Abstract
With the European Union (EU) Health Technology Assessment (HTA) regulation, Joint Clinical Assessments (JCA) are now required for oncological and advanced therapy medicinal products. The JCA assessment scope is determined through the PICO framework (Population, Intervention, Comparator, Outcome). Given the tight JCA timelines, Health Technology Developers (HTD) must anticipate PICO elements early to prepare dossiers effectively. This study investigates whether PICO can be predicted across EU member states using publicly available information. A systematic literature review was conducted to identify relevant peer-reviewed articles. Additionally, an extensive search of publicly available HTA documents, including reports, methodological guidelines, submission templates, and market access information was performed across 29 European countries. Relevant information for PICO anticipation was extracted. For many member states, a wealth of relevant information is publicly accessible: 66% have HTA reports publicly available, 79% have HTA methodological guidelines, 69% have dossier templates, and 100% have market access status lists. Between countries, the requirements for population and outcomes are largely aligned, making comparator the central element in PICO anticipation. PICO can be anticipated reliably based on public information. HTDs must be prepared to adjust their strategies as national procedures adapt, ensuring alignment with both current and emerging EU and national requirements.
1. Introduction
In Europe, Health Technology Assessment (HTA) has been a strictly national process for decades. As the European Union (EU) HTA regulation has entered into force, oncological medicinal products and advanced therapy medicinal products (ATMP) with initial market authorization applications, will need to pass through a Joint Clinical Assessment (JCA). From 2028 any orphan medicinal products and from 2030 all medicinal products will follow. The course and the contents of JCA are laid down in Regulation 2021/2282 and in Commission Implementing Regulation 2024/1381 [1,2].
1.1. Different HTA Systems Across Europe
EU member states have implemented different value assessment frameworks and notable differences exist in how HTA is implemented across the EU [3,4,5,6]. Differences include for instance methodological preference, patient-involvement, timing of HTA, special conditions for orphan drugs, consideration of uncertainties and unmet medical need, and legal decision-making power on reimbursement and price [7,8,9,10,11]. In some countries, such as Sweden and the Netherlands, cost-effectiveness analysis plays a central role in healthcare decision-making, while others, such as Germany and Austria, predominantly evaluate clinical effectiveness and safety. A third group of countries, including, e.g., France and Slovakia, integrate both clinical and economic aspects in their assessments [12,13,14]. Not surprisingly, these differences also lead to differences in the national appraisal of the added therapeutic value [15]. A comparative analysis of 191 HTA decisions in France and Germany indicated, for instance, only a 50% concordance in added value rating [16]. Together, these findings demonstrate the large differences in HTA systems and HTA outcomes across Europe.
Despite their differences, from 2025 the clinical evidence will be evaluated in a centralized EU HTA process, the JCA. Economic evaluations as well as the decision on the additional benefit and the amount of reimbursement of a new technology will, however, remain on the national level.
