J. Biol. Macromol. 2023, 230, 123298. [Google Scholar] [CrossRef]
- Bostancı, N.S.; Büyüksungur, S.; Hasirci, N.; Tezcaner, A. pH responsive release of curcumin from photocrosslinked pectin/gelatin hydrogel wound dressings. Biomater. Adv. 2022, 134, 112717. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Li, Y.; Li, H.; Li, L.; Xia, Q. Encapsulation of resveratrol-loaded Pickering emulsions in alginate/pectin hydrogel beads: Improved stability and modification of digestive behavior in the gastrointestinal tract. Int. J. Biol. Macromol. 2022, 222, 337–347. [Google Scholar] [CrossRef]
- Mala, T.; Anal, A.K. Protection and Controlled Gastrointestinal Release of Bromelain by Encapsulating in Pectin-Resistant Starch Based Hydrogel Beads. Front. Bioeng. Biotechnol. 2021, 9, 757176. [Google Scholar] [CrossRef]
- Alsakhawy, M.A.; Abdelmonsif, D.A.; Haroun, M.; Sabra, S.A. Naringin-loaded Arabic gum/pectin hydrogel as a potential wound healing material. Int. J. Biol. Macromol. 2022, 222, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Phonrachom, O.; Charoensuk, P.; Kiti, K.; Saichana, N.; Kakumyan, P.; Suwantong, O. Potential use of propolis-loaded quaternized chitosan/pectin hydrogel films as wound dressings: Preparation, characterization, antibacterial evaluation, and in vitro healing assay. Int. J. Biol. Macromol. 2023, 241, 124633. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.T.; Morgan, J.L.; Zimmer, J. A molecular description of cellulose biosynthesis. Annu. Rev. Biochem. 2015, 84, 895–921. [Google Scholar] [CrossRef] [PubMed]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.; Mohd, N.; Suhaili, N.; Anuar, F.; Lazim, A.M.; Othaman, R. Preparation of Cellulose-based Hydrogel: A Review. J. Mater. Res. Technol. 2020, 10, 935–952. [Google Scholar] [CrossRef]
- Chang, C.; Na, N. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Silva, I.G.R.d.; Pantoja, B.T.d.S.; Almeida, G.H.D.R.; Carreira, A.C.O.; Miglino, M.A. Bacterial Cellulose and ECM Hydrogels: An Innovative Approach for Cardiovascular Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 3955. [Google Scholar] [CrossRef]
- Phan, H.L.; Tran, N.C.T.; Le, T.H.Y.; Le, Q.V.; Le, T.T.; Thach, U.D. Fabrication of polydopamine-modified cellulose hydrogel for controlled release of α-mangostin. Biopolymers 2024, 115, e23613. [Google Scholar] [CrossRef]
- Gami, P.; Kundu, D.; Seera, S.D.K.; Banerjee, T. Chemically crosslinked xylan-β-Cyclodextrin hydrogel for the in vitro delivery of curcumin and 5-Fluorouracil. Int. J. Biol. Macromol. 2020, 158, 18–31. [Google Scholar] [CrossRef]
- Islam, F.; Wong, S.Y.; Li, X.; Arafat, M.T. Pectin and mucin modified cellulose-based superabsorbent hydrogel for controlled curcumin release. Cellulose 2022, 29, 5207–5222. [Google Scholar] [CrossRef]
- Anagha, B.; George, D.; Maheswari, P.U.; Begum, K.M.M.S. Biomass Derived Antimicrobial Hybrid Cellulose Hydrogel with Green ZnO Nanoparticles for Curcumin Delivery and its Kinetic Modelling. J. Polym. Environ. 2019, 27, 2054–2067. [Google Scholar] [CrossRef]
- Tortorella, S.; Maturi, M.; Vetri Buratti, V.; Vozzolo, G.; Locatelli, E.; Sambri, L.; Comes Franchini, M. Zein as a versatile biopolymer: Different shapes for different biomedical applications. RSC Adv. 2021, 11, 39004–39026. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Yao, J.; Shao, Z.; Chen, X. Zein/chitosan composite nanospheres for controlled release of fragrances. Mater. Lett. 2023, 349, 134863. [Google Scholar] [CrossRef]
- Yu, X.; Wu, H.; Hu, H.; Dong, Z.; Dang, Y.; Qi, Q.; Wang, Y.; Du, S.; Lu, Y. Zein nanoparticles as nontoxic delivery system for maytansine in the treatment of non-small cell lung cancer. Drug Deliv. 2020, 27, 100–109. [Google Scholar] [CrossRef]
- Wang, H.; Hao, L.; Wang, P.; Chen, M.; Jiang, S.; Jiang, S. Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocoll. 2017, 63, 437–446. [Google Scholar] [CrossRef]
