Abstract
Systemic chemotherapy is still the first-line treatment for cancer, and it’s associated with toxic side effects, chemoresistance, and ultimately cancer recurrence. Rapid gelling hydrogels can overcome this limitation by providing localized delivery of anti-cancer agents to solid tumors. Silk hydrogels are extremely biocompatible and suitable for anti-cancer drug delivery, but faster gelling formulations are needed. In this study, we introduce a rapid gelling hydrogel formulation (<3 min gelling time) due to chemical crosslinking between silk fibroin and curcumin, initiated by the addition of minute quantities of horseradish peroxidase (HRP) and hydrogen peroxide (H2O2). The novel observation in this study is that curcumin, while being a free-radical scavenger, also participates in accelerating silk di-tyrosine crosslinking in the presence of HRP and H2O2. Using UV-Vis, rheology, and time-lapse videos, we convincingly show that curcumin accelerates silk di-tyrosine crosslinking reaction in a concentration-dependent manner, and curcumin remains entrapped in the hydrogel post-crosslinking. FTIR results show an increase in secondary beta-sheet structures within hydrogels, with increasing concentrations of curcumin. Furthermore, we show that curcumin-silk di-tyrosine hydrogels are toxic to U2OS osteosarcoma cells, and most cancer cells are dead within short time scales of 4 h post-encapsulation.
1. Introduction
Cancer is a significant healthcare burden, and it is the second leading cause of mortality in the United States, with ~2 million new cases and 0.6 million projected deaths in 2025 [1]. Chemotherapeutics are still the first line of treatment for most solid tumors, and they are delivered intravenously, resulting in toxic side effects, causing chemoresistance, ineffective cancer-killing, and ultimately leading to cancer recurrence. This is especially true for osteosarcoma patients, where recurrence rates are up to 40–50% even after aggressive systemic chemotherapy and surgery [2,3]. Therefore, targeted delivery of anti-cancer drugs is a much more effective strategy for killing solid tumors, especially prior to metastasis.
Hydrogel drug delivery vehicles are used for treating solid tumors, owing to their biocompatibility, mechanical properties, degradability, and ability to provide localized delivery of anti-cancer drugs over long periods of time [4,5]. Nanoparticles have also been widely used for anti-cancer drug delivery, but they are often sought out in conjunction with hydrogels as combinatory systems [6,7]. Smart thermoplastic hydrogels are often preferred because they undergo a quick sol-gel transition at 37 °C and can be delivered in liquid form, filling the area surrounding tumors easily [8,9]. Silk fibroin protein hydrogels are well-suited for treating solid tumors, as they are smart thermoplastics capable of gelling in vivo, with excellent biocompatibility. Silk fibroin hydrogels are better suited than other natural extracellular matrix hydrogels (e.g., collagen, gelatin, hyaluronic acid, etc.), as their amino acid composition promotes degradability, but prevents cancer cell growth due to the lack of cell binding sites. There have been many previous studies utilizing silk fibroin hydrogel systems for anti-cancer drug delivery; however, most of these studies do not support a quick gelling hydrogel formulation that can be delivered to the target tumor site [10,11,12,13,14,15,16]. Those studies that developed quick gelling hydrogels (<5 min gelling time), suffered from other limitations such as (1) high silk concentrations, which can be difficult to administer, or (2) combinatory products, which can be difficult to translate in the clinic.
A review of the recent literature shows that chemical crosslinking methods consistently delivered the quickest gelling silk hydrogel formulations. Yan et al. used minute quantities of horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) to induce silk hydrogel gelation [11]. Rapid gelling hydrogels from 4–40 min were fabricated, but very high concentrations (~16% w/v) of silk fibroin and higher concentrations of HRP were required to achieve shorter crosslinking times. The fabricated hydrogels in this study were able to kill chondrogenic cancer cells in vitro and in vivo within 7 days, coinciding with beta-sheet formation. Li et al., fabricated silk hydrogels by the physical drying process, with or without curcumin, a well-known anti-cancer drug [16]. Interestingly, silk hydrogel gelation time decreased with increasing concentration of curcumin, and hydrogel gelation times between 30 min to 5 h are reported. Because the study was not focused on treating cancer, the authors did not investigate faster gelation times. In another study that utilized physical crosslinking methods, Chaala et al. prepared silk fibroin/hyaluronic acid composite hydrogels via sonication, and a sonication time of at least 5 s was required to initiate gelling, taking anywhere from 30 min to 2 h to achieve complete gelation [12,13]. Peng et al. fabricated silk/PEG composite hydrogels with gelling times closer to 30 min [14]. These hydrogels could be mixed with polyvinylpyrrolidone iodine (PVP-I) to kill osteosarcoma cells both in vitro and in vivo. Another simple gelling formulation was developed by Laomeephol et al., by mixing silk fibroin with Dimyristoyl glycerophosphorylglycerol (DMPG)-based liposomes [15]. Hydrogels produced using this technique had extremely rapid gelation times of 3 min, but the gelation effect was only observed with certain liposomal formulations. Hydrogels prepared with curcumin encapsulating liposomes displayed anti-cancer properties and killed MDA-MB-231 breast cancer cells.
Herein, we introduce a rapid gelling hydrogel formulation (<3-min setting time) due to chemical crosslinking between silk fibroin and curcumin. Curcumin is a yellow-orange polyphenolic compound taken from the Curcuma longa plant that is known to have potent anti-cancer and anti-oxidant properties [17]. But curcumin has limited therapeutic efficacy because of its very low bioavailability, and poor ADME (adsorption, distribution, metabolism, excretion) properties [18]. A human Phase I clinical trial showed curcumin is non-toxic up to 8 mg/day, but this dosage will only produce plasma concentrations of 0.5–1 µM within 1–2-h post administration [19,20]. Through the new rapid gelling hydrogel formulation observed in this study, curcumin could be encapsulated and released slowly via hydrogel degradation, potentially improving its bioavailability over the long term in the human body. Encapsulating curcumin (e.g., liposomes, micelles, exosomes, and polymer nanoparticles) is a proven strategy to increase curcumin bioavailability in the body [21].
The novel observation in this study is that curcumin, while being a free-radical scavenger, also participates in accelerating silk di-tyrosine crosslinking in the presence of HRP and H2O2. Using UV-Vis and rheological characterizations, we show that curcumin participates in the silk di-tyrosine crosslinking reaction, and it also accelerates the rate of the chemical reaction at the tested concentrations (i.e., 50 µM–2 mM). Hydrogels produced using this technique could have extremely rapid gelling times of 3 min or less, dependent on curcumin and substrate concentrations. Furthermore, we show that curcumin is immobilized within the hydrogel matrix, and does not leak post crosslinking, suggesting chemical bonding with the hydrogel network. Using FTIR, we show that hydrogels formed by this chemistry display increased beta-sheet structures with increasing concentrations of curcumin. Finally, we show that curcumin-silk crosslinked hydrogels are potent anti-cancer agents, and they are extremely toxic to osteosarcoma cells in vitro even at the lowest tested concentrations.