1.2. EU HTA Process and PICO Scoping
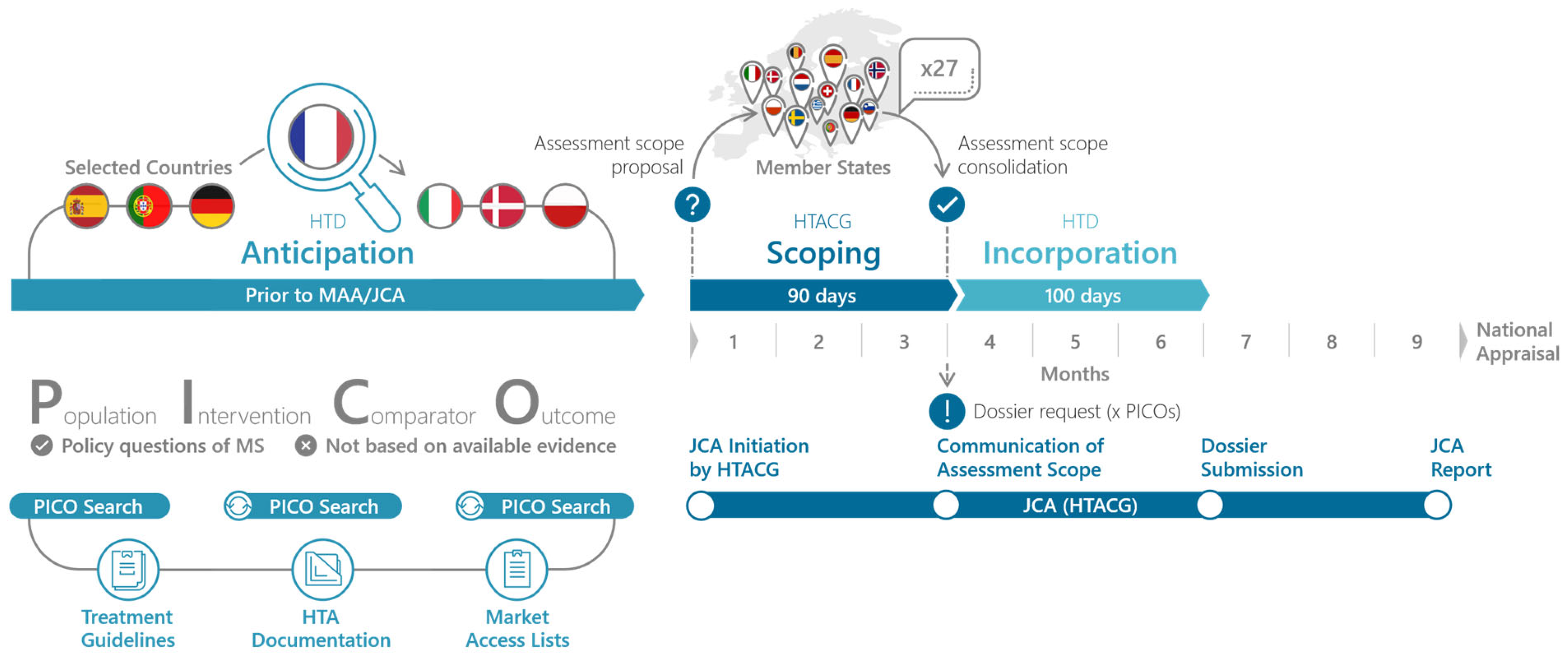
The goal of the EU HTA process is to facilitate access of new therapeutic innovation to patients, streamline the HTA process, and reduce the duplication of work for HTDs and HTA bodies [17]. Thereby, the JCA should account for the needs of all member states. To ensure this, the JCA process starts with a PICO (Population, Intervention, Comparator, Outcome) Scoping phase that queries the relevant PICO for their national HTA appraisals. This scoping processes is outlined in the HTA Coordination Group’s Guidance on the scoping process (see Figure 1) [18]. Importantly PICO scoping should not be based on the available evidence but on the policy questions of the member states. A PICO scoping exercise conducted in preparation for the JCA revealed that 10 and more PICO can be expected, with complexity arising mainly from differences in populations and comparators between member states [19,20,21]. Given the complexity of the JCA and the expected high number of PICO, HTD have to start the preparation of the JCA submission dossier prior to the communication of the assessment scope. Ideally, this preparation already includes the evidence syntheses and results for all PICO. In order to do so effectively, it is essential that the HTD anticipates the assessment scope of the JCA submission dossier.
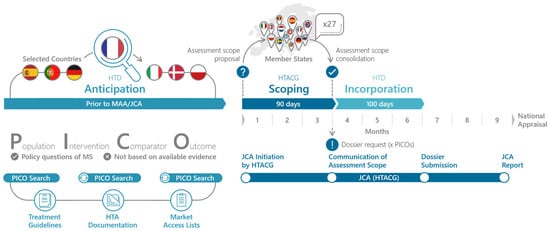
Figure 1.