- Zhan, X.; Dai, L.; Zhang, L.; Gao, Y. Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocoll. 2020, 106, 105839. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Huang, J.; Dai, L.; Du, J.; McClements, D.J.; Mao, L.; Liu, J.; Gao, Y. Fabrication and Characterization of Layer-by-Layer Composite Nanoparticles Based on Zein and Hyaluronic Acid for Codelivery of Curcumin and Quercetagetin. ACS Appl. Mater. Interfaces 2019, 11, 16922–16933. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Del-Toro-Sánchez, C.L.; Javier Cinco-Moroyoqui, F.; Juárez, J.; Ruiz-Cruz, S.; López-Ahumada, G.A.; Carvajal-Millan, E.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Preparation and Characterization of Quercetin-Loaded Zein Nanoparticles by Electrospraying and Study of In Vitro Bioavailability. J. Food Sci. 2019, 84, 2883–2897. [Google Scholar] [CrossRef]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019, 279, 223–230. [Google Scholar] [CrossRef]
- Gali, L.; Bedjou, F.; Ferrari, G.; Donsì, F. Formulation and characterization of zein/gum arabic nanoparticles for the encapsulation of a rutin-rich extract from Ruta chalepensis L. Food Chem. 2022, 367, 129982. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Y.; Chen, G.; Shi, Y.; Li, X.; Zhang, H.; Shen, Y. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Wang, Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J. Agric. Food Chem. 2012, 60, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-E.; Park, D.-J.; Kim, B.-K. Effects of a chitosan coating on properties of retinol-encapsulated zein nanoparticles. Food Sci. Biotechnol. 2015, 24, 1725–1733. [Google Scholar] [CrossRef]
- Seok, H.Y.; Sanoj Rejinold, N.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Atif, M. Polysaccharides based biopolymers for biomedical applications: A review. Polym. Adv. Technol. 2024, 35, e6203. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Pinteala, M.; Simionescu, N. Dextran Formulations as Effective Delivery Systems of Therapeutic Agents. Molecules 2023, 28, 1086. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic Modification of Polysaccharides: Mechanisms, Properties, and Potential Applications: A Review. Enzyme Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef]
- Yadav, N.; Francis, A.P.; Priya, V.V.; Patil, S.; Mustaq, S.; Khan, S.S.; Alzahrani, K.J.; Banjer, H.J.; Mohan, S.K.; Mony, U.; et al. Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy. Polymers 2022, 14, 950. [Google Scholar] [CrossRef]
- Ng, J.Y.; Obuobi, S.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Biomimicry of microbial polysaccharide hydrogels for tissue engineering and regenerative medicine—A review. Carbohydr. Polym. 2020, 241, 116345. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Łuczak, J.; Habibzadeh, S.; Hasanin, M.S.; Mohammadi, A.; Esmaeili, A.; Kim, S.-J.; Khodadadi Yazdi, M.; Rabiee, N.; Badawi, M.; et al. Polysaccharide nanocomposites in wastewater treatment: A review. Chemosphere 2024, 347, 140578. [Google Scholar] [CrossRef]
- Dangre, P.V.; Gurumukhi, V.C.; Meshram, S.S.; Zade, S.M. Chapter 8—Hydrogels Based on Dextran. In Polysaccharide Hydrogels for Drug Delivery and Regenerative Medicine; Giri, T.K., Ghosh, B., Badwaik, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 129–137. ISBN 978-0-323-95351-1. [Google Scholar]
