Streamlining Endoscopy Cleaning: The Impact of a New Detergent on Time and Water Use
Abstract
1. Introduction
1.1. Water Usage and Environmental Impact of Endoscopy Reprocessing
1.2. Challenges in Meeting Reprocessing Demand
1.3. The Significance of Manual Cleaning
1.4. EndoPreZyme™ Will Henceforth Be Referred to as the ‘New Detergent’
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Observations
2.4. Data Collection
2.4.1. Phase 1: Define the Manual Cleaning Process Using the Standard Detergent
2.4.2. Phase 2: Training, Familiarisation with, and Introduction of the New Detergent
2.4.3. Phase 3: Define the Manual Cleaning Process Using the New Detergent
2.4.4. Measuring Decontamination Technicians’ Experience
2.5. Analysis
2.5.1. Standard Minute Values
- Relaxation Allowance (RA): Accounts for fatigue and short pauses during tasks, following established time study principles to reflect sustainable performance across longer shifts.
- Contingency Allowance (CA): Captures unplanned variations in the cleaning process, such as minor workflow interruptions, additional scrubbing due to visible debris, and variation in endoscope complexity. It also accounts for any residual impact of the observer effect, which was minimised through familiarisation and routine workflow monitoring.
2.5.2. Resource Cost Analysis
2.6. Ethics Approval and Consent
3. Results
3.1. Manual Cleaning Time
3.2. Utilisation Analysis
3.2.1. Current Utilisation (April 2023 to March 2024)
3.2.2. Projected Utilisation (April 2024 to March 2025)
3.2.3. Time Requirements for Decontamination Technicians
3.3. Water Usage During Manual Cleaning
3.4. Resource Cost Analysis
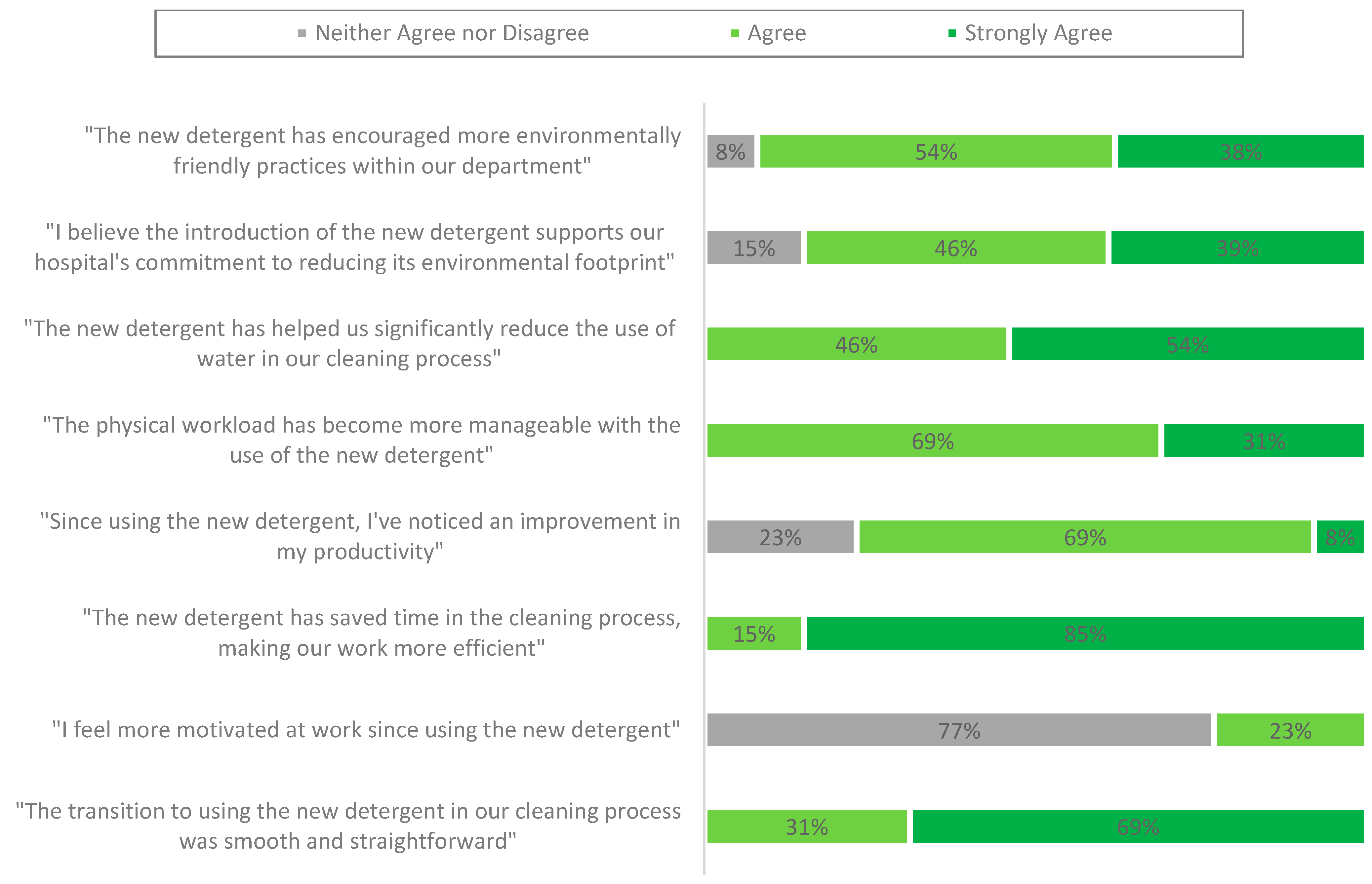
3.5. Decontamination Technicians’ Experience
3.5.1. Decontamination Technicians’ Experience of Using the Standard Detergent
3.5.2. Decontamination Technicians’ Experience of Using the New Detergent
4. Discussion
4.1. Water Consumption and Environmental Impact
4.2. Cost Savings and Efficiency Gains
4.3. Enhanced Productivity and Staff Awareness:
- NHS water management practices involve upgrading equipment to minimise water waste and raising staff awareness about efficient water use [7,33,46]. Our survey revealed that 77% of respondents reported improved productivity with the new detergent, while 100% acknowledged a more manageable workload. This increased efficiency allowed technicians to focus on other critical tasks. All respondents agreed or strongly agreed that the new detergent significantly reduced water usage during the manual cleaning process and contributed to time savings, thus enhancing the efficiency of decontamination technicians.
- Interestingly, 77% of respondents selected ‘neither agree nor disagree’ when asked if they felt more motivated at work since using the new detergent. This may have indicated that while the change improved workflow efficiency, it had a limited impact on perceived job satisfaction or motivation. This neutrality could be attributed to role constraints, the indirect nature of the intervention, or the timing of the post-survey, which occurred shortly after training.
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Metric | Standard Detergent | New Detergent |
| Weekly Hours Allocated | 112.5 | 112.5 |
| Weekly Minutes Allocated | 6750 | 6750 |
| Annual Minutes Allocated | 351,000 | 351,000 |
| Weighted Average Scope Manual Cleaning Time (minutes, seconds) | 13 min, 10.2 s | 11 min, 10.7 s |
| Manually Cleaned Reusable Flexible Endoscopes (April 2023 to March 2024) | 24,217 | 24,217 |
| Current Reusable Flexible Endoscope Manual Cleaning Capacity | 26,652 | 26,652 |
| Utilisation (%) | 90.86% | 77.12% |
| Forecasted Reusable Flexible Endoscopes (March 2024 to April 2025) | 29,000 | 29,000 |
| Forecasted Utilisation (March 2024 to April 2025) (%) | 108.81% | 92.35% |
References
- British Medical Association. More Support Needed to Help the NHS Reach Net Zero. 2024. Available online: https://www.bma.org.uk/what-we-do/population-health/protecting-people-from-threats-to-health/more-support-needed-to-help-the-nhs-reach-net-zero (accessed on 27 January 2025).