2. Results and Discussion
2.1. Curcumin Participates in Di-Tyrosine Crosslinking of Silk Fibroin Hydrogels
To investigate whether UV-Vis spectroscopy could be used to study silk di-tyrosine crosslinking, wavelength sweeps were conducted on aqueous silk + HRP (S + H) and silk + HRP + curcumin (S + H + C). Neither of these solutions contained hydrogen peroxide (H2O2) which initiates the chemical crosslinking reaction. Figure 1a shows the absorption spectrum of silk + HRP (S + H) with two maxima, one below 300 nm and the other at 410 nm. This is consistent with previous reports showing absorption maxima for pure silk fibroin and HRP at 280 nm and 400 nm, respectively [22]. Previous studies also show that curcumin has a strong absorption maxima at ~430 nm [23]. Corroborating these results, we found curcumin to have a similar absorption maxima at ~430 nm when co-mixed with aqueous silk fibroin and horseradish peroxidase (HRP) (S + H + C in Figure 1a). The absorption maximum of pure di-tyrosine is reported at 315 nm [24,25]. Because silk fibroin, curcumin, and HRP do not have overlapping absorption maxima at 315 nm, we decided to use UV-Vis time sweeps to monitor the progression of di-tyrosine crosslinking in silk fibroin hydrogels.
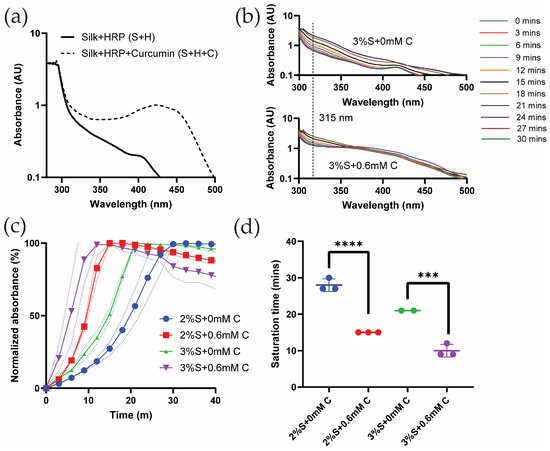
Figure 1.
UV-Vis absorbance spectra of silk and curcumin-silk di-tyrosine crosslinked hydrogels: (a) UV-Vis absorbance of silk + HRP (S + H) and silk + HRP + curcumin (S + H + C) solutions without hydrogen peroxide (H2O2) (b) UV-Vis wavelength and time sweeps of di-tyrosine crosslinked silk and curcumin-silk hydrogels (c) UV-Vis absorbance time sweeps and (d) UV-Vis saturation times of di-tyrosine crosslinked silk (S + 0 mM C) and curcumin-silk hydrogels (S + 0.6 mM C). (2/3%S + 0 mM C—2/3% silk + HRP + H2O2 + no curcumin; 2/3%S + 0.6 mM C—2/3% silk + HRP + H2O2 + 0.6 mM curcumin; *** p < 0.001, **** p < 0.0001).
Figure 1b shows UV-Vis wavelength and time sweeps of silk di-tyrosine hydrogel gelation without curcumin over a time period of 30 min. To initiate gelation, minute quantities of hydrogen peroxide (H2O2) were added to the reaction mixture before recording the UV-Vis spectra in these experiments. The results are presented in a log-axis scale to better visualize the absorption bands across all wavelengths. In this experiment, UV-Vis spectra clearly captured the di-tyrosine crosslinking reaction of silk fibroin as seen in Figure 1b. Variations in absorbance spectra were not observed when no H2O2 was added to the reaction mixture. Saturation of the reaction was observed at 21 min, as subsequent absorption bands overlapped with each other and no further increase in absorbance was observed (Figure 1b). Interestingly, the absorption maximum at 415 nm reduced in intensity as the di-tyrosine crosslinking reaction neared completion, suggesting the inactivation of HRP during the chemical reaction [26].
Figure 1b shows UV-Vis wavelength and time sweeps of silk di-tyrosine hydrogel gelation with 0.6 mM curcumin over a period of 30 min. Once again UV-Vis spectra clearly captured the di-tyrosine crosslinking reaction. The addition of hydrogen peroxide (H2O2) shifted the UV-Vis minima and maxima to 338 nm and 360 nm, from 350 and 430 nm (i.e., absorbance maxima for the S + H + C reaction mixture in Figure 1a). As the reaction progressed, the 315 nm di-tyrosine absorption value increased, while the absorption maxima at 360 nm decreased as a function of time. Saturation of the di-tyrosine crosslinking reaction was observed at 18 min, as subsequent absorption bands overlapped with each other and no further increase in absorbance was observed (Figure 1c).
To better understand the effect of curcumin on di-tyrosine crosslinking dynamics, and to get more accurate estimates of di-tyrosine reaction saturation times, UV-Vis time sweeps were conducted at the 315 nm di-tyrosine absorbance. Results from these experiments are shown in Figure 1c,d, where di-tyrosine saturation times are compared between the groups. Figure 1c shows normalized absorbance as a function of time across different groups and Figure 1d shows the saturation time across different groups. As seen from these figures, the addition of 0.6 mM curcumin significantly (p < 0.0002) reduced silk di-tyrosine crosslinking saturation time, independent of silk concentration (2% or 3%). Taken together, we conclude that curcumin participates in the silk di-tyrosine crosslinking reaction, and reaction saturation is faster in the presence of curcumin.
The observation that curcumin promotes di-tyrosine crosslinking in the presence of HRP and H2O2 is a novel observation because curcumin is a well-known anti-oxidant. Previous research has showcased that curcumin can act as a free radical scavenger when introduced into an environment containing hydrogen peroxide [27,28,29,30]. The anti-oxidant capacity of curcumin has been attributed to the diketone group and the two phenolic rings, but curcumin can promote anti-oxidant activity by inducing enzymes and related cell signaling pathways [27,28]. The anti-oxidant behavior of curcumin has primarily been observed at lower concentrations (<20 µm) [27,29], but at higher concentrations, this effect disappeared, and moderate cell-type specific cytotoxicity was observed [30]. It is possible that the rate-promoting activity of curcumin observed in this study could be concentration dependent, although even at the lowest concentration of 50 µM, curcumin still promoted di-tyrosine crosslinking. Li et al. [16], suggested that curcumin could bind to the hydrophobic domains of silk to accelerate silk hydrogel gelling, although we did not observe an increase in absorbance within S + H + C (silk + HRP + curcumin) reaction mixtures in the absence of H2O2 in shorter time scales (<30 min). The exact mechanism by which curcumin promotes free-radical dependent di-tyrosine crosslinking requires further investigation, and this could have novel implications for its use as a therapeutic.
For this study, we tested hydrogel formation using different concentrations of silk (i.e., 1–3%) and these were part of calibration experiments. We did not get results using 1% silk, as they formed inconsistent gels, or they were too soft and could not be handled easily. Silk concentrations ≥ 2% resulted in consistent hydrogels that could be handled well and were used for all subsequent experiments.