PICO Anticipation and PICO Scoping in the Joint Clinical Assessment process. The PICO scoping phase begins with the initiation of the JCA, which is time-linked to the start of the EMA’s assessment of the marketing authorization application. In the PICO Scoping phase, the assessor and co-assessor, appointed by the JCA subgroup, develop the draft PICO. The member states are then asked to review and confirm or adjust the PICO survey and report back to the assessors. Finally, the assessors will consolidate the PICO to the minimal possible number and this assessment scope and communicate it to the HTD [18]. Once the HTD knows the final assessment scope, they have 100 days to prepare the JCA submission dossier (EMA standard procedure) [1].
1.3. PICO Anticipation
PICO can be anticipated using various methods. From our experience, the preferred way for HTD might be to involve local country affiliates with expert knowledge on the national HTA system and requirements. However, this approach might not always be feasible, especially if HTD do not have local affiliates in all EU markets. Yet much information can also be gained from public sources, such as previous assessments in the same indication, national treatment guidelines or published information about national HTA systems and requirements. In some countries, such as Germany, there are even specific rules to determine some of the PICO elements [22,23].
Here we ask whether PICO can be anticipated for all member states based on public information? We find that, for many member states, a wealth of relevant information is publicly accessible and conclude that PICO can be anticipated reliably based on public information.
2. Materials and Methods
2.1. Systematic Literature Research
We conducted a systematic literature review (SLR) which included a thorough search strategy, screening process, and data extraction (for details see Supplementary Materials). We developed search strategies for the following electronic databases: Embase, MEDLINE, and the Cochrane Library. Searches were conducted on 5 February 2025, without language restrictions, to ensure a comprehensive capture of available evidence. We screened titles and abstracts for relevance using a customized screening tool. After abstract screening, full-text articles were retrieved for detailed evaluation. Included studies were peer-reviewed publications that focused on HTA requirements in Europe, differences between HTA systems in European countries, or the determination of population, comparator, or outcomes relevant to EU HTA. Excluded were conference abstracts, commentaries, editorials, and publications that focused solely on non-European countries or did not address HTA requirements. Articles that were selected for the full text review were inspected in detail and relevant information on country-specific requirements on PICO elements relevant to the JCA process were extracted. Descriptive statistics were used to quantify the publication characteristics and results, where applicable (for details see Supplementary Materials).
2.2. Identifying Publicly Available Documents
In addition to the SLR, we conducted an online search for publicly available documents for each Member State. We also included Norway and Iceland in our analysis, as both countries participate in the EU HTA system through their European Economic Area (EEA) membership. For clarity, we will henceforth refer to these countries as ‘member states’. Specifically, we assessed whether member states have publicly available (1) HTA reports, (2) methodological guidelines, (3) HTA submission templates, (4) market access or reimbursement lists, and (5) national oncological treatment guidelines. While national treatment guidelines are not strictly necessary for information sourcing in the context of PICO anticipation, they may serve as a useful starting point for oncological indications. We first identified the HTA bodies of all member states and reviewed their websites for publicly available HTA reports, methodological guidelines, and submission dossier templates. If this did not yield results, we conducted a broader search using Google, combining the respective Member State or HTA body name with the keywords ‘HTA report’, ‘HTA methodology’, or ‘HTA template’. Similarly, to identify country-specific information on market access status lists and oncological guidelines an online search was conducted. With regard to the treatment guidelines, we specifically searched for landing pages that provide national guidelines for multiple oncological indications. Any documents that were not available in either English or German were translated to English using the DeepL translator tool.
2.3. Data Analysis
To quantify the availability of information, we compiled a list with references of all identified documents. We then calculated the absolute and relative number of member states with available information per document type (HTA report, methodological guideline, HTA submission template, market access status list, oncological treatment guideline). Furthermore, we selected the member states with available HTA reports, HTA methodological guidelines, and HTA submission templates and systematically searched in all the available documents to answer the following questions:
- Which population is relevant for HTA?
- How is/are relevant comparator(s) determined?
- Are there any preferred outcomes for HTA?
Note that we did not include intervention in our analysis as it “reflects the intervention to be assessed in the indication for which the HTD applied in the regulatory submission dossier” [18].