- Dhaneshwar, S.; Bhilare, N.; Roy, S. Dextran Pharmaceutical Applications. In Polysaccharides of Microbial Origin: Biomedical Applications; Oliveira, J., Radhouani, H., Reis, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Patenaude, M.; Smeets, N.M.; Hoare, T. Designing injectable, covalently cross-linked hydrogels for biomedical applications. Macromol. Rapid Commun. 2014, 35, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, J.; Ding, J.; Chen, L.; Chen, X. Acid-sensitive dextran prodrug: A higher molecular weight makes a better efficacy. Carbohyd. Polym. 2017, 161, 33–41. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Li, X.; Lin, Z.; Chen, L.; Chen, H.; Jin, Y.; Zhang, T.; Xia, H.; Lu, Y.; et al. Ultrafast In-Situ Forming Halloysite Nanotube-Doped Chitosan/Oxidized Dextran Hydrogels for Hemostasis and Wound Repair. Carbohyd. Polym. 2021, 267, 118155. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Wang, Y.; Li, J.; Bao, J.; Xu, X.; Zhang, C.; Li, Y.; Wu, H.; Gu, Z. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydr. Polym. 2021, 257, 117598. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Majumder, S.; Gupta, K.C.; Kumar, A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids Surf. B Biointerfaces 2020, 195, 111263. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, M.; Liu, Q.; Yan, S.; Feng, L.; Lan, Y.; Shan, G.; Xue, W.; Guo, R. Enhanced healing activity of burn wound infection by a dextran-HA hydrogel enriched with sanguinarine. Biomater. Sci. 2018, 6, 2472–2486. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, K.; Cui, L.; Liang, J.; Li, J.; Guan, F. Injectable gelatin/oxidized dextran hydrogel loaded with apocynin for skin tissue regeneration. Biomater. Adv. 2022, 133, 112604. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, Q.; Wei, X.; Ma, Q.; Li, D.; Zhao, J. Rosmarinic Acid-Grafted Dextran/Gelatin Hydrogel as a Wound Dressing with Improved Properties: Strong Tissue Adhesion, Antibacterial, Antioxidant and Anti-Inflammatory. Molecules 2023, 28, 4034. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, B.; Liu, Y.; Wan, J.-B. Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior. Int. J. Mol. Sci. 2021, 22, 13516. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Seo, S.R.; Kim, J.C. A β-cyclodextrin, polyethyleneimine and silk fibroin hydrogel containing Centella asiatica extract and hydrocortisone acetate: Releasing properties and in vivo efficacy for healing of pressure sores. Clin. Exp. Dermatol. 2012, 37, 762–771. [Google Scholar] [CrossRef]
- Pinho, E.; Henriques, M.; Soares, G. Cyclodextrin/cellulose hydrogel with gallic acid to prevent wound infection. Cellulose 2014, 21, 4519–4530. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Y.; Li, C.; Chen, X.; Lin, L. Antibacterial efficacy of Satureja montana L. essential oil encapsulated in methyl-β-cyclodextrin/soy soluble polysaccharide hydrogel and its assessment as meat preservative. LWT 2021, 152, 112427. [Google Scholar] [CrossRef]
- Ponnusami, V.; Gunasekar, V. Production of Pullulan by Microbial Fermentation. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 581–596. [Google Scholar]
- Sugumaran, K.R.; Ponnusami, V. Review on production, downstream processing and characterization of microbial pullulan. Carbohydr. Polym. 2017, 173, 573–591. [Google Scholar] [CrossRef]
- Teixeira, M.O.; Marinho, E.; Silva, C.; Antunes, J.C.; Felgueiras, H.P. Pullulan hydrogels as drug release platforms in biomedicine. J. Drug Deliv. Sci. Technol. 2023, 89, 105066. [Google Scholar] [CrossRef]
- Rai, M.; Wypij, M.; Ingle, A.P.; Trzcińska-Wencel, J.; Golińska, P. Emerging Trends in Pullulan-Based Antimicrobial Systems for Various Applications. Int. J. Mol. Sci. 2021, 22, 13596. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.; Martin, C.; Radecka, I. The production and application of hydrogels for Wound Management: A Review. Eur. Polym. J. 2018, 111, 134–151. [Google Scholar] [CrossRef]