2.2. Curcumin Accelerates Silk Di-Tyrosine Hydrogel Gelation
To characterize the setting time, di-tyrosine crosslinking of hydrogels was studied using rheology at 1% oscillatory strain and a rate of 1 Hz. The storage and the loss modulus of the hydrogels are plotted below as a function of time. Storage modulus (G′) represents the energy stored and recovered per strain cycle, and it is indicative of the hydrogel’s solid-like elastic character [31]. On the other hand, the loss modulus (G″) represents the energy dissipated as heat, and it is indicative of the hydrogel’s liquid-like viscous character [31].
Figure 2a shows rheological time sweeps of silk di-tyrosine crosslinking reaction with no curcumin added to the mixture (3%S + 0 mM C). As per the results, the storage modulus (G′) starts to increase around 5 min and continues to increase until 20 min, when the modulus reaches saturation. The loss modulus (G″) was very low compared to the storage modulus (G′) throughout the crosslinking reaction, and no crossover was observed between the two. Crossover was previously utilized to study the setting time (gel-sol transformation) of polymer-based hydrogels [32]. However, there are limitations to this measurement, as crossing over was not observed in quick-setting gels in some studies [33,34]. Furthermore, rheological time sweeps conducted on similar biomaterial hydrogels such as agarose, collagen, and fibrin did not show crossing-over behavior [33].
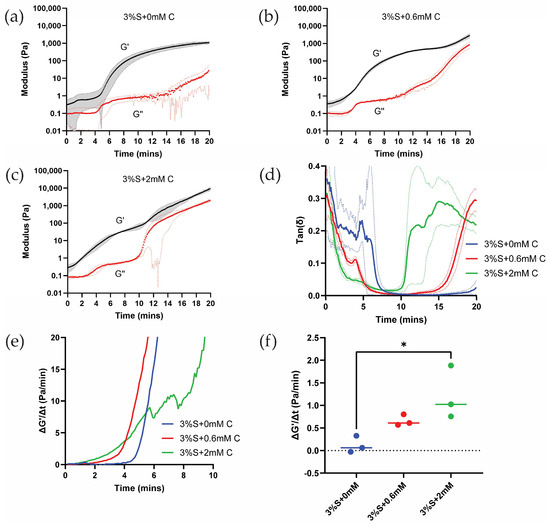
Figure 2.
Rheology time sweeps of silk and curcumin-silk di-tyrosine crosslinked hydrogels: (a) Rheological time sweeps of silk di-tyrosine crosslinking hydrogels with no curcumin (b,c) Rheological time sweeps of di-tyrosine crosslinked silk hydrogels with 0.6 mM and 2 mM curcumin (d) Tan(δ) vs. time for different hydrogel groups (e) First derivative of G′ as a function of time for different hydrogel groups (f) Rate of increase of G′ at 4 min for silk and curcumin-silk di-tyrosine crosslinked hydrogels. Dotted lines in panels (a-d) represent standard deviation. Dotted line in panel (f) is used to highlight positive vs. negative values. (3%S + 0 mM C—3% silk + HRP + H2O2 + no curcumin; 3%S + 0.6 mM C—3% silk + HRP + H2O2 + 0.6 mM curcumin; 3%S + 2 mM C—3% silk + HRP + H2O2 + 2 mM curcumin; * p < 0.05).
Figure 2b shows rheological time sweeps of silk di-tyrosine crosslinking containing curcumin (3%S + 0.6 mM C). The addition of 0.6 mM curcumin caused the storage modulus (G′) to increase around 3 min, and it continued to increase up until 16 min when it reached a pseudo-plateau point before entering a transition zone and increasing further. This is in contrast to silk di-tyrosine crosslinked hydrogels containing no curcumin (3%S + 0 mM C), where the storage modulus starts to increase at 5 min and reaches saturation at 20 min, with no clear transition zone. Here again, the loss modulus (G″) of the hydrogels remained well below the storage modulus (G′), and no crossing-over was observed.
When the concentration of curcumin was increased to 2 mM (3%S + 2 mM C), the storage modulus started to increase even earlier, between 0 to 2 min, reaching a pseudo-plateau point around 10 min, and then entering a transition zone and increasing further (Figure 2c). The rate of increase of the storage modulus is greater and occurs earlier in these hydrogels compared to the other hydrogel groups (i.e., 3%S + 0 mM C and 3%S + 0.6 mM C).
The values of G″ (loss modulus) remain below 1 Pascal for up to 10 min across all treatment groups. The low G″ values combined with limits of instrument sensitivity and increased G′ (storage modulus) values within the same time frame resulted in the inability to use G′/G″ crossover to characterize the gelling time of hydrogel. Therefore, we plotted tan(δ) to characterize gelation time. Figure 2d shows a faster drop in tan(δ) values as a function of time in the 2 mM treatment group compared to the other groups, indicating a greater increase in G′ at the same time (i.e., tan(δ) = G″/G′). To quantitatively assess relative differences in gelation time across the different treatment groups, we obtained the first derivative of G′ for all groups (Figure 2e). Comparing the initial reaction rates (~4 min) across all three hydrogel groups, a significant (p < 0.0232) difference is observed between di-tyrosine crosslinking reactions of silk and curcumin-silk hydrogels. However, the reaction rates between the two groups containing increasing concentrations of curcumin were not significant (Figure 2f). Based on these results we conclude that curcumin accelerates silk di-tyrosine crosslinking reaction.
To further confirm that curcumin accelerates silk di-tyrosine crosslinking, a time-lapse video was captured with periodic inversion testing of hydrogel gelation (Video S1). For this experiment, we tested 3 different concentrations of curcumin 0.05 mM, 0.6 mM, and 2 mM. The hydrogels were crosslinked at 37 °C, identical to the conditions used for the UV-Vis and rheological studies. The video was started immediately after adding H2O2 to all the groups, which is the start of the di-tyrosine crosslinking reaction. As seen in the video (Video S1), the 2 mM curcumin-silk di-tyrosine hydrogels (S + 2 mM C) were set quickly, at approximately 2 min and 26 s. At this time, all the remaining hydrogels are still in liquid form, as seen in the video. With continued incubation at 37 °C, the setting time of other hydrogel groups could be approximated. Silk hydrogels containing 0.6 mM and 0.05 mM curcumin were set at approximately 3 min 23 s and 4 min 16 s, respectively. Silk hydrogels containing no curcumin had a setting time of approximately 5 min and 16 s. The video of the hydrogels undergoing phase change is available within Supplementary Materials (Video S1). The video clearly shows that hydrogels with curcumin have a much faster setting time compared to hydrogels without curcumin, once again confirming that the silk di-tyrosine crosslinking reaction is accelerated by the addition of curcumin. A snapshot of the video showcasing the difference is included in Figure 3a. Figure 3b confirms curcumin remained immobilized within the hydrogel matrix after di-tyrosine crosslinking, and no curcumin can be seen leaching out the hydrogel into water post-crosslinking.