- NHS England. Delivering a Net Zero National Health Service. 2022. Available online: https://www.england.nhs.uk/greenernhs/wp-content/uploads/sites/51/2020/10/delivering-a-net-zero-national-health-service.pdf (accessed on 27 January 2025).
- Hospital Times. Efficient Water Delivery Is Vital for Healthcare Climate Resilience. 2021. Available online: https://hospitaltimes.co.uk/efficient-water-delivery-is-vital-for-healthcare-climate-resilience/ (accessed on 27 January 2025).
- Clear, C.; Hardi, J.; Thorne, A. Sustainability Strategies for Healthcare Estates: Lessons from University College London Hospitals. October 2021. Available online: https://www.researchgate.net/publication/275020700_S (accessed on 27 January 2025).
- TaxPayers’ Alliance. The NHS Wasted £41.4 Million on Energy Innovative Water Use. 2024. Available online: https://www.imperial.nhs.uk/about-us/our-strategy/green-plan/innovative-water-use (accessed on 16 January 2025).
- NHS Business Services Authority. Our Core Priorities. Available online: https://www.nhsbsa.nhs.uk/what-we-do/safety-health-and-environment/our-environment-strategy-2022-2025/our-core-priorities (accessed on 1 January 2025).
- NHS Innovation. Delivering a Net Zero Health Service. Available online: https://innovation.nhs.uk/innovation-guides/commissioning-and-adoption/delivering-a-net-zero-health-service (accessed on 22 January 2025).
- Spaner, S.J.; Warnock, G.L. A brief history of endoscopy, laparoscopy, and laparoscopic surgery. J. Laparoendosc. Adv. Surg. Tech. 1997, 7, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.; Cool, C. Costs involved in compliance with new endoscope reprocessing guidelines. Clin. Endosc. 2024, 57, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Gayam, S. Environmental Impact of Endoscopy: “Scope” of the Problem. Am. J. Gastroenterol. 2020, 115, 1931–1932. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Morais, R.; Monteiro, C.; Carvalho, A.; Barros, S.; Fernando, A.; Pioche, M.; de Santiago, E.R.; Macedo, G. Estimating the environmental impact of endoscopic activity at a tertiary center: A pilot study. Eur. J. Gastroenterol. Hepatol. 2024, 36, 39–44. [Google Scholar] [CrossRef]
- Oates, B. Gastroenterology: GIRFT Programme National Specialty Report. London: Getting It Right First Time. 2021. Available online: https://gettingitrightfirsttime.co.uk/wp-content/uploads/2021/11/Gastroenterology-overview.pdf (accessed on 27 January 2025).
