Ion-Specific Gelation and Internal Dynamics of Nanocellulose Biocompatible Hybrid Hydrogels: Insights from Fluctuation Analysis
Abstract
1. Introduction
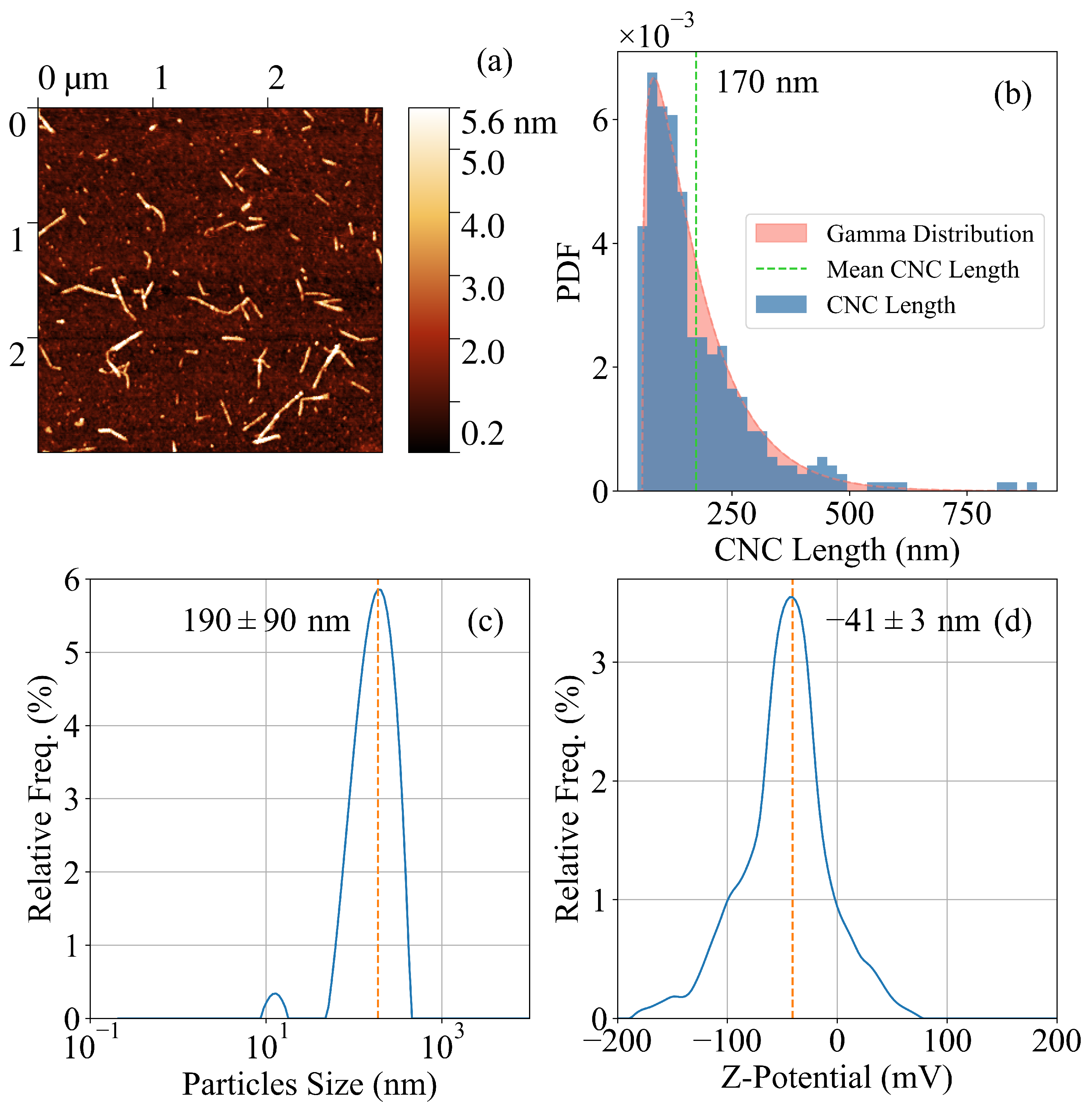