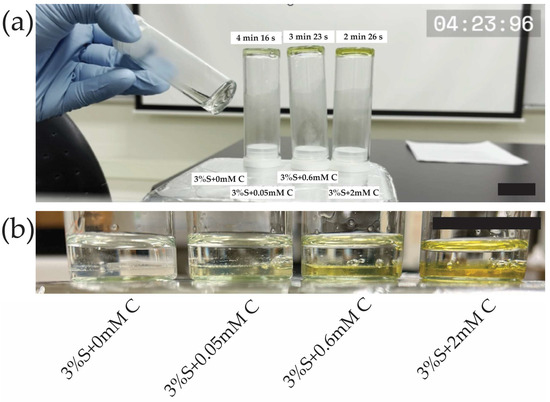
Figure 3.
Curcumin-silk di-tyrosine crosslinking hydrogels (a) Snapshot of Video S1 showing earlier setting times for silk di-tyrosine hydrogels containing curcumin compared to silk di-tyrosine hydrogels containing no curcumin, (b) Snapshot confirming curcumin is immobilized inside the hydrogels with no leakage into surrounding liquid. The scale bars are 1 cm. (3%S + 0 mM C—3% silk + HRP + H2O2 + no curcumin; 3%S + 0.6 mM C—3% silk + HRP + H2O2 + 0.6 mM curcumin; 3%S + 2 mM C—3% silk + HRP + H2O2 + 2 mM curcumin).
2.3. ATR-FTIR Spectra Shows Characteristic Beta-Sheet Crystalline Peaks in Curcumin-Silk Di-Tyrosine Crosslinked Hydrogels
Attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR) was used to study the conformational states of silk fibroin following the sol-gel transition. For this experiment, all samples were lyophilized at the same time and subjected to the same experimental conditions. Lyophilized silk fibroin (S) which did not undergo di-tyrosine crosslinking (no HRP, H2O2, or curcumin were added) was used as the reference, and compared to lyophilized silk di-tyrosine crosslinked hydrogels with or without curcumin (i.e., 3%S + 0 mM C, 3%S + 0.05 mM C, 3%S + 0.6 mM C, and 3%S + 2 mM C).
Figure 4a shows stacked ATR-FTIR spectra of the different treatment groups for comparison. Across all samples, there are clear amide I, II, and III absorption bands, each representing the characteristic vibration modes C=O stretching, N-H deformation, and C-N stretching/N-H bending, respectively [35]. While the profiles are similar across samples, there are a few differences in the peaks observed between the samples. The amide I peak had higher wavenumbers for silk fibroin (S; 1635 cm−1) and silk di-tyrosine hydrogels (3%S + 0 mM C; 1620 cm−1), and amide I is used as a marker for studying the secondary structure of silk fibroin proteins [35,36,37].
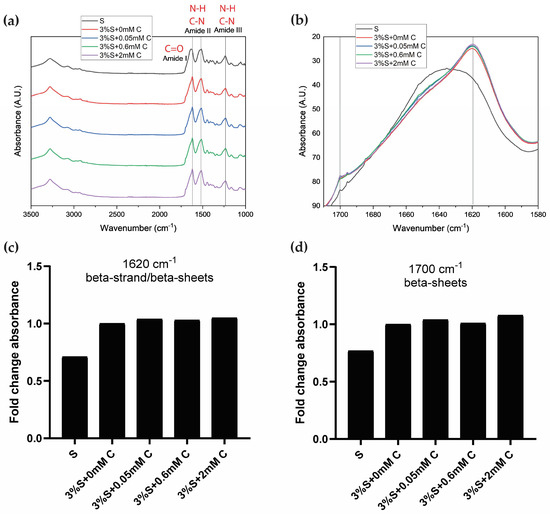
Figure 4.
ATR-FTIR spectra of freeze-dried silk di-tyrosine hydrogels with or without curcumin, plotted as normalized absorbance versus wavenumber (a) Entire spectra between 1000 cm−1 and 3500 cm−1, showcasing characteristic amide I, II, and III peaks of silk fibroin (b) Overlapped amide I band (1580–1720 cm−1) across all samples showcasing relative differences. All samples were freeze-dried simultaneously under identical experimental conditions (c) Absorbance at 1620 cm−1 (beta-strands/beta-sheets) across all samples (d) Absorbance at 1700 cm−1 (beta-sheets) across all samples. Vertical lines in panel (b) highlight 1620 and 1700 cm−1 wavenumbers. Legend: (Silk fibroin (S); 3%S + 0 mM C—3% silk + HRP + H2O2 + no curcumin; 3%S + 0.6 mM C—3% silk + HRP + H2O2 + 0.6 mM curcumin; 3%S + 2 mM C—3% silk + HRP + H2O2 + 2 mM curcumin).
To further study the amide I band and understand relative differences across samples, the band is extracted from the spectra and presented separately in an overlapped fashion in Figure 4b. As seen from the figure, silk fibroin (S) has a relatively lower Amide I peak at 1635 cm−1, suggesting a mixture of beta-sheet and random coils, compared to di-tyrosine crosslinked silk hydrogels (i.e., 3%S + 0 mM C, 3%S + 0.05 mM C, 3%S + 0.6 mM C, and 3%S + 2 mM C), which display a strong peak at 1619 cm−1, corresponding with beta-strand and beta-sheet structures [36,38]. In addition to the characteristic 1619 cm−1 peak, silk di-tyrosine crosslinked hydrogels also displayed a strong shoulder peak between 1697–1703 cm−1, indicating the formation of crystalline beta-sheet structures [36]. The peak height at 1619 cm−1 and 1697 cm−1, increased with increasing concentrations of curcumin, and curcumin-silk di-tyrosine hydrogels containing 2 mM curcumin had the highest peak intensity in both these regions, while silk di-tyrosine hydrogels containing no curcumin (S + 0 mM C) had the lowest (Figure 4c,d). These results suggest that curcumin could increase the percentage of beta-sheet crystalline structures formed in silk di-tyrosine hydrogels post-crosslinking. This could also explain the bi-phasic transition zone in rheology time sweeps, and higher storage moduli observed in curcumin-silk di-tyrosine hydrogels (Figure 2b–d). Further studies are required to delineate the underlying mechanisms.
2.4. Curcumin-Silk Di-Tyrosine Crosslinked Hydrogels Are Toxic to Ostoeosarcoma U2OS Cancer Cells
Curcumin is a well-known anti-cancer agent, and it has been investigated in clinical trials for many different types of cancers [39,40]. Curcumin has proven toxicity against osteosarcoma cells but is limited by its poor bioavailability [19,41,42]. Because hydrogels can improve the bioavailability and also provide targeted delivery of curcumin, we studied the effect of curcumin-silk di-tyrosine crosslinked hydrogels on encapsulated U2OS—osteosarcoma cells in an in vitro study. The cells were suspended in the hydrogel mixture pre-crosslinking and their viability was studied post-crosslinking using fluorescence confocal microscopy.