- Shenbagaraj, L.; Thomas-Gibson, S.; Stebbing, J.; Broughton, R.; Dron, M.; Johnston, D.; Shaw, T.; Haboubi, H.N.; Green, J.T. Endoscopy in 2017: A National Survey of Practice in the UK. Frontline Gastroenterol. 2019, 10, 7–15. [Google Scholar] [CrossRef]
- Forte, L.; Shum, C. Comparative Cost-Efficiency of the EVOTECH Endoscope Cleaner and Reprocessor versus Manual Cleaning Plus Automated Endoscope Reprocessing in a Real-World Canadian Hospital Endoscopy Setting. BMC Gastroenterol. 2011, 11, 105. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, J.S. Clinical Practice Guidelines for Endoscope Reprocessing. Clin. Endosc. 2015, 48, 364–368. [Google Scholar] [CrossRef]
- Beilenhoff, U.; Neumann, C.S.; Rey, J.F.; Biering, H.; Blum, R.; Cimbro, M.; Kampf, B.; Rogers, M.; Schmidt, V. ESGE-ESGENA Guideline: Cleaning and Disinfection in Gastrointestinal Endoscopy. Endoscopy 2008, 40, 939–957. [Google Scholar] [CrossRef]
- ASGE Quality Assurance in Endoscopy Committee; Petersen, B.T.; Chennat, J.; Cohen, J.; Cotton, P.B.; Greenwald, D.A.; Kowalski, T.E.; Krinsky, M.L.; Park, W.G.; Pike, I.M.; et al. Multisociety Guideline on Reprocessing Flexible Gastrointestinal Endoscopes: 2011. Gastrointest. Endosc. 2011, 73, 1075–1084. [Google Scholar] [CrossRef]
- Loveday, H.P.; Wilson, J.A.; Pratt, R.J.; Golsorkhi, M.; Tingle, A.; Bak, A.; Browne, J.; Prieto, J.; Wilcox, M. Epic3: National Evidence-Based Guidelines for Preventing Healthcare-Associated Infections in NHS Hospitals in England. J. Hosp. Infect. 2014, 86 (Suppl. 1), S1–S70. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities; Centers for Disease Control: Washington, DC, USA, 2008. [Google Scholar]
- Rey, J.; Bjorkman, D.; Nelson, N.; Duforest-Rey, D.; Axon, A.; Sáenz, R.; Fried, M.; Mine, T.; Ogoshi, K.; Krabshuis, J.; et al. Endoscope Disinfection: A Resource-Sensitive Approach. Milwaukee: World Gastroenterology Organisation. 2011. Available online: https://www.worldgastroenterology.org/UserFiles/file/guidelines/endoscope-disinfection-english-2011.pdf (accessed on 10 September 2015).
- ANSI/AAMI ST91:2015; Flexible and Semi-Rigid Endoscope Processing in Health Care Facilities. AAMI: Arlington, VA, USA, 2015; pp. 1–70.
- Marie, A.; Bashaw, D.N.P. Guideline for Processing Flexible Endoscopes. AORN J. 2016, 104, 675–758. [Google Scholar] [CrossRef]
- Society of Gastroenterology Nurses and Associates (SGNA). Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes. 2015. Available online: https://www.sgna.org/Portals/0/SGNA%20Standards%20of%20infection%20prevention%20in%20reprocessing_FINAL.pdf (accessed on 27 January 2025).
- Ofstead, C.L.; Quick, M.R.; Eiland, J.E.; Adams, S.J. A Glimpse at the True Cost of Reprocessing Endoscopes: Results of A Pilot Project. Communiqué. International Association of Healthcare Central Service Materiel Management. May 2017. Available online: https://www.bostonscientific.com/content/dam/bostonscientific/uro-wh/portfolio-group/LithoVue/pdfs/Sterilization-Resource-Handout.pdf (accessed on 19 June 2024).
- XE.com. Exchange Rates. 2025. Available online: https://www.xe.com/currencytables/?from=USD&date=2025-03-11#table-section (accessed on 11 March 2025).
- Chemische Fabrik Dr. Weigert GmbH & Co. KG. Instructions for Use: EndoPreZyme Detergent; Chemische Fabrik Dr. Weigert GmbH & Co. KG: Hamburg, Germany; Olympus Winter & Ibe GmbH: Hamburg, Germany, 2021. [Google Scholar]
- Olympus Winter & Ibe GmbH. Instructions for Use: ETD Double Washer-Disinfector (Advanced User); Olympus Winter & Ibe GmbH: Hamburg, Germany, 2016. [Google Scholar]
- Kalne, P.S.; Mehendale, A.M. The Purpose of Time-Motion Studies (TMSs) in Healthcare: A Literature Review. Cureus 2022, 14, e29869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.; Dwivedi, N.; Dongre, A.; Deshmukh, P.; Dey, D.; Kumar, V.; Upadhyaya, S. Functioning and time utilisation by female multi-purpose health workers in South India: A time and motion study. Hum. Resour. Health 2018, 16, 64. [Google Scholar] [CrossRef]
- Admin_coats. Defining Standard Time in the Garment Industry. 2021. Available online: https://www.coatsdigital.com/en/blog/defining-standard-time/ (accessed on 27 January 2025).