- Park, T.H.; Lee, S.; Amatya, R.; Maharjan, P.; Kim, H.-J.; Park, W.S.; Ahn, M.-J.; Kim, S.Y.; Moon, C.; Cheong, H.; et al. Development and characterization of a superabsorbing hydrogel film containing Ulmus davidiana var. Japonica root bark and pullulan for skin wound healing. Saudi Pharm. J. 2020, 28, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Back, S.; An, J.; Lee, N.-s.; Kim, D.K.; Na, C.; Jeong, Y.G.; Han, S. Topical film prepared with Rhus verniciflua extract-loaded pullulan hydrogel for atopic dermatitis treatment. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2325–2334. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Mahmood, A.; Kousar, M.; Thu, H.E.; Abbasi, M.; Kashif, M.U.R. Curcumin-laden hyaluronic acid-co-Pullulan-based biomaterials as a potential platform to synergistically enhance the diabetic wound repair. Int. J. Biol. Macromol. 2021, 185, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Suflet, D.M.; Constantin, M.; Pelin, I.M.; Popescu, I.; Rimbu, C.M.; Horhogea, C.E.; Fundueanu, G. Chitosan-Oxidized Pullulan Hydrogels Loaded with Essential Clove Oil: Synthesis, Characterization, Antioxidant and Antimicrobial Properties. Gels 2024, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Pelin, I.M.; Silion, M.; Popescu, I.; Rîmbu, C.M.; Fundueanu, G.; Constantin, M. Pullulan/Poly(vinyl alcohol) Hydrogels Loaded with Calendula officinalis Extract: Design and In Vitro Evaluation for Wound Healing Applications. Pharmaceutics 2023, 15, 1674. [Google Scholar] [CrossRef]
- Lierova, A.; Kasparova, J.; Filipova, A.; Cizkova, J.; Pekarova, L.; Korecka, L.; Mannova, N.; Bilkova, Z.; Sinkorova, Z. Hyaluronic Acid: Known for Almost a Century, but Still in Vogue. Pharmaceutics 2022, 14, 838. [Google Scholar] [CrossRef]
- Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. [Google Scholar] [CrossRef]
- Sprott, H.; Fleck, C. Hyaluronic Acid in Rheumatology. Pharmaceutics 2023, 15, 2247. [Google Scholar] [CrossRef]
- Valachová, K.; Šoltés, L. Hyaluronan as a Prominent Biomolecule with Numerous Applications in Medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose-hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wei, C.; Li, K.; Yin, P.; Wu, C.; Zhang, W. Polyphenol-mediated hyaluronic acid/tannic acid hydrogel with short gelation time and high adhesion strength for accelerating wound healing. Carbohydr. Polym. 2024, 342, 122372. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, G.; Cione, E.; Serra, R.; Leo, A.; Citraro, R.; Matricardi, P.; Di Meo, C.; Bisceglia, F.; Caroleo, M.C.; Basile, S.; et al. Nano-hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int. Wound J. 2020, 17, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Valentino, A.; De Luca, I.; Soares Pontes, G.; Calarco, A.; Cerruti, P. Thermo-Responsive Hydrogel Containing Microfluidic Chitosan Nanoparticles Loaded with Opuntia ficus-indica Extract for Periodontitis Treatment. Int. J. Mol. Sci. 2024, 25, 9374. [Google Scholar] [CrossRef]
- Conte, R.; De Luca, I.; Valentino, A.; Cerruti, P.; Pedram, P.; Cabrera-Barjas, G.; Moeini, A.; Calarco, A. Hyaluronic Acid Hydrogel Containing Resveratrol-Loaded Chitosan Nanoparticles as an Adjuvant in Atopic Dermatitis Treatment. J. Funct. Biomater. 2023, 14, 82. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Zhou, H. Biomimetic Hydrogel Applications and Challenges in Bone, Cartilage, and Nerve Repair. Pharmaceutics 2023, 15, 2405. [Google Scholar] [CrossRef]
- Fuchs, S.; Shariati, K.; Ma, M. Specialty Tough Hydrogels and Their Biomedical Applications. Adv. Healthc. Mater. 2020, 9, e1901396. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Pineda, C.; Castañeda Hernández, G.; Jacobs, I.A.; Alvarez, D.F.; Carini, C. Assessing the Immunogenicity of Biopharmaceuticals. BioDrugs 2016, 30, 195–206. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Kumi, M.; Ejeromedoghene, O.; Sudane, W.D.; Zhang, Z. Unlocking the biological response of smart Stimuli-Responsive hydrogels and their application in biological systems. Eur. Polym. J. 2024, 209, 112906. [Google Scholar] [CrossRef]
- Negrete-Bolagay, D.; Guerrero, V.H. Opportunities and Challenges in the Application of Bioplastics: Perspectives from Formulation, Processing, and Performance. Polymers 2024, 16, 2561. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar] [CrossRef] [PubMed]