3. Results
3.1. Results of the Systematic Literature Research
The SLR revealed 571 abstracts screened for relevance (for detailed information see Supplementary Materials). Based on the abstract screening, 39 publications were selected for further inspection. Of these 39, 26 were deemed as irrelevant for our research question after reading the full publication. The remaining 13 articles entailed relevant information about country-specific HTA requirements or about at least one of the PICO elements. For some countries, particularly France, Germany, and the Netherlands, relevant publications were identified in the SLR (for an overview of available references per country see Supplementary Materials). However, most member states were represented in a very limited number of published articles only or were not included at all (see Supplementary Materials).
Thus, overall, the publications identified in the SLR did not reveal sufficient information to answer our research questions.
3.2. Availability of Documents
HTA reports are accessible in 19 out of 29 countries (66%), however not all HTA bodies with available reports publish the reports of all their assessments, but a selection. Published guidelines for HTA methodologies are available in 23 countries (79%). Additionally, 20 countries (69%) provide a standardised template for HTA submissions. Market access status information is publicly available in all 29 assessed countries. Furthermore, 20 of the 29 countries (69%) have publicly accessible national oncology treatment guidelines. In 45%, i.e., 13 of the 29 analysed countries, HTA reports, HTA methodological guidelines, and dossier templates are publicly available. However, the documents differ in length and depth between countries, ranging from concise overviews to very detailed documents (e.g., short HTA reports in Netherlands [24] to detailed submission dossier templates in Germany [25]).
Table 1 provides an overview of which specific documents are publicly available in each Member State.

Table 1.
Overview of document availability per member state.
3.3. PICO Anticipation Based on Public Information
Based on the outcomes summarized in Table 1 we selected countries with publicly available HTA reports, HTA methodological guidelines, dossier templates, and market access status lists for further analysis. In particular we inspected the documents to identify criteria for population, comparator and outcome requirements in the national HTA procedure. The intervention is the indication-applied-for or the intended use of the medicinal product. Table 2 provides a summary of population, comparator, and outcome requirements for all member states that we included in our detailed analysis.

Table 2.
Comparator Requirements and Study Outcomes Across European Countries. The table provides an overview of comparator requirements and study outcome considerations for selected European countries. The selection of countries is based on the availability of publicly accessible documents, including HTA reports, methodological guidelines, dossier templates, market access status lists and relevant publications.
3.4. Population
Generally, the requested population for HTA evaluation will be aligned with the label population. However, some countries specify additional considerations for subpopulations. Belgium, Finland, Germany, and Italy explicitly require subpopulations to be considered when different comparators exist or when clinically relevant differences in effectiveness, safety, or cost-effectiveness are expected. Notably, this does not necessarily mean that other member states are less likely to request subpopulations, it is however not explicitly mentioned in the available documents. Italy also considers the study population in addition to the label population and might therefore request results analysis specifically for the study population. According to the Guidance on the scoping process [18], potential reasons to define separate subpopulations, i.e., separate PICO for each subpopulation, could be (a) different comparators are deemed appropriate for the different subpopulations, (b) the therapeutic indication explicitly comprises different subpopulations, e.g., defined by certain tumor entities, or (c) the subpopulations have different prognoses and therefore different effectiveness is expected. The definition of subgroups by contrast will not lead to a new PICO. Subgroup analyses in the context of a JCA are performed within a given PICO [18]. This analysis suggests that differences in requested population between countries are likely driven by variations in comparators for certain subpopulations
3.5. Comparator
The choice of comparator varies significantly between member states, reflecting national clinical practices and preferences within the national HTA systems. In general, all member states prefer comparing new interventions to the current clinical practice. However, there are notable differences in emphasis and level of detail in the selection of comparators: Several countries, including Denmark, Finland, Germany, Italy, and the Netherlands, explicitly require national clinical practice to be taken into account.