- Personal Social Services Research Unit. Unit Costs of Health and Social Care Programme (2022–2027): The New Home for the Unit Costs of Health and Social Care Report. 2022. Available online: https://www.pssru.ac.uk/unitcostsreport/ (accessed on 11 March 2024).
- Building Better Healthcare. Safety and Sustainability Are Pulling Water Safety Groups. Available online: https://buildingbetterhealthcare.com/safety-and-sustainability-are-pulling-water-safety-groups (accessed on 22 January 2025).
- World Economic Forum. England Is Set to Run Out of Water in Just 25 Years. 2019. Available online: https://www.weforum.org/stories/2019/03/england-faces-jaws-of-death-as-taps-set-to-run-dry-in-25-years/ (accessed on 16 January 2025).
- Environment Agency. Meeting Our Water Needs for the Next 25 Years—Creating a Better Place. 2024. Available online: https://environmentagency.blog.gov.uk/2024/03/21/meeting-our-water-needs-for-the-next-25-years/ (accessed on 27 January 2025).
- Boucheron, T.; Lechevallier, E.; Gondran-Tellier, B.; Michel, F.; Bastide, C.; Martin, N.; Baboudjian, M. Cost and Environmental Impact of Disposable Flexible Cystoscopes Compared to Reusable Devices. J. Endourol. 2022, 36, 1317–1321. [Google Scholar] [CrossRef]
- Sebastian, S.; Dhar, A.; Baddeley, R.; Donnelly, L.; Haddock, R.; Arasaradnam, R.; Coulter, A.; Disney, B.R.; Griffiths, H.; Healey, C.; et al. Green Endoscopy: British Society of Gastroenterology (BSG), Joint Accreditation Group (JAG) and Centre for Sustainable Health (CSH) Joint Consensus on Practical Measures for Environmental Sustainability in Endoscopy. Gut 2023, 72, 12–26. [Google Scholar] [CrossRef]
- Maida, M.; Vitello, A.; Shahini, E.; Vassallo, R.; Sinagra, E.; Pallio, S.; Melita, G.; Ramai, D.; Spadaccini, M.; Hassan, C.; et al. Green endoscopy, one step toward a sustainable future: Literature review. Endosc. Int. Open 2024, 12, E968–E980. [Google Scholar] [CrossRef]
- NHS Digital. Cancer Services Profiles: 2024 Annual Update. 2025. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/cancer-services-profiles/2024-annual-update (accessed on 22 January 2025).
- Beilenhoff, U.; Biering, H.; Blum, R.; Brljak, J.; Cimbro, M.; Dumonceau, J.-M.; Hassan, C.; Jung, M.; Neumann, C.; Pietsch, M.; et al. Prevention of Multidrug-Resistant Infections from Contaminated Duodenoscopes: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA). Endoscopy 2017, 49, 1098–1106. [Google Scholar] [CrossRef]
- Rodríguez de Santiago, E.; Dinis-Ribeiro, M.; Pohl, H.; Agrawal, D.; Arvanitakis, M.; Baddeley, R.; Bak, E.; Bhandari, P.; Bretthauer, M.; Burga, P.; et al. Reducing the Environmental Footprint of Gastrointestinal Endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) Position Statement. Endoscopy 2022, 54, 797–826. [Google Scholar] [CrossRef]
- Maurice, J.B.; Siau, K.; Sebastian, S.; Ahuja, N.; Wesley, E.; Stableforth, W.; Hayee, B.; Green Endoscopy Network. Green Endoscopy: A Call for Sustainability in the Midst of COVID-19. Lancet Gastroenterol. Hepatol. 2020, 5, 636–638. [Google Scholar] [CrossRef]
- NHS England. Health Technical Memorandum 07-04: Water Management and Water Efficiency—Best Practice Advice for Healthcare Organisations. 2021. Available online: https://www.england.nhs.uk/wp-content/uploads/2021/05/HTM_07-04_Final.pdf (accessed on 22 January 2025).