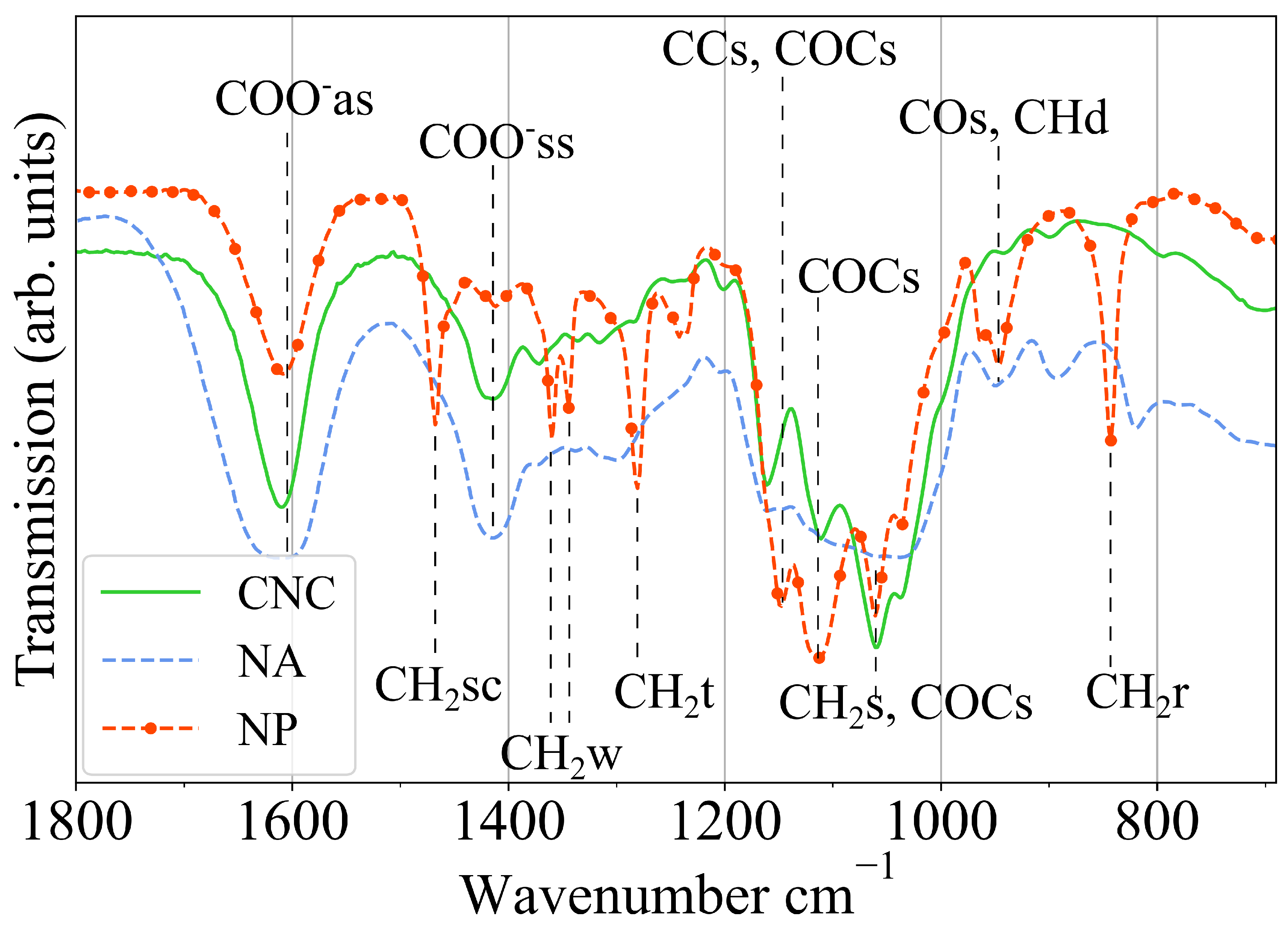
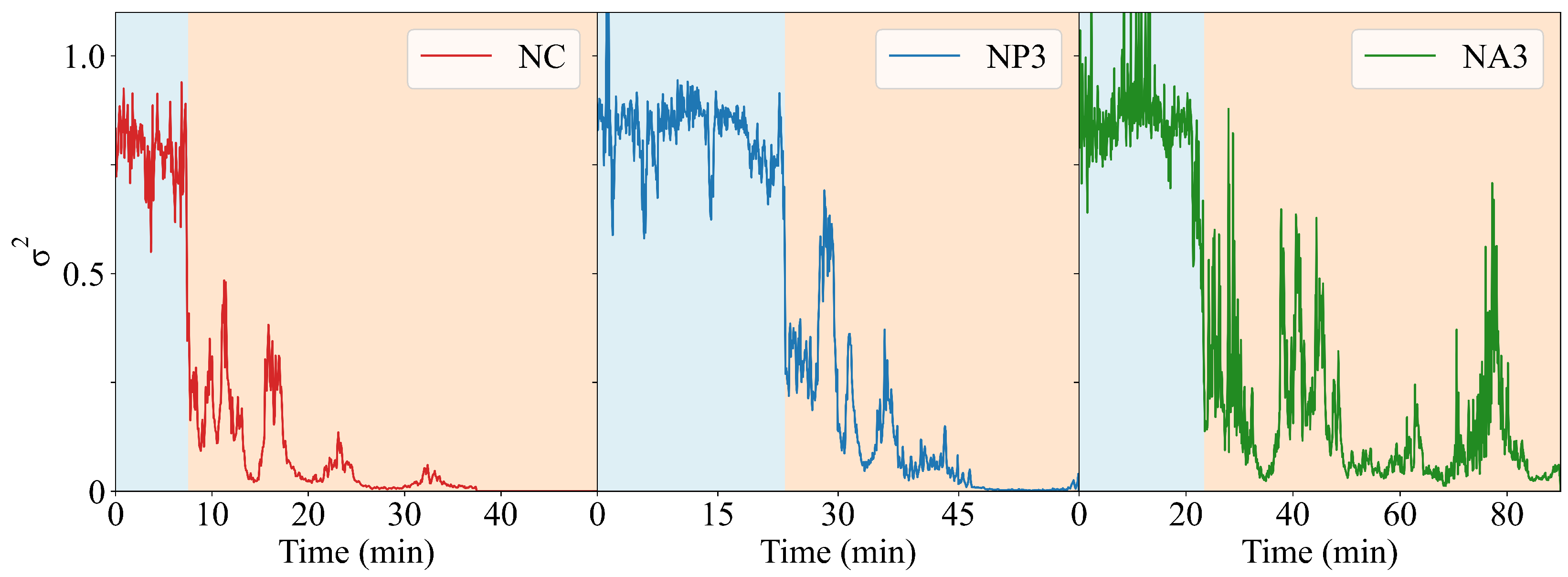
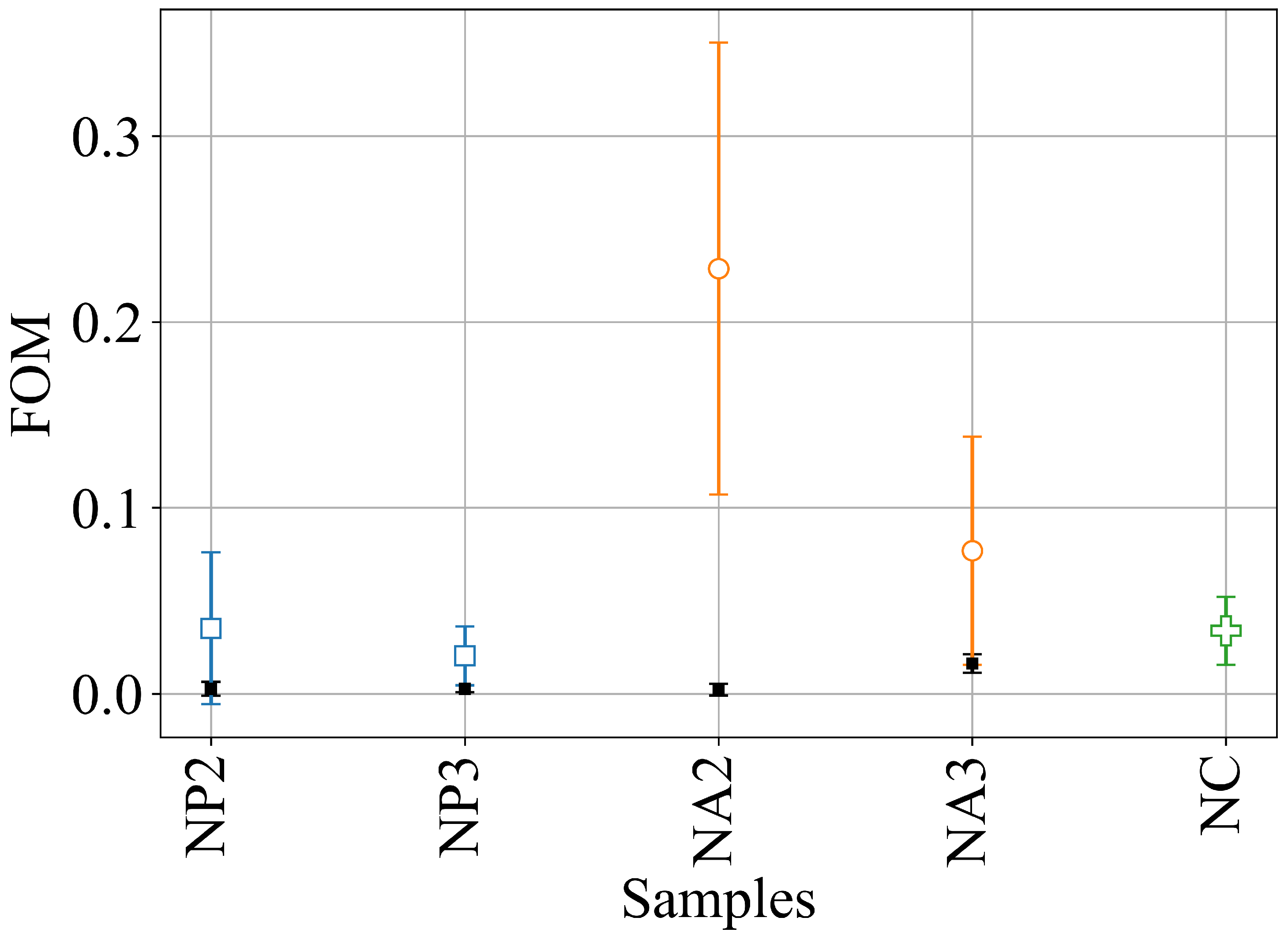
2. Results and Discussion
3. Conclusions
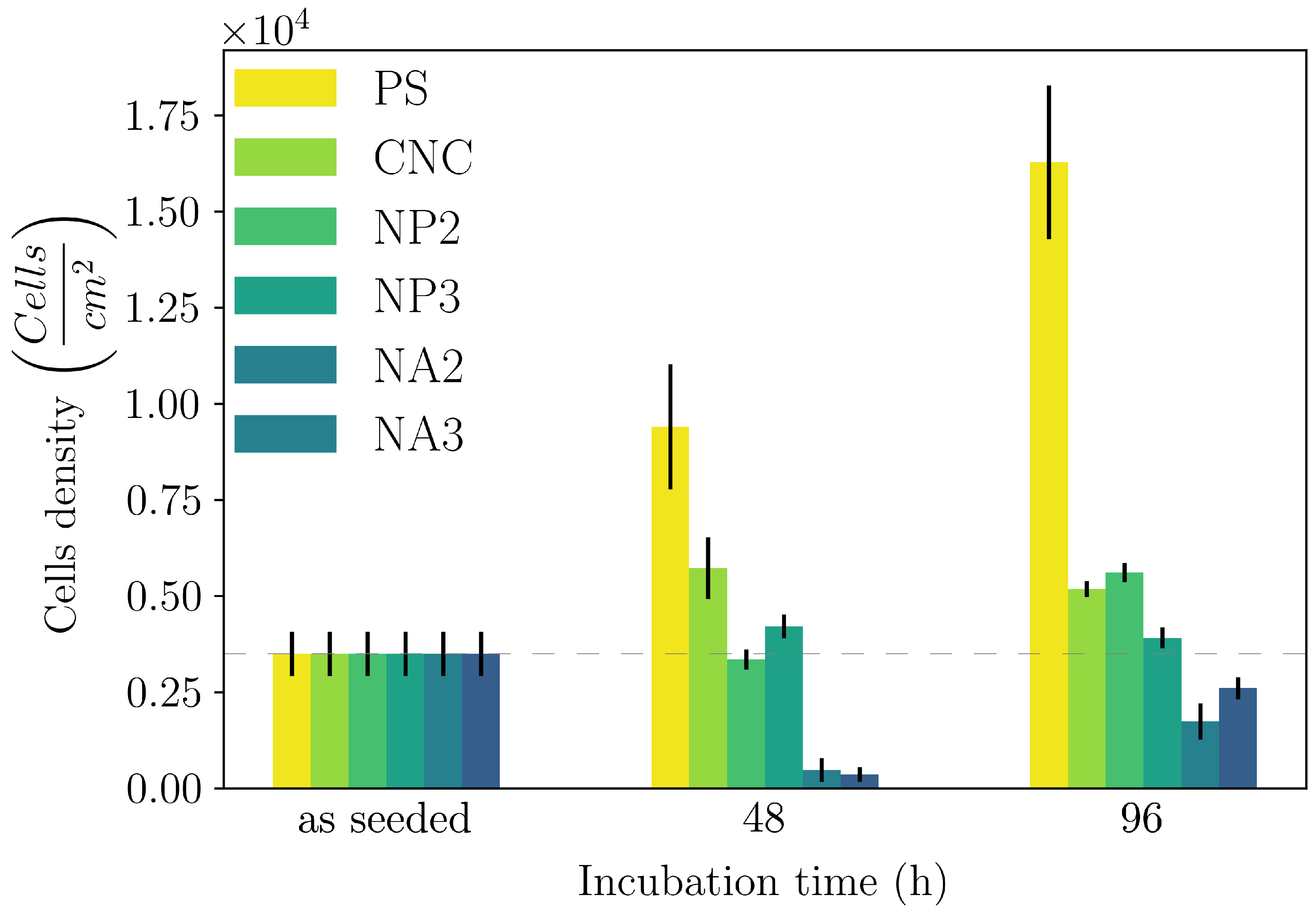
- We showed that both the composite hydrogels made of CNC/linear amino–PEG and CNC–alginate were good substrates for growth in a model cell line. However, CNC/amino–PEG induced much faster cell proliferation, despite the similar physical macroscopic properties of the two tested materials. This result suggests that the cells also responded to dynamic environmental cues not detectable by conventional techniques.