Many previous studies have tested the effect of curcumin on cancers such as osteosarcomas, and toxicity was observed at dosages ranging from 10–100 µM [19,42]. It is important to note that curcumin was directly added to the media in most of these studies, unlike the current study where curcumin is encapsulated within the hydrogel matrix. In this study, we decided to test a larger range of curcumin dosages (0, 50 µM, 0.6 mM, and 2 mM), as newer formulations of curcumin have been shown to have a bioavailability ranging from 30 nM–2 mM, and dosages of 2 g/day to 12 g/day are well tolerated in human clinical studies [19,43].
Figure 5a–d shows 3D fluorescence maximum projections of U2OS cells encapsulated within silk di-tyrosine hydrogels containing different concentrations of curcumin (0–2 mM). Immunofluorescence signals from Cell Tracker-CMFDA dyes and DAPI clearly showcase a dose response in viability across the different treatment groups within 4 h of initial seeding. The 4-h time point was chosen to allow sufficient time for cells to recover or undergo apoptosis. Very few viable cells (green) were observed within the 2 mM curcumin treatment group (Figure 5d) at 4 h. Silk di-tyrosine hydrogels containing no curcumin (S + 0 mM C) had the highest number of viable cells as seen in Figure 5a. Figure 5e shows the percentage of cellular viability across the different treatment groups, and treatment with curcumin, even at the lowest concentration of 50 µM displayed significant toxicity (p < 0.0016) compared to the control group. Almost all osteosarcoma cells were killed in the treatment groups containing 0.6 and 2 mM curcumin (<10% viable cells).
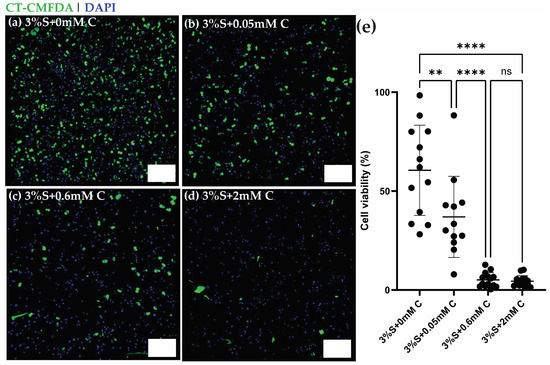
Figure 5.
Viability of U2OS—Osteosarcoma cells encapsulated within curcumin-silk di-tyrosine hydrogels (a–d) Representative confocal 3D maximum projections of U2OS cells seeded within silk di-tyrosine hydrogels with different concentrations of curcumin (0, 0.05, 0.6 and 2 mM curcumin) 4 h after cell seeding. All cells are stained with Cell Tracker-CMFDA green dye (live) and DAPI (dead). (e) Percent cell viability of osteosarcoma cells across different groups. Scale bars are 200 µm (** p < 0.01, **** p < 0.0001).
2.5. Limitations of This Study
One of the limitations of this study is that we did not show degradation of curcumin-silk tyrosine crosslinked hydrogels. This is because many previous studies have extensively studied the degradation properties of silk fibroin materials [11,44,45]. Silk fibroin materials are amino acid-based structures, and protease enzymes can easily degrade silk materials into by-products that are metabolized by the human body. Yan et al., conducted degradation studies of silk tyrosine crosslinked hydrogels in the presence of protease XIV and found that these materials degrade rapidly over a time-scale of 12 h within in vitro studies, with 50% loss of hydrogel weight observed within 4-h of incubation [11]. Longer release profiles over the time scale of days could also be achieved by tuning the concentration of silk. Furthermore, non-invasive ultrasound waves could also be used to degrade silk biomaterials [44], and this could be another strategy to modulate the degradation of curcumin-silk tyrosine crosslinked hydrogels as needed.
The current study did not showcase the improved bioavailability of curcumin following encapsulation. We postulate that curcumin will have enhanced bioavailability post-encapsulation based on many previous studies that have encapsulated curcumin within liposomes, micelles, exosomes, and polymer nanoparticles [21,46,47]. Encapsulation of curcumin has been shown to prevent it from being rapidly metabolized or eliminated [21,46,47]. Encapsulation can also protect curcumin against rapid changes in pH and temperature, which can protect it against keto-enol tautomerism and degradation [21,47]. Furthermore, previous studies have confirmed curcumin’s ability to maintain its antioxidant activity for up to 28 days while being encapsulated within silk films [16]. Taken together, we strongly believe that encapsulation within silk hydrogels coupled with tunable degradation will improve curcumin bioavailability.
While we did not include any data to confirm the injectability of the hydrogels, we have tested the ability to extrude curcumin-silk formulations from syringes at the tested concentrations (i.e., 0–2 mM curcumin and 2–3% silk) in our laboratory, and based on this information we do believe that injectable formulations could be developed for treatments.
3. Conclusions
In summary, we convincingly show that curcumin accelerates silk di-tyrosine crosslinking reaction in the presence of HRP and H2O2. We show this rate-promoting effect of curcumin using three different methodologies—UV-Vis spectroscopy, rheology, and time-lapse videos. To better understand the underlying mechanism, we performed FTIR, and this shows an increase in silk beta-sheet structures with increasing concentrations of curcumin. Finally, using fluorescence confocal microscopy, we also show that curcumin-silk di-tyrosine crosslinked hydrogels are extremely toxic to U2Os-osteosarcoma cells in short time scales. While there are many previous studies on curcumin and silk, our study is the first to showcase the rate-promoting activity of curcumin during di-tyrosine crosslinking. This is a novel observation because curcumin is also a well-known free radical scavenger, and one would expect curcumin to slow down di-tyrosine crosslinking. It is unclear why curcumin can accelerate a chemical reaction with hydrogen peroxide as the substrate. We suggest that this could be a concentration-dependent effect, and it could be an important consideration while manufacturing curcumin-based therapeutics. Taken together, we showcase rapidly gelling curcumin-silk hydrogel formulations that can undergo sol-gel transitions in 3 min or less for anti-cancer drug delivery to solid tumors.
4. Materials and Methods
4.1. Preparation of Aqueous Silk Solution
Aqueous silk solutions were prepared using well-established protocols reported earlier [48]. Briefly, 5 g of B. mori silkworm cocoons were placed in 2 L of boiling water containing 0.02 M Na2CO3 (Sigma-Aldrich, St. Louis, MO, USA) solution for 30 min. The procedure is conducted to separate pure silk fibroin proteins from sericin. Subsequently, the silk fibroin fibers were washed three times in distilled water, and each wash step included a 15–20-min soak time to remove the separated sericin protein. Air-dried fibers were then solubilized in 9.3 M Lithium Bromide (LiBr; Sigma-Aldrich, St. Louis, MO, USA) at 60 °C for 4 h, followed by dialysis in 2 L of distilled water (water changes at 1, 3, 6, 24, 36, and 48 h) using a regenerated cellulose membrane (3.5 kDa MWCO, Spectrum Laboratories, Rancho Dominguez, CA, USA). The solubilized silk fibroin protein was then centrifuged at the maximum setting to remove any particulate waste material from the silk fibroin solution. Silk fibroin solutions were filtered using a 0.2 µm filter for sterilization prior to use in cell culture experiments.