- Pioche, M.; Pohl, H.; Cunha, A.; Laporte, A.; Mochet, J.M.; Rivory, J.; Grau, R.; Jacques, J.; Grinberg, D.; Boube, M.; et al. Environmental Impact of Single-Use versus Reusable Gastroscopes. Gut 2024, 73, 1816. [Google Scholar] [CrossRef]
- Tai, F.W.D.; Healy, A.; Thokala, P.; Chetcuti Zammit, S.; Sidhu, R.; McAlindon, M. Cost Comparison of Oral, Transnasal and Magnet Assisted Capsule Endoscopy in the Examination of the Upper Gastrointestinal Tract in Patients with Dyspepsia. Frontline Gastroenterol. 2022, 14, 300–305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- XE.com. Exchange Rates. 2025. Available online: https://www.xe.com/currencytables/?from=USD&date=2025-03-24#table-section (accessed on 24 March 2025).
- NHS Property Services. Water Efficiency Pledge: NHS Property Services. Available online: https://www.property.nhs.uk/media/1710/c-users-katherinemassie-onedrive-nhs-property-services-ltd-documents-directorates-energy-and-environment-final-versions-water-efficiency-pledge.pdf (accessed on 22 January 2025).

| Sub-Category | Type of Reusable Flexible Endoscope | Average SMV Per Flexible Endoscope (New Detergent) (min, s) | Average SMV Per Flexible Endoscope (Standard Detergent) (min, s) | SMV Reduction with New Detergent (min, s) | SMV Reduction (%) |
|---|---|---|---|---|---|
| A1 | Colonoscope, Gastroscope, Paediatric | 12 min, 42.4 s | 15 min, 12.7 s | 2 min, 30 s | 16% |
| A2 | Urology Scopes (Cystoscope) | 10 min, 55.8 s | 12 min, 17.2 s | 1 min, 21 s | 11% |
| A3 | Inpatient (Ureteroscope) | 11 min, 13.5 s | 14 min, 28.6 s | 3 min, 15 s | 22% |
| B | ENT, Cardiac Probes | 6 min, 4.8 s | 6 min, 46.0 s | 41 s | 10% |
| C1 | Therapeutic/Echo Scopes | 14 min, 30.2 s | 18 min, 30.3 s | 4 min | 22% |
| C2 | Pulmonary and Ultrasound Scopes | 10 min, 16.2 s | 14 min, 10.4 s | 3 min, 55 s | 28% |
| C3 | Video Bronchoscope | 9 min, 20.4 s | 11 min, 51.2 s | 2 min, 31 s | 21% |
| Subcategories | Flexible Endoscopes Manually Cleaned from 1 April 2023 to 31 March 2024 | Percentage of Total (%) |
|---|---|---|
| A1 | 15,174 | 63% |
| A2 | 4253 | 18% |
| A3 | 346 | 1% |
| B | 4351 | 18% |
| C | 92 | <1% |
| Total | 24,217 | 100% |
| SMV Per Flexible Endoscope (New Detergent) (min, s) | SMV Per Flexible Endoscope (Standard Detergent) (min, s) | SMV Reduction with New Detergent (min, s) | SMV Reduction (%) |
|---|---|---|---|
| 11 min, 10.7 s | 13 min, 10.2 s | 1 min, 59.47 s | 15% |
| Category | Weighted Average SMV (New Detergent) (min, s) | Weighted Average SMV (Standard Detergent) (min, s) | SMV Reduction with the New Detergent (min, s) | SMV Reduction (%) |
|---|---|---|---|---|
| A | 12 min, 17.9 s | 14 min, 34.2 s | 2 min, 6.0 s | 16% |
| B | 6 min, 4.8 s | 6 min, 46.0 s | 41.0 s | 10% |