- We introduced a novel DLS analysis implemented with commercial, single-angle apparatus that was able to discriminate between gels with similar macroscopic mechanical properties but differing chemical composition and microscopic structural dynamics. As representative tests, we investigated the dynamics of CNC–PEG and CNC–alginate materials. To prove the relationship between the dynamic DLS data and the microscopic hydrogel structure, we exploited the peculiar characteristic of alginates jellifying in the presence of but not with [42], since smaller , differently from , does not fit into the G boxes and establishes with alginate chains a weaker affinity-driven interaction ruled basically by Manning’s theory [69]. By defining a phenomenological Figure of Merit (FOM), based on the short-time values of the second-order correlation function, we showed that its statistical properties contained information about the sol–gel transition and the material composition. We observed that the nanoscale dynamical properties were in some way retained when the hydrogels attained both stable and similar macroscopic properties. We suppose that the differences in their internal dynamics causally affected the hydrogel interaction with cells in the culture. In fact, while CNC–PEG composites formed a stable gel structure in a relatively short time and it induced faster cell proliferation, the slowly equilibrating CNC–alginate hydrogels showed longer induction time. Notably, cell proliferation depends on the stability of cell adhesion to the support and our results indicate that this stability is affected by nanoscale phenomena. Cell adhesion and proliferation are fundamental issues for the design of innovative hydrogels for human use (e.g., they are invaluable for tissue regeneration, wound healing, drug delivery, and organoids), and at present, nanoscale behavior is often neglected. Conversely, this behavior seems to play an important role, and here, we provide a simple approach for its monitoring. Thus, by combining the DLS approach to current state-of-the-art techniques (i.e., NMR, SAXS, and rheometry) we could obtain more exhaustive information on the gelation mechanism. In fact, DLS is characterized by a significantly better temporal (tens of s) and spatial (tens of m) resolution and it provides short-timescale dynamic information for small size clusters of a gel. The other advantage of the proposed approach is its easy implementation using conventional DLS equipment. While phenomenological in nature, our approach is the only one reported able to fully exploit the spatial and temporal resolution provided by DLS. It provides quantitative information about differences between materials that vary in specific parameters, like component ratios. Although DLS is a well-known technique, this is the first report where the statistical properties of the initial values of (t) are used to derive information about the chemical composition and the dynamical state of a material. Our proof-of-principle demonstration will pave the way for a much broader use of our approach. Dedicated models and computational tools are needed to fully understand the method’s possibilities and limitations and will be developed in the future.
4. Materials and Methods
4.1. Materials
4.2. Synthesis of CNC
4.3. Synthesis of Hybrid Gels
| Name | CNC | PEG | Alginate |
|---|---|---|---|
| CNC | 1 | 0 | 0 |
| NP2 | 2 | 1 | 0 |
| NP3 | 3 | 1 | 0 |
| NA2 | 2 | 0 | 1 |
| NA3 | 3 | 0 | 1 |
4.4. Hydrogel Characterization
4.5. Cell Culture and Cell Viability
4.6. DLS Analysis
- in solutions (ergodic systems).
- in gels.
- in glasses (completely frozen systems).