4.2. Preparation of Curcumin-Silk and Silk Di-Tyrosine Hydrogels
Silk di-tyrosine hydrogels were prepared as per previous protocols [37,49,50]. For di-tyrosine crosslinking silk fibroin, 10 µL of 1000 U/mL stock solution of HRP (horseradish peroxidase type IV; Sigma-Aldrich, St. Louis, MO, USA) and 10 µL of 165 mM hydrogen peroxide (H2O2; Sigma Aldrich, St. Louis, MO, USA) were added to 1 mL of 2/3% (w/v) silk solution (final concentration of 10 U/mL of HRP and 1.65 mM H2O2) at 37 °C. Silk fibroin can change mechanical properties depending on the extraction procedure, silk fibroin type, and other variables such as storage time post-extraction, etc. Therefore, while 2/3% (w/v) is suggested as a guide to repeat these experiments, silk concentration should be calibrated each time, to identify a working concentration for your target application and experiments. Curcumin (Sigma Aldrich, St. Louis, MO, USA), the yellow-colored polyphenol found in Curcuma longa was utilized to prepare curcumin-silk di-tyrosine crosslinked hydrogels. Curcumin solubilized in pure ethanol was co-mixed at appropriate volumes with silk fibroin to achieve final curcumin concentrations of 0.05 mM, 0.6 mM, and 2 mM, respectively. To initiate crosslinking, HRP and H2O2 were added at the concentrations described above, followed by incubation at 37 °C.
4.3. UV-Vis Spectroscopy
UV-Vis measurements were made using a Cary 60 UV-Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) fitted with a Quantum Northwest (Quantum Northwest, Liberty Lake, WA, USA) TC125 Peltier unit for temperature control or with a TECAN Spark Cyto plate reader (Tecan Inc., Morrisville, NC, USA). The same protocol was followed for all UV-Vis experiments. Prior to each experiment, the temperature was set to 37 °C. A blank was performed using water at the beginning for baseline correction. Either 1.5 mL (cuvette format) or 100 µL/well (96 well plate format) of silk di-tyrosine crosslinking mixture with or without curcumin was added and placed inside the instrument. Wavelength time sweeps recorded 250–500 nm at 1 nm increments every 3 min for 30 min or until saturation was observed. Endpoint kinetic measurements recorded 315 nm absorbance every 3 min for 30 min or until saturation was observed. Results are plotted as a function of absorbance versus wavelength.
4.4. Rheology
Rheological measurements were performed on silk and curcumin-silk di-tyrosine crosslinked hydrogels using a TA Instruments Discovery HR-2 rheometer (Waters Corporation, Milford, MA, USA). The setup included a 40 mm stainless steel conical plate (angle: 0.0349 rad) and a temperature-controlled Peltier plate set to 37 °C. Briefly, silk or curcumin-silk di-tyrosine crosslinking mixture was prepared by mixing 600 µL of the different constituents, at the concentrations mentioned above, and 420 µL of the mixture was dispensed on the tester. Immediately, the cone geometry was lowered to the trimming gap for trimming excess liquid and then lowered to the specified final testing gap. Low-viscosity silicone oil was placed around the outside edge of the cone geometry to prevent water evaporation. Dynamic time sweeps were conducted as per previously established protocols [37,49], at a frequency of 1 Hz (6.283 rad/s) and 1% strain until the sample reached a plateau modulus. Preliminary experiments showed that storage moduli of silk di-tyrosine crosslinked hydrogels reached a plateau within 20 min, and so all subsequent experiments were run for 20 min.
4.5. Time Lapse Video
To study the setting time of silk and curcumin-silk hydrogels, time-lapse videos were recorded. Hydrogels were placed inside glass vials on a hot plate set to 37 °C. Videos were recorded once H2O2 was added to the hydrogel mixture. The setting time of hydrogels was studied by conducting a partial inversion test at regular time intervals. Upon visual observation, if the hydrogels remained in a solution state, they were placed back onto the hotplate to continue gelation. If the hydrogels appeared to have undergone sol-gel transition, they were inverted, and the time of inversion was recorded as their setting time. All samples were tested at each point to enable relative comparison of setting times.
4.6. Fourier Transform Infrared (FTIR) Spectroscopy
Silk fibroin (S) without HRP, H2O2, or curcumin, and silk di-tyrosine crosslinked hydrogels with or without curcumin (i.e., S + 0 mM C, S + 0.05 mM C, S + 0.6 mM C, and S + 2 mM C) were frozen at −80 °C and then placed inside a custom-made freeze-drying apparatus. A vacuum pump and cold trap were set up to keep the pressure and temperature well below the triple point of water. The freeze-dried silk fibroin (S) served as the positive control, as the FTIR spectra for lyophilized silk fibroin are well-established by other studies [51]. Attenuated Total Reflectance Fourier-Transform Infrared Spectra (ATR-FTIR) were recorded using a Nicolet iS50R FTIR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), using previously well-established protocols [35,51]. The spectrometer was equipped with a single bounce diamond attenuated total reflectance (ATR) module with a refractive index of 2.4 and an active sample area diameter of 2 mm. Spectra of each of the samples were acquired by pressing the samples on the ATR crystal. The air was used as the reference and automatically subtracted from sample readings. Samples were analyzed in the frequency range from 650–4000 cm−1, with each measurement adding 25 interferograms at a resolution of 1 cm−1. The amide 1, amide 2, and amide 3 regions were identified based on wavenumber ranges specified in previous publications [51]. The presence of secondary structures, including random coils, alpha-helices, and beta sheets, was also based on wavelength numbers within the amide I region [36].
4.7. Cell Culture and Cellular Viability Staining
Human osteosarcoma cell line (U-2 OS; HTB-96; ATCC, Manassas, VA, USA) was cultured in DMEM/F12 medium (Life Technologies, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) and 1× penicillin/streptomycin (Penn/Strep). A master stock of the U2OS cell line was obtained from ATCC, and experiments were conducted using early passaged cells. For viability experiments, U2OS cells were co-mixed with silk and curcumin-silk di-tyrosine hydrogel precursor solutions at a concentration of 1 × 106 cells/mL. Cell-laden hydrogel precursor solutions were then incubated at 37 °C until hydrogel gelation, and a warm cell culture medium was quickly added to the cell-laden hydrogels. Cellular viability and distribution were studied by incubating cell-laden hydrogels in 10 µM of CellTracker Green CMFDA (CT-CMFDA) in serum-free media for 20 min at 37 °C. Hydrogels were then fixed using 4% paraformaldehyde (PFA) in 1× PBS for 30 min and washed 3× in 1× PBS. Subsequently, hydrogels were stained with nuclear stain DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride; Life Technologies, Grand Island, NY, USA) at a final concentration of 1 μg/mL. While all cells are stained with DAPI, only live cells retain the CT-CMFDA dye.