| C | 12 min, 22.3 s | 14 min, 50.6 s | 3 min, 28.0 s | 23% |
| Cleaning Materials | Unit Price (GBP) | Quantity Used per Flexible Endoscope | Cost per Manually Cleaned Flexible Endoscope (GBP) 3 | Total Annual Cost (GBP) 2 |
|---|---|---|---|---|
| Detergent (10 L) | GBP 59.00 | 100 mL | GBP 0.59 | GBP 17,110 |
| Water (50 L) | GBP 0.07 | 50 L | GBP 0.07 | GBP 2001 |
| Disposable lint-free cloth | GBP 94.28 | 1 per flexible endoscope | GBP 0.12 | GBP 3418 |
| Single-use channel brush | GBP 99.00 | 1 per flexible endoscope | GBP 0.99 | GBP 18,710 |
| Disinfectant wipes for sinks and counters | GBP 21.49 | 1 per flexible endoscope | GBP 0.11 | GBP 3116 |
| Total material costs (GBP): | GBP 1.87 | GBP 54,355 | ||
| Personnel costs (GBP): 1 | GBP 3.03 | GBP 87,841 | ||
| Total cost per manually cleaned reusable flexible endoscope (GBP): * | GBP 4.90 | GBP 142,196 |
| Cleaning Materials | Unit Price (GBP) | Quantity Used per Flexible Endoscope | Cost per Manually Cleaned Flexible Endoscope (GBP) 3 | Total Annual Cost (GBP) 2 |
|---|---|---|---|---|
| Detergent (10 L) | GBP 86.24 | 125 mL | GBP 1.08 | GBP 31,262 |
| Water (25 L) | GBP 0.07 | 25 L | GBP 0.03 | GBP 1001 |
| Disposable lint-free cloth | GBP 94.28 | 1 per flexible endoscope | GBP 0.12 | GBP 3418 |
| Single-use channel brush | GBP 99.00 | 1 per endoscope | GBP 0.99 | GBP 28,710 |
| Disinfectant wipes for sinks and counters | GBP 21.49 | 1 per flexible endoscope | GBP 0.11 | GBP 3116 |
| Total material costs (GBP): | GBP 2.33 | GBP 67,506 | ||
| Personnel costs (GBP): 1 | GBP 2.55 | GBP 74,083 | ||
| Total cost per manually cleaned reusable flexible endoscope (GBP): * | GBP 4.88 | GBP 141,590 |
| Standard Detergent (GBP) | New Detergent (GBP) | |
|---|---|---|
| Total Cost per Flexible Endoscope | GBP 4.90 | GBP 4.88 |
| Total Annual Cost (29,000 Endoscopes) | GBP 142,196 | GBP 141,590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hicks, J.; Mutowo, M. Streamlining Endoscopy Cleaning: The Impact of a New Detergent on Time and Water Use. J. Mark. Access Health Policy 2025, 13, 23. https://doi.org/10.3390/jmahp13020023
Hicks J, Mutowo M. Streamlining Endoscopy Cleaning: The Impact of a New Detergent on Time and Water Use. Journal of Market Access & Health Policy. 2025; 13(2):23. https://doi.org/10.3390/jmahp13020023
Chicago/Turabian StyleHicks, Joshua, and Mutsa Mutowo. 2025. "Streamlining Endoscopy Cleaning: The Impact of a New Detergent on Time and Water Use" Journal of Market Access & Health Policy 13, no. 2: 23. https://doi.org/10.3390/jmahp13020023
APA StyleHicks, J., & Mutowo, M. (2025). Streamlining Endoscopy Cleaning: The Impact of a New Detergent on Time and Water Use. Journal of Market Access & Health Policy, 13(2), 23. https://doi.org/10.3390/jmahp13020023