We assume that all member states generally prefer comparators that align with their national clinical practices. Notably, Norway and Belgium explicitly state that the comparator should be the treatment most likely to be replaced. The Belgian guideline further states that particular attention will be given to the comparator(s) used in the studies [35,108]. Sweden and the Netherlands require the comparator to be the most cost-effective option, highlighting a strong economic focus in their HTA evaluations. In Romania, a relevant comparator must be listed among reimbursed medicinal products under the social health insurance system or national healthcare programs, with the same approved indication for the same patient group [149]. Slovakia also requests that the comparator must be reimbursed within the national health care system. Furthermore, a relevant comparator must represent at least 20% of clinical practice [123]. Finland specifies that the comparator should belong to the same treatment group, with the example that beta blockers should be compared to beta blockers [150]. Further, Norway and Denmark require that all commonly used treatments are considered when multiple options exist. Germany follows a unique approach, with strictly defined rules for determining the appropriate comparator based on the national regulatory framework [23]. Bulgaria also stands out, recommending the best standard treatment to ensure international comparability, although the current national clinical practice might be accepted [41].
3.6. Outcome
All member states consider efficacy, quality of life (QoL) or health-related quality of life (HRQoL), and safety as fundamental outcome measures, however, the emphasis and level of detail vary across countries. Belgium, Bulgaria, France, and Norway specify that both primary and secondary clinical study outcomes should be assessed, with Denmark also explicitly requesting explorative endpoints. Denmark and Estonia specifically recommend using the EQ-5D-5L questionnaire or a measure that is mapped to the EQ-5D-5L to evaluate QoL. Finland prefers efficacy metrics such as overall survival (OS), progression-free survival (PFS), and treatment response [66]. Germany, Italy, and the Netherlands underscore the importance of patient-relevant, clinically significant, and validated outcomes, with France focusing on patient-centred outcomes. Furthermore, Germany and the Netherlands provide detailed hierarchies to guide outcome prioritization: Germany emphasizes mortality, morbidity, HRQoL, and patient-reported outcome measures (PROMs), while the Netherlands categorize outcomes into clinically relevant endpoints, adverse events, and composite measures [148].
4. Discussion
Here we show that PICO can be anticipated for member states based on public information. The previous literature has predominantly focused on comparing HTA systems in countries with highly transparent processes (e.g., [3,11,15,19,151,152,153,154,155,156,157]). While this allows for deeper analysis for these countries due to the availability of extensive data, it also introduces a significant limitation: the comprehensive requirements of all member states are not fully represented. Additionally, the published literature focused on a specific product within a particular indication [19] or compared HTA requirements with market authorization requirements [158,159,160,161]. Thus, relying solely on previous peer-reviewed articles seems inefficient to accurately predict PICO elements for the JCA.
Here we adopted a novel approach: we systematically searched for relevant publicly available HTA information about all EU Member states plus Iceland and Norway. This inclusive approach provides, for the first time, a clearer picture of the diversity in HTA practices and improves our ability to evaluate how feasible it is to predict PICO elements across the EU.
4.1. Availability of Documents as a Basis for PICO Anticipation
Our findings reveal that, for many member states, a wealth of relevant information is publicly accessible (see Table 1). Therefore, we are confident that it is possible to anticipate PICO elements for the majority of member states. Nevertheless, our analysis of thirteen states with well-documented information reveals that, despite some apparent similarities, nuanced differences exist in how each country defines and prioritizes PICO elements. Notably, the depth of information varies across HTA systems. Some countries (e.g., Sweden, Poland, Ireland) publish only selected assessment reports, while others (e.g., Germany, France) provide full transparency. This selective reporting can make drawing conclusions about PICO more difficult and potentially biased. To anticipate PICO elements, it requires a holistic understanding of the diverse HTA systems, especially in member states where only limited information is accessible, and the combination of multiple sources. Note however, that in some case especially new HTA systems seem very well thought through and matched to the EU HTA requirements (e.g., Slovakia, Malta, and Spain). The observed differences in the availability, type, and level of detail of publicly accessible documents reflect the variability in national HTA practices and requirements. Importantly, even if one of the document types is missing, it might still be possible to acquire a good understanding about the HTA requirements, due to redundancies, various structures and informative values. For instance, if the methodological guideline is detailed enough the dossier content, structure and HTA requirements can be well anticipated even without a dossier template (e.g., Portugal [117]).