4.8. Confocal Microscopy
Cell Tracker CMFDA and DAPI-stained cell-laden hydrogels were imaged using a Nikon A1R MP confocal microscope (Nikon, Melville, NY, USA) equipped with a tunable laser. Excitation lasers and filters were chosen to enable the detection of fluorescent emissions for CT-CMFDA (MW: 464.9 g/mol ex: 492 nm; em: 517 nm) and DAPI (MW: 320 g/mol; ex: 364 nm; em: 454 nm). Using the Z-stack feature, image stacks of 500 µm depth were acquired using a 10× objective. The step size was set to 30 µm for all groups based on the average cell size. Each sample was imaged and multiple samples/group were used to achieve statistical significance. Images obtained from the green and blue channels were analyzed using ImageJ software (v. 1.54f). After an initial thresholding step, the Analyze Particles feature was used to count the total number of cells in each z-stack across all groups. The percentage of viable cells in each group was obtained from this data and plotted for each treatment group. Fluorescence 3D maximum projection images with both fluorescent channels were visualized using the 3D Viewer plugin in Fiji ImageJ (v. 1.54f) for relative comparison.
4.9. Statistical Analysis
Data are expressed as mean, and error bars denote standard deviation. The unpaired student’s t-test was used for comparisons of the two groups. For experimental comparisons of more than two groups, two-way analysis of variance (ANOVA) and Tukey post-hoc analysis were used to determine statistically significant differences. Statistical significance was accepted at the p < 0.05 level and indicated in figures as * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
4.10. Editing
Microsoft Co-pilot integrated with Word and Office 365 was used to edit this article. Co-pilot was used to (1) generate a list of acronyms (2) identify and correct typos, and (3) identify and correct grammatical errors in the manuscript. The article writing and data interpretation were conducted by the author based on subject matter expertise.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gels11040288/s1, Video S1: Gelling time of curcumin silk-tyrosine crosslinked hydrogels.
Funding
Experiments and research work were funded by the Start-up Faculty grant provided through the Materials Science & Biomedical Engineering Resource Center at the University of Wisconsin Eau Claire.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The author thanks undergraduate students Andres Jimenez, Trixie Tah, and Seth Waalen for their technical help with experiments and lab work related to this project.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADME | Adsorption, Distribution, Metabolism, Excretion |
| ANOVA | Analysis of Variance |
| ATR-FTIR | Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy |
| CMFDA | 5-chloromethylfluorescein diacetate |
| DAPI | 4′,6-diamidino-2-phenylindole, dihydrochloride |
| DMEM/F12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| FBS | Fetal Bovine Serum |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HRP | Horseradish Peroxidase |
| H2O2 | Hydrogen Peroxide |
| LiBr | Lithium Bromide |
| MWCO | Molecular Weight Cut-Off |
| Na2CO3 | Sodium Carbonate |
| PBS | Phosphate Buffered Saline |
| Penn/Strep | Penicillin/Streptomycin |
| PFA | Paraformaldehyde |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA A Cancer J. Clin 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Kaneuchi, Y.; Hakozaki, M.; Yamada, H.; Hasegawa, O.; Yamada, S.; Oka, Y.; Watanabe, K.; Konno, S. Very late relapse of high-grade osteosarcoma: A case report and review of the literature. Med. Baltim. 2020, 99, e21206. [Google Scholar] [CrossRef] [PubMed]
- Daw, N.C.; Chou, A.J.; Jaffe, N.; Rao, B.N.; Billups, C.A.; Rodriguez-Galindo, C.; Meyers, P.A.; Huh, W.W. Recurrent osteosarcoma with a single pulmonary metastasis: A multi-institutional review. Br. J. Cancer 2015, 112, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Tan, B.; Wu, Y.; Wu, Y.; Shi, K.; Han, R.; Li, Y.; Qian, Z.; Liao, J. Curcumin-Microsphere/IR820 Hybrid Bifunctional Hydrogels for In Situ Osteosarcoma Chemo-co-Thermal Therapy and Bone Reconstruction. ACS Appl. Mater. Interfaces 2021, 13, 31542–31553. [Google Scholar] [CrossRef]
- Freeman, F.E.; Dosta, P.; Shanley, L.C.; Ramirez Tamez, N.; Riojas Javelly, C.J.; Mahon, O.R.; Kelly, D.J.; Artzi, N. Localized Nanoparticle-Mediated Delivery of miR-29b Normalizes the Dysregulation of Bone Homeostasis Caused by Osteosarcoma whilst Simultaneously Inhibiting Tumor Growth. Adv. Mater. 2023, 35, 2207877. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Wang, Z.; Nie, Y.; Yang, Y.; Li, C.; Zhang, Y.; Jiang, Y.; Kou, Y.; Zhang, W.; et al. Injectable and adhesive MgO2-potentiated hydrogel with sequential tumor synergistic therapy and osteogenesis for challenging postsurgical osteosarcoma treatment. Biomaterials 2025, 315, 122959. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, C.; Zhang, M.; Ding, X.; Zhang, L.; Jiang, X.; Li, M.; Gao, J.; Wu, J. Application of nanostructure-loaded hydrogels for cancer treatment and tissue regeneration. Appl. Mater. Today 2024, 37, 102086. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Xu, M.; Geng, Z.; Ji, P.; Liu, Y. Hydrogel systems for targeted cancer therapy. Front. Bioeng. Biotechnol. 2023, 11, 1140436. [Google Scholar] [CrossRef]
- Jaiswal, C.; Gupta, T.; Jadi, P.K.; Moses, J.C.; Mandal, B.B. Injectable anti-cancer drug loaded silk-based hydrogel for the prevention of cancer recurrence and post-lumpectomy tissue regeneration aiding triple-negative breast cancer therapy. Biomater. Adv. 2023, 145, 213224. [Google Scholar] [CrossRef]
- Yan, L.-P.; Silva-Correia, J.; Ribeiro, V.P.; Miranda-Gonçalves, V.; Correia, C.; da Silva Morais, A.; Sousa, R.A.; Reis, R.M.; Oliveira, A.L.; Oliveira, J.M.; et al. Tumor Growth Suppression Induced by Biomimetic Silk Fibroin Hydrogels. Sci. Rep. 2016, 6, 31037. [Google Scholar] [CrossRef] [PubMed]
- Chaala, M.; Sebba, F.Z.; Fuster, M.G.; Moulefera, I.; Montalbán, M.G.; Carissimi, G.; Víllora, G. Accelerated Simple Preparation of Curcumin-Loaded Silk Fibroin/Hyaluronic Acid Hydrogels for Biomedical Applications. Polymers 2023, 15, 504. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef]