- One coming from the “frozen”, averaged configurations of the scatterers;
- One due to the fluctuations around their average position.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D.; et al. Hydrogel-Based Flexible Electronics. Adv. Mater. 2023, 35, 2205326. [Google Scholar] [CrossRef]
- Mikhailidi, A.; Ungureanu, E.; Tofanica, B.M.; Ungureanu, O.C.; Fortună, M.E.; Belosinschi, D.; Volf, I. Agriculture 4.0: Polymer Hydrogels as Delivery Agents of Active Ingredients. Gels 2024, 10, 368. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in biomedical and biosensing applications: A review. Int. J. Biol. Macromol. 2021, 166, 587–600. [Google Scholar] [CrossRef]
- Maestri, C.A.; Abrami, M.; Hazan, S.; Chistè, E.; Golan, Y.; Rohrer, J.; Bernkop-Schnürch, A.; Grassi, M.; Scarpa, M.; Bettotti, P. Role of sonication pre-treatment and cation valence in the sol-gel transition of nano-cellulose suspensions. Sci. Rep. 2017, 7, 11129. [Google Scholar] [CrossRef]
- Maestri, C.A.; Bettotti, P.; Scarpa, M. Fabrication of complex-shaped hydrogels by diffusion controlled gelation of nanocellulose crystallites. J. Mater. Chem. B 2017, 5, 8096–8104. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Bettotti, P.; Scarpa, M. Nanocellulose and Its Interface: On the Road to the Design of Emerging Materials. Adv. Mater. Interfaces 2022, 9, 2101593. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Nunes, Y.L.; Figueirêdo, M.C.B.; de Azeredo, H.M.C.; Aouada, F.A.; Feitosa, J.P.A.; Rosa, M.F.; Dufresne, A. Nanocellulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Hu, S.; Zhi, Y.; Shan, S.; Ni, Y. Research progress of smart response composite hydrogels based on nanocellulose. Carbohydr. Polym. 2022, 275, 118741. [Google Scholar] [CrossRef]
- Deng, Y.; Xi, J.; Meng, L.; Lou, Y.; Seidi, F.; Wu, W.; Xiao, H. Stimuli-Responsive nanocellulose Hydrogels: An overview. Eur. Polym. J. 2022, 180, 111591. [Google Scholar] [CrossRef]
- Strnad, S.; Zemljič, L. Cellulose–Chitosan Functional Biocomposites. Polymers 2023, 15, 425. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The Application Status of Nanoscale Cellulose-Based Hydrogels in Tissue Engineering and Regenerative Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 732513. [Google Scholar] [CrossRef]
- Heidarian, P.; Kaynak, A.; Paulino, M.; Zolfagharian, A.; Varley, R.J.; Kouzani, A.Z. Dynamic nanocellulose hydrogels: Recent advancements and future outlook. Carbohydr. Polym. 2021, 270, 118357. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qamar, S.A.; Qamar, M.; Basharat, K.; Bilal, M. Engineered nanocellulose-based hydrogels for smart drug delivery applications. Int. J. Biol. Macromol. 2021, 181, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Roman, M. Fluorescently Labeled Cellulose Nanocrystals for Bioimaging Applications. J. Am. Chem. Soc. 2007, 129, 13810–13811. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, W.; Xu, Q.; Sun, Q. Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 2015, 22, 2443–2456. [Google Scholar] [CrossRef]
- Palaganas, N.B.; Mangadlao, J.D.; de Leon, A.C.C.; Palaganas, J.O.; Pangilinan, K.D.; Lee, Y.J.; Advincula, R.C. 3D Printing of Photocurable Cellulose Nanocrystal Composite for Fabrication of Complex Architectures via Stereolithography. ACS Appl. Mater. Interfaces 2017, 9, 34314–34324. [Google Scholar] [CrossRef]
- Kalossaka, L.M.; Mohammed, A.A.; Sena, G.; Barter, L.; Myant, C. 3D printing nanocomposite hydrogels with lattice vascular networks using stereolithography. J. Mater. Res. 2021, 36, 4249–4261. [Google Scholar] [CrossRef]
- Bai, C.; Tang, A.; Zhao, S.; Liu, W. Flexible nanocellulose/poly(ethylene glycol) diacrylate hydrogels with tunable Poisson’s ratios by masking and photocuring. BioResources 2020, 15, 3307–3319. [Google Scholar] [CrossRef]
- Tang, A.; Ji, J.; Li, J.; Liu, W.; Wang, J.; Sun, Q.; Li, Q. Nanocellulose/PEGDA Aerogels with Tunable Poisson’s Ratio Fabricated by Stereolithography for Mouse Bone Marrow Mesenchymal Stem Cell Culture. Nanomaterials 2021, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Monfared, M.; Mawad, D.; Rnjak-Kovacina, J.; Stenzel, M.H. 3D bioprinting of dual-crosslinked nanocellulose hydrogels for tissue engineering applications. J. Mater. Chem. B 2021, 9, 6163–6175. [Google Scholar] [CrossRef] [PubMed]