4.2. PICO Anticipation
Previous studies show differences between HTA requirements and EMA requirements, compared HTA systems, or outcomes of assessments between countries for individual products [7,19,153]. Yet, the selection of countries appeared to follow a non-systematic approach. Our selection was based on a thorough analysis of publicly available information to provide a framework for deriving PICO independent of product or indication. This led to the inclusion of often-overlooked countries like Slovakia and Romania. The analysis enables reliable PICO prediction using only public data. We assume that the requested population for HTA evaluation will be generally aligned with the label population and that any division of the population in subpopulations will be driven by the availability and choice of comparators. This makes the comparator the central and presumably most important element in PICO anticipation. Predicting the right comparator is key to study planning and evidence generation planning. Our analysis shows, that it is generally the standard of care in each country. Notably, the standard of care can also be an “individualised treatment”, which is a valid comparator option, if a treatment suitable for all patients in a given Population does not exist, or if clinical guidelines recommend a range of different treatment options [18,162]. In general, the standard of care, being a specific or individualized treatment, can most likely be identified by studying local treatment guidelines or, if local guidelines are not available, by European guidelines. These guidelines will indicate if subpopulations are needed, for example, to treat patients with a specific gene mutation using a targeted approach. This may seem straightforward, but we believe it’s crucial to adopt each country’s HTA mindset, especially for comparator prediction. Our analysis shows that countries differ in their approach to selecting comparators for HTA. Some prefer multiple treatment options (e.g., Norway and Denmark), others focus on most economic standard of care (e.g., Sweden and the Netherlands) or treatments likely to be replaced (e.g., Norway and Belgium), while some follow specific national rules for comparator selection (e.g., Germany). A comprehensive approach is required, integrating all available information, including national HTA perspectives, economic considerations, and country-specific comparator selection rules, to ensure a complete and accurate comparator anticipation (see also Supplementary Figure S2 for a step-by-step approach for selecting country-specific comparators).
With regard to outcomes, all member states consider efficacy, quality of life (QoL) or health-related quality of life (HRQoL), and safety as fundamental outcome measures, however, the emphasis and level of detail vary across countries. While some countries particularly highlight the need of patient-relevance or patient-centred outcomes (e.g., Germany, France), others focus on clinical study outcome measures (e.g., Belgium and Bulgaria). Additionally, whereas some countries explicitly require specific HRQoL questionnaires (e.g., Denmark), most allow for flexibility in the selection of questionnaires.
4.3. Methodological Considerations
Our study is based exclusively on publicly available documents from HTA bodies and the published literature. While this approach enables a broad overview of the publicly accessible information on national HTA practices, it also has several limitations. First, internal procedures, proprietary practices, or the most up-to-date developments within individual HTA bodies may not be captured. The absence of data from expert interviews or direct surveys means that nuanced, non-public aspects of national processes remain unexplored. Future studies could enrich these findings by integrating qualitative insights from practitioners and policy experts. Second, the depth and clarity of public reporting vary considerably across member states. Third, we cannot be certain that anticipating PICO based on public documentation will ultimately enable accurate predictions of the JCA assessment scope, as there are currently no published JCA reports available for external validation of this approach. Fourth, the consolidation process introduces an additional layer of complexity that may influence the predictability of the final PICO. While national PICO can be inferred as described, the consolidated final PICO might be more difficult to anticipate. Finally, in many member states HTA systems and processes are evolving rapidly and might undergo more changes with the full implementation of the JCA process. Our analysis is a snapshot in time. Notably, some countries are already aligning their HTA practices more closely with the evolving EU framework. For instance, recent developments in Spain [163] suggest that new or reformed HTA frameworks are in closer alignment with EU HTA objectives, and the HTA collaboration such as JNHB [146] and Beneluxa [147] indicates a move toward more integrated, joint assessments among EU countries. These examples might suggest a gradual convergence toward greater harmonization across diverse national systems. As national procedures adapt, country specific demands for PICO elements may also shift. Thus, it is important to review and reflect on these findings in the future.