- Peng, Z.; Li, M.; Wang, Y.; Yang, H.; Wei, W.; Liang, M.; Shi, J.; Liu, R.; Li, R.; Zhang, Y.; et al. Self-Assembling Imageable Silk Hydrogels for the Focal Treatment of Osteosarcoma. Front. Cell Dev. Biol. 2022, 10, 698282. [Google Scholar] [CrossRef]
- Laomeephol, C.; Ferreira, H.; Kanokpanont, S.; Neves, N.M.; Kobayashi, H.; Damrongsakkul, S. Dual-functional liposomes for curcumin delivery and accelerating silk fibroin hydrogel formation. Int. J. Pharm. 2020, 589, 119844. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, T.; Zheng, Z.; Murphy, A.R.; Wang, X.; Kaplan, D.L. Curcumin-functionalized silk materials for enhancing adipogenic differentiation of bone marrow-derived human mesenchymal stem cells. Acta Biomater. 2015, 11, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Montalbán, M.G.; Coburn, J.M.; Lozano-Pérez, A.A.; Cenis, J.L.; Víllora, G.; Kaplan, D.L. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef]
- Stachowiak, M.; Mlynarczyk, D.T.; Dlugaszewska, J. Wondrous Yellow Molecule: Are Hydrogels a Successful Strategy to Overcome the Limitations of Curcumin? Molecules 2024, 29, 1757. [Google Scholar] [CrossRef]
- Lu, K.H.; Lu, P.W.; Lu, E.W.; Lin, C.W.; Yang, S.F. Curcumin and its Analogs and Carriers: Potential Therapeutic Strategies for Human Osteosarcoma. Int. J. Biol. Sci. 2023, 19, 1241–1265. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.-M. Enhancing the bioavailability and bioactivity of curcumin for disease prevention and treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Ai, S.; Shi, W.; Zhu, L. A novel hydrogen peroxide biosensor based on horseradish peroxidase immobilized on gold nanoparticles–silk fibroin modified glassy carbon electrode and direct electrochemistry of horseradish peroxidase. Sens. Actuators B Chem. 2009, 137, 747–753. [Google Scholar] [CrossRef]
- Subhan, M.; Alam, K.; Rahaman, M.; Rahman, M.; Awal, R. Synthesis and characterization of metal complexes containing curcumin (C21H20O6) and study of their anti-microbial activities and DNA binding properties. J. Sci. Res. 2014, 6, 97–109. [Google Scholar] [CrossRef]
- Correia, M.; Neves-Petersen, M.T.; Jeppesen, P.B.; Gregersen, S.; Petersen, S.B. UV-Light Exposure of Insulin: Pharmaceutical Implications upon Covalent Insulin Dityrosine Dimerization and Disulphide Bond Photolysis. PLoS ONE 2012, 7, e50733. [Google Scholar] [CrossRef] [PubMed]
- Keyes, E.D.; Kauser, K.; Warner, K.S.; Roberts, A.G. Photosensitized Oxidative Dimerization at Tyrosine by a Water-Soluble 4-Amino-1,8-naphthalimide. Chembiochem 2021, 22, 2703–2710. [Google Scholar] [CrossRef]
- Morales-Urrea, D.; López-Córdoba, A.; Contreras, E.M. Inactivation kinetics of horseradish peroxidase (HRP) by hydrogen peroxide. Sci. Rep. 2023, 13, 13363. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, L.; Lin, L.; Ho, K.-T.; Huang, S.-Y.; Li, J.; Liu, J.; Zhang, Z.; Li, G. Curcumin Protects Pheochromocytoma Cells Against Hydrogen Peroxide-Induced Oxidative Stress. Nat. Prod. Commun. 2023, 18, 1–13. [Google Scholar] [CrossRef]
- Purushothaman, A.; Teena Rose, K.S.; Jacob, J.M.; Varatharaj, R.; Shashikala, K.; Janardanan, D. Curcumin analogues with improved antioxidant properties: A theoretical exploration. Food Chem. 2022, 373, 131499. [Google Scholar] [CrossRef]
- Pan, T.; Zhang, S.; Fei, H.; Hu, Y. Curcumin Protects Human Dermal Fibroblasts Exposed to Hydrogen Peroxide by Regulating Autophagy Level and Reactive Oxygen Species Generation. J. Burn Care Res. 2023, 44, 1208–1215. [Google Scholar] [CrossRef]
- JiaQiang, X.; Gao, R.; Liang, W.; Wu, C.; Li, F.; Huang, S.; Zhang, B.; Qi, Q. Oxidative Stress Reducing Osteogenesis Reversed by Curcumin via NF-κB Signaling and Had a Role in Anti-Osteoporosis. Res. Sq. Durh. NC USA 2021. [Google Scholar]
- Ramli, H.; Zainal, N.F.A.; Hess, M.; Chan, C.H. Basic principle and good practices of rheology for polymers for teachers and beginners. Chem. Teach. Int. 2022, 4, 307–326. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Soman, D.; Payanam, U.; Laurent, A.; Labarre, D.; Jayakrishnan, A. A novel injectable tissue adhesive based on oxidized dextran and chitosan. Acta Biomater. 2017, 53, 343–354. [Google Scholar] [CrossRef]
- Woltje, M.; Kolbel, A.; Aibibu, D.; Cherif, C. A Fast and Reliable Process to Fabricate Regenerated Silk Fibroin Solution from Degummed Silk in 4 Hours. Int. J. Mol. Sci. 2021, 22, 10565. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef]
- Partlow, B.P.; Bagheri, M.; Harden, J.L.; Kaplan, D.L. Tyrosine Templating in the Self-Assembly and Crystallization of Silk Fibroin. Biomacromolecules 2016, 17, 3570–3579. [Google Scholar] [CrossRef]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Zahedipour, F.; Bolourinezhad, M.; Teng, Y.; Sahebkar, A. The Multifaceted Therapeutic Mechanisms of Curcumin in Osteosarcoma: State-of-the-Art. J. Oncol. 2021, 2021, 3006853. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Xu, H.-g.; Shen, J.-n.; Chen, X.-w.; Wang, H.; Zhou, J.-g. Apoptotic effects of curcumin on human osteosarcoma U2OS cells. Orthop. Surg. 2009, 1, 144–152. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef] [PubMed]
- DeBari, M.K.; Niu, X.; Scott, J.V.; Griffin, M.D.; Pereira, S.R.; Cook, K.E.; He, B.; Abbott, R.D. Therapeutic Ultrasound Triggered Silk Fibroin Scaffold Degradation. Adv. Healthc. Mater. 2021, 10, 2100048. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, B.; Li, M.; Zuo, B.; Kaplan, D.L.; Huang, Y.; Zhu, H. Degradation Mechanism and Control of Silk Fibroin. Biomacromolecules 2011, 12, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Ali Redha, A.; Kodikara, C.; Cozzolino, D. Does Encapsulation Improve the Bioavailability of Polyphenols in Humans? A Concise Review Based on In Vivo Human Studies. Nutrients 2024, 16, 3625. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Sundarakrishnan, A.; Herrero Acero, E.; Coburn, J.; Chwalek, K.; Partlow, B.; Kaplan, D.L. Phenol red-silk tyrosine cross-linked hydrogels. Acta Biomater. 2016, 42, 102–113. [Google Scholar] [CrossRef]
- Sundarakrishnan, A.; Chen, Y.; Black, L.D.; Aldridge, B.B.; Kaplan, D.L. Engineered cell and tissue models of pulmonary fibrosis. Adv. Drug Deliv. Rev. 2018, 129, 78–94. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Abdullah, N.; Kaplan, D.L. Microporous drug-eluting large silk particles through cryo-granulation. Adv. Eng. Mater. 2019, 21, 1801242. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).