- Iman, M.; Barati, A.; Safari, S. Characterization, in vitro antibacterial activity, and toxicity for rat of tetracycline in a nanocomposite hydrogel based on PEG and cellulose. Cellulose 2020, 27, 347–356. [Google Scholar] [CrossRef]
- Yin, A.; Yang, J. Cross-Linking Dynamics of Cellulose Nanofibrils-Based Transient Network Hydrogels: A Study of pH Dependence. Macromol. Chem. Phys. 2017, 218, 1600584. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, M.; Xu, F. Modulation of Assembly and Dynamics in Colloidal Hydrogels via Ionic Bridge from Cellulose Nanofibrils and Poly(ethylene glycol). Acs Macro Lett. 2015, 4, 829–833. [Google Scholar] [CrossRef]
- Tehrani, Z.; Nordli, H.R.; Pukstad, B.; Gethin, D.T.; Chinga-Carrasco, G. Translucent and ductile nanocellulose-PEG bionanocomposites—A novel substrate with potential to be functionalized by printing for wound dressing applications. Ind. Crops Prod. 2016, 93, 193–202. [Google Scholar] [CrossRef]
- Silva, R.D.; Sierakowski, M.R.; Bassani, H.P.; Zawadzki, S.F.; Pirich, C.L.; Ono, L.; de Freitas, R.A. Hydrophilicity improvement of mercerized bacterial cellulose films by polyethylene glycol graft. Int. J. Biol. Macromol. 2016, 86, 599–605. [Google Scholar] [CrossRef]
- Donati, I.; Christensen, B.E. Alginate-metal cation interactions: Macromolecular approach. Carbohydr. Polym. 2023, 321, 121280. [Google Scholar] [CrossRef]
- Donati, I.; Asaron, F.; Paoletti, S. Experimental evidence of counterion affinity in alginates: The case of nongelling ion Mg2+. J. Phys. Chem. 2009, 113, 12877–12886. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Batchelor, W.; Tabor, R.F.; Garnier, G. Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective. J. Colloid Interface Sci. 2018, 509, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Beris, A.N.; Rogers, S.A.; Wagner, N.J. Dynamic shear rheology of a thixotropic suspension: Comparison of an improved structure-based model with large amplitude oscillatory shear experiments. J. Rheol. 2016, 60, 433–450. [Google Scholar] [CrossRef]
- Jakeman, E. Photon Correlation. In Photon Correlation and Light Beating Spectroscopy; Cummins, H.Z., Pike, E.R., Eds.; Springer Science + Business Media, LLC: New York, NY, USA, 1974. [Google Scholar]
- Pusey, P.; Van Megen, W. Dynamic light scattering by non-ergodic media. Phys. A Stat. Mech. Its Appl. 1989, 157, 705–741. [Google Scholar] [CrossRef]
- Shibayama, M.; Norisuye, T. Gel formation analyses by dynamic light scattering. Bull. Chem. Soc. Jpn. 2002, 75, 641–659. [Google Scholar] [CrossRef]
- Abou, B.; Bonn, D.; Meunier, J. Aging dynamics in a colloidal glass. Phys. Rev. E 2001, 64, 021510. [Google Scholar] [CrossRef]
- Scheffold, F.; Skipetrov, S.; Romer, S.; Schurtenberger, P. Diffusing-wave spectroscopy of nonergodic media. Phys. Rev. E-Stat. Nonlinear Soft Matter Phys. 2001, 63, 061404/1–061404/11. [Google Scholar] [CrossRef]
- Urquidi, O.; Barbosa, N.; Brazard, J.; Adachi, T.B.M. Toward time resolved dynamic light scattering microscopy: Retrieving particle size distributions at high temporal resolutions. Rev. Sci. Instrum. 2023, 94, 083101. [Google Scholar] [CrossRef]
- Fahimi, Z.; Aangenendt, F.J.; Voudouris, P.; Mattsson, J.; Wyss, H.M. Diffusing-wave spectroscopy in a standard dynamic light scattering setup. Phys. Rev. E 2017, 96, 062611. [Google Scholar] [CrossRef] [PubMed]
- Badruddoza, A.Z.M.; MacWilliams, S.V.; Sebben, D.A.; Krasowska, M.; Beattie, D.; Durian, D.J.; Ferri, J.K. Diffusing wave spectroscopy (DWS) methods applied to double emulsions. Curr. Opin. Colloid Interface Sci. 2018, 37, 74–87. [Google Scholar] [CrossRef]
- Hassan, P.A.; Rana, S.; Verma, G. Making Sense of Brownian Motion: Colloid Characterization by Dynamic Light Scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef]