| Application | Administration | Starch-Based Formulation | Benefits | References |
|---|---|---|---|---|
| Drug delivery | Oral route | Poly(starch/acrylic acid) (1:10 wt) hydrogel for rutin delivery | Inhibition of colonic inflammation with reduced toxicity | [37] |
| Drug delivery | Oral route | Glycerol-starch suspension with green tea extract | Steady and sustained release of these compounds over time | [38] |
| Drug delivery | Oral route | Phosphorylated starch with sodium tripolyphosphate as a crosslinking agent containing anthocyanins from purple maize | High encapsulation efficiency | [39] |
| Drug delivery | Oral route | Starch modified with octenyl succinic anhydride (OSA) containing tea polyphenols and catechins | Gradual release during digestion | [40] |
| Application | Administration | Pectin-Based Formulation | Benefits | References |
|---|---|---|---|---|
| Drug delivery | Oral route | pH sensitive polyacrylamide grafted pectin to deliver natural substances | Better gelling and film forming ability than pectin | [36] |
| Colon localized release | Oral route | Calcium carbonate microparticles within a pectin/polyethylene glycol hydrogel blend for the targeted delivery of bovine serum albumin (BSA) | High encapsulation efficiency | [37] |
| Drug delivery | Oral route | Pectin hydrogel beads reinforced with caseinate (PCHG-CAS) to improve the stability of (−)-epigallocatechin (EGC) | Delayed EGC release in water and providing controlled release under simulated gastrointestinal conditions. EGC remained chemically stable over a 6-day storage period at 37 °C, preventing epimerization, oxidation, dimerization, and trimerization | [38] |
| Drug delivery | Oral route | Photo-crosslinked methacrylated derivatives of pectin/gelatin hydrogels containing curcumin | Stabilization of bioactive compounds | [49] |
| Drug delivery | Oral route | Hydrogel beads with varying alginate and pectin ratios to encapsulate Pickering emulsions loaded with resveratrol | pH-responsive behavior | [40] |
| Gastric delivery device | Oral route | Hydrogel beads made from pectin and acid-resistant maize starch containing bromelain | Better swelling properties, sustained release, and greater gastric stability compared to hydrogels made with pectin alone | [51] |
| Wound healing | Topical | Hydrogel with Arabic gum and pectin encapsulating naringenin | Acceleration of wound healing by promoting angiogenesis and collagen deposition and reduction in the mRNA expression of inflammatory markers and apoptosis-related factors | [52] |
| Wound healing | Topical | Quaternized chitosan and pectin hydrogel containing propolis | Strong antioxidant and antibacterial activity | [53] |
| Application | Administration | Cellulose-Based Formulation | Benefits | References |
|---|---|---|---|---|
| Treatment of ankylosing spondylitis | Scaffold | Cellulose hydrogel by physical cross-linking in a NaOH/urea medium with α-mangostin employed as an active pharmaceutical ingredient | Inhibitory activity against the growth of MC3T3-E1 cells | [59] |
| Drug delivery | Scaffold | Xylan-β-cyclodextrin cellulose hydrogel with curcumin | Curcumin forms inclusion complexes with β-cyclodextrin within the gel, prolonging the release | [60] |
| Drug delivery | Scaffold | Cellulose hydrogel matrix with pectin and mucin cross-linked with epichlorohydrin to develop superabsorbent cellulose-based hydrogels | Greater swelling and reduced matrix erosion; Slower drug release due to an extended diffusion pathway | [61] |
| Skin infection | Topical | Hydrogel of regenerated cellulose derived from sugarcane bagasse with zinc oxide nanoparticles delivering musk melon seed extract and curcumin | Strong antimicrobial efficacy | [62] |
| Application | Administration | Zein-Based Formulation | Benefits | References |
|---|---|---|---|---|
| Antimicrobial device | Oral route | Composite carrier made of zein and chitosan containing cinnamaldehyde | Interaction of drug and zein-chitosan composite reduces volatilization rate with good encapsulation efficiency, release behavior and antimicrobial efficacy | [64] |
| Anticancer delivery system | Oral route | Maytansine-loaded zein devices | Enhanced tumor targeting for non-small cell lung cancer (A549 cells) and reduced toxic side effects of maytansine | [55] |
| Drug delivery | Oral route | Curcumin-loaded zein devices | Enhanced antibacterial activity | [66] |
| Drug delivery | Oral route | Whey protein isolate (WPI)-zein composite nanogels containing curcumin | Improved dispersibility of Curcumin | [67] |
| Drug delivey | Oral route | Zein-hyaluronic acid composite nanogels containing curcumin and quercetagetin | Preparation using a layer-by-layer assembly technique; increase in the light, thermal, and storage stability of drugs | [68] |
| Carriers | Oral route | Zein hydrogel containing quercetin | Good encapsulation efficiency | [69] |
| Carriers | Oral route | Zein hydrogel containing resveratrol | Good encapsulation efficiency | [70] |
| Carriers | Oral route | Zein hydrogel containing rutin | Good encapsulation efficiency | [71] |
| Carriers | Oral route | Zein hydrogel containing β-carotene | Good encapsulation efficiency | [62] |
| Carriers | Oral route | Zein hydrogel containing Vitamin D3 | Good encapsulation efficiency | [73] |
| Carriers | Oral route | Zein hydrogel containing retinol | Good encapsulation efficiency | [74] |
| Localized drug delivery to colorectal cells | Oral route | Curcumin-loaded zein–hyaluronic acid gel | Strong anticancer activity against CT26 colorectal cancer cells, attributed to the enhanced targeting of the CD44 receptor | [75] |
| Application | Administration | Dextran-Based Formulation | Benefits | References |
|---|---|---|---|---|
| Antibacterial device for wound healing | Topical | Hydrogel of halloysite nanotubes mixed with chitosan/oxidized dextran. | Strong antibacterial capacity; Improvement of S. aureus-infected resistant wounds | [88] |
| Antimicrobial device for wound healing | Topical | Dextran/chitosan hydrogels loaded with polydopamine nanoparticles | Strong antimicrobial capacity | [89] |
| Antimicrobial device for wound healing | Topical | Oxidizing dextran and gelatin hydrogel, loaded with nanoparticles of cerium oxide and curcumin | Strong antimicrobial capacity | [90] |
| Wound healing | Topical | Combined hydrogels based on dextran-hyaluronic acid loaded with Sanguinarine | Antibacterial efficacy against S. aureus and E. coli; Long-duration drug release; Wound regeneration ability | [91] |
| Wound healing | Topical | Injectable hydrogel composed of gelatin and oxidized dextran loaded with apocynin | Good injectability, self-healing and hemostatic properties | [92] |
| Wound healing | Topical | Rosmarinic acid-grafted dextran/gelatin hydrogel | High biocompatibility, very rapid gelation times and good mechanical properties | [93] |
| Melanoma-directed delivery device | Topical | Hypromellose hydrogels with different dextran based cyclodextrins incorporating 3-O-Methylquercetin. | Improved skin release | [95] |
| Localized drug delivery | Oral route | Hydrogel composed of dextran β-CD, polyethylenimine and silk fibroin loaded with Centella asiatica extract and hydrocortisone acetate | pH dependent release | [96] |
| Localized drug delivery | Topical | Dextran cyclodextrin-based hydrogel loaded with gallic acid | Slow release; Antibacterial activity against S. epidermidis, S. aureus and K. pneumoniae without causing any damage to the surrounding tissue | [97] |