4.4. Recommendations
Accurate PICO anticipation for a specific indication can be achieved by leveraging data from a subset of countries. Based on our analysis, we recommend including countries from various regions of Europe, such as Northern, Southern, Eastern, Western, and Central Europe. It is crucial to incorporate diverse markets that emphasize different aspects of HTA and reimbursement systems, such as those with a strong focus on economic considerations (e.g., the Netherlands) and those that prioritize clinical factors (e.g., Germany). The selection of markets should also take into account the average time required for drug availability, ensuring a balanced representation of both slower-access markets (e.g., Romania) and faster-access markets (e.g., Germany). Moreover, PICO for the JCA should not be anticipated with an exclusive focus on clinical assessment, as in many countries, clinical evaluation serves merely as the foundation for economic assessments. Importantly, engaging with local or country-specific stakeholders, when possible, can enhance accuracy and relevance of PICO anticipation. This is particularly relevant when no previous HTA exists, no comparators or guidelines are available, e.g., in rare diseases, or the existing information is outdated. Involving stakeholders is likely to yield more precise and reliable PICO predictions, ensuring that HTDs are well-prepared for the diverse and evolving requirements of the JCA process. Importantly, with the expected adjustment in national systems and the ever-changing therapy landscape, we recommend that HTDs who want to anticipate PICO remain agile—regularly verifying, cross-checking, and updating their anticipated PICO elements throughout both the EU submission process and the preparation of national dossiers.
5. Conclusions
Ultimately, our analysis underscores the importance of a foundational understanding of each Member State’s HTA system to accurately anticipate PICO elements. While relying on publicly available documents provides a robust starting point, continuous monitoring of policy changes and direct engagement with local experts will be essential to maintain an up-to-date and effective strategy.
In conclusion, although anticipating PICO based on public information is feasible for many EU member states, the evolving nature of HTA practices calls for a flexible and iterative approach. HTDs must be prepared to adjust their strategies as national procedures adapt, ensuring that submissions remain aligned with both current and emerging EU and national requirements. Future research should explore integrating real-time stakeholder insights with document analysis to further refine PICO predictions, ultimately facilitating smoother and more efficient pathways to patient access for innovative therapies. Finally, only future experience with JCAs will reveal how accurately pharmaceutical companies are able to predict PICO scopes and which methods prove most effective.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmahp13030032/s1, Figure S1: Data extraction flowchart; Table S1: List of relevant publications and countries included within each publication; Table S2: Frequency of inclusion in a publication per country; Figure S2: Step-by-step approach for selecting country-specific comparators based on publicly available data.
Author Contributions
Conceptualization: K.E., L-M-H. and E.-M.R. Methodology and investigation: K.E., L.-M.H., M.K.S. and M.J. Writing—original draft preparation: K.E., L-M-H., J.M. and E.-M.R. Writing—review and editing: K.E., L-M-H., J.M., A.C. and E.-M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank Stefan Schleibner and Selina Onoh for providing valuable feedback during preparation of the manuscript; Fiona Sprung and Vanessa Schimek for providing support during information sourcing, and Anna-Lena Vogel for preparing the Figure.
Conflicts of Interest
All authors were employed by AMS Advanced Medical Services GmbH. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- European Commission. Commission Implementing Regulation (EU) 2024/1381 of 23 May 2024. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202401381 (accessed on 26 March 2025).
- European Commission. Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2282 (accessed on 26 March 2025).
- Blonda, A.; Barcina Lacosta, T.; Toumi, M.; Simoens, S. Assessing the value of Nusinersen for spinal muscular atrophy: A comparative analysis of reimbursement submission and appraisal in European countries. Front. Pharmacol. 2022, 12, 750742. [Google Scholar] [CrossRef]
- Akehurst, R.L.; Abadie, E.; Renaudin, N.; Sarkozy, F. Variation in health technology assessment and reimbursement processes in Europe. Value Health 2017, 20, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Jaksa, A.; Louder, A.; Maksymiuk, C.; Vondeling, G.T.; Martin, L.; Gatto, N.; Richards, E.; Yver, A.; Rosenlund, M. A Comparison of Seven Oncology External Control Arm Case Studies