- Höhler, R.; Cohen-Addad, S.; Durian, D.J. Multiple light scattering as a probe of foams and emulsions. Curr. Opin. Colloid Interface Sci. 2014, 19, 242–252. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Kotov, N.; Larsson, P.A.; Jain, K.; Abitbol, T.; Cernescu, A.; Wågberg, L.; Johnson, C.M. Elucidating the fine-scale structural morphology of nanocellulose by nano infrared spectroscopy. Carbohydr. Polym. 2023, 302, 120320. [Google Scholar] [CrossRef]
- Saito, K.; Xu, T.; Ishikita, H. Correlation between C=O Stretching Vibrational Frequency and pKa Shift of Carboxylic Acids. J. Phys. Chem. B 2022, 126, 4999–5006. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Brogly, M.; Bistac, S.; Bindel, D. Advanced surface FTIR spectroscopy analysis of poly(ethylene)-block-poly(ethylene oxide) thin film adsorbed on gold substrate. Appl. Surf. Sci. 2022, 603, 154428. [Google Scholar] [CrossRef]
- Fukushima, K.; Matsuura, H. Infrared spectroscopic study of solid films of poly(ethylene glycol) doped with cations. J. Mol. Struct. 1995, 350, 215–219. [Google Scholar] [CrossRef]
- Li, M.C.; Wu, Q.; Song, K.; Qing, Y.; Wu, Y. Cellulose Nanoparticles as Modifiers for Rheology and Fluid Loss in Bentonite Water-based Fluids. Acs Appl. Mater. Interfaces 2015, 7, 5006–5016. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Mijlkovic, A.; Malafronte, L.; Stevanic, J.S.; Larsson, P.T.; Lopez-Sanchez, P. Cellulose nanocrystal/low methoxyl pectin gels produced by internal ionotropic gelation. Carbohydr. Polym. 2021, 260, 117345. [Google Scholar] [CrossRef]
- Elosegui-Artola, A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr. Opin. Cell Biol. 2021, 72, 10–18. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tan, Y.; Feng, J.; Huang, C.; Liu, B.; Fan, Z.; Xu, B.; Lu, T. Exploration of the Effects of Substrate Stiffness on Biological Responses of Neural Cells and Their Mechanisms. ACS Omega 2020, 5, 31115–31125. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, Q.; Li, X.; Feng, J.; Ao, Z.; Li, X.; Wang, J. Substrate Stiffness Modulates the Growth, Phenotype, and Chemoresistance of Ovarian Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 7118834. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Wang, B.; Lyu, C.; Li, W.; Wu, Y.; Zhu, L.; Bi, R.; Huang, C.; Li, J.J.; Du, Y. Consistent apparent Young’s modulus of human embryonic stem cells and derived cell types stabilized by substrate stiffness regulation promotes lineage specificity maintenance. Cell Regen. 2020, 9, 15. [Google Scholar] [CrossRef]
- Borgogna, M.; Skjåk-Bræk, G.; Paoletti, S.; Donati, I. On the Initial Binding of Alginate by Calcium Ions. The Tilted Egg-Box Hypothesis. J. Phys. Chem. B 2013, 117, 7277–7282. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Capes-Davis, A.; Freshney, R. (Eds.) Freshney’s Culture of Animal Cells, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2021. [Google Scholar]
- Truong, C.; Oudre, L.; Vayatis, N. Selective review of offline change point detection methods. Signal Process. 2020, 167, 107299. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomei, A.; D’Amato, E.; Scarpa, M.; Bergamaschi, G.; Gori, A.; Bettotti, P. Ion-Specific Gelation and Internal Dynamics of Nanocellulose Biocompatible Hybrid Hydrogels: Insights from Fluctuation Analysis. Gels 2025, 11, 197. https://doi.org/10.3390/gels11030197
Bartolomei A, D’Amato E, Scarpa M, Bergamaschi G, Gori A, Bettotti P. Ion-Specific Gelation and Internal Dynamics of Nanocellulose Biocompatible Hybrid Hydrogels: Insights from Fluctuation Analysis. Gels. 2025; 11(3):197. https://doi.org/10.3390/gels11030197
Chicago/Turabian StyleBartolomei, Arianna, Elvira D’Amato, Marina Scarpa, Greta Bergamaschi, Alessandro Gori, and Paolo Bettotti. 2025. "Ion-Specific Gelation and Internal Dynamics of Nanocellulose Biocompatible Hybrid Hydrogels: Insights from Fluctuation Analysis" Gels 11, no. 3: 197. https://doi.org/10.3390/gels11030197
APA StyleBartolomei, A., D’Amato, E., Scarpa, M., Bergamaschi, G., Gori, A., & Bettotti, P. (2025). Ion-Specific Gelation and Internal Dynamics of Nanocellulose Biocompatible Hybrid Hydrogels: Insights from Fluctuation Analysis. Gels, 11(3), 197. https://doi.org/10.3390/gels11030197