| Antibacterial device | Oral route (in association with ingested food) | Hydrogel based on dextran methyl-β-cyclodextrin and soluble soy polysaccharide loaded with Satureja montana L. essential oil. | Strong antibacterial action | [98] |
| Application | Administration | Pullulan-Based Formulation | Benefits | References |
|---|---|---|---|---|
| Wound healing | Topical | Pullulan hydrogel with Ulmus davidiana var. japonica root bark | Improved skin adherence | [105] |
| Anti-scratch film on the skin for the treatment of atopic dermatitis | Topical | Pullulan-based hydrogel loaded with an aqueous extract of Rhus verniciflua | Immunostimulant, antioxidant, anticancer, anti-inflammatory and antimicrobial | [106] |
| Repair diabetic wounds | Topical | Injectable hydrogel platform of hyaluronic acid-pullulan and pluronic containing curcumin | Controlled release of curcumin | [107] |
| Wound dressing | Topical | Clove oil-loaded pullulan hydrogel | Excellent mechanical properties, good swelling ability combined with rapid shape recovery; Sustained release of the oil | [108] |
| Wound healing | Topical | Pullulan/poly(vinyl alcohol) hydrogels loaded with the hydroalcoholic extract of Calendula officinalis | High loading efficiency; Good mechanical properties; Increased bioadhesiveness | [109] |
| Application | Administration | Hyaluronic Acid-Based Formulation | Benefits | References |
|---|---|---|---|---|
| Transdermal drug delivery | Topical | pH-sensitive hydrogel formed of hydroxyethyl cellulose and hyaluronic acid, containing isoliquiritigenin | pH-dependent drug release | [115] |
| Wound healing | Topical | Hyaluronic acid based composite hydrogel incorporating tannic acid and dopamine-coated carbon particles rich in phenols | Rapid gelation time; Good adhesive strength, Low hemolytic activity; Minimal cytotoxicity; Ability to promote fibroblast proliferation and migration | [116] |
| Treatment of lower limb skin wound in patients with diabetes mellitus | Topical | Hyaluronic acid-based nanohydrogel embedded with quercetin and oleic acid | Reduction in wound healing time without developing adverse drug reactions | [117] |
| Localized and sustained release in periodontal pockets | Scaffold | Opuntia ficus-indica extract encapsulated in chitosan nanoparticles embedded in pluronic–hyaluronic thermo-responsive hydrogel | System able to eradicate biofilms of S. mutans, P. aeruginosa and P. gingivalis and disrupt extracellular polymeric substance formation. | [118] |
| Treatment of atopic dermatitis | Topical | Hyaluronic acid hydrogel containing resveratrol-loaded chitosan (CS) nanoparticles | Delayed hydrolytic degradation; Decreased oxidative damage; Reduced secretion and gene expression of proinflammatory cytokines | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Calarco, A.; Margarucci, S.; Peluso, G.; Conte, R. Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Molecules: From Tissue Regeneration to Infection Control. Gels 2025, 11, 198. https://doi.org/10.3390/gels11030198
Sepe F, Valentino A, Marcolongo L, Petillo O, Calarco A, Margarucci S, Peluso G, Conte R. Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Molecules: From Tissue Regeneration to Infection Control. Gels. 2025; 11(3):198. https://doi.org/10.3390/gels11030198
Chicago/Turabian StyleSepe, Fabrizia, Anna Valentino, Loredana Marcolongo, Orsolina Petillo, Anna Calarco, Sabrina Margarucci, Gianfranco Peluso, and Raffaele Conte. 2025. "Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Molecules: From Tissue Regeneration to Infection Control" Gels 11, no. 3: 198. https://doi.org/10.3390/gels11030198
APA StyleSepe, F., Valentino, A., Marcolongo, L., Petillo, O., Calarco, A., Margarucci, S., Peluso, G., & Conte, R. (2025). Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Molecules: From Tissue Regeneration to Infection Control. Gels, 11(3), 198. https://doi.org/10.3390/gels11030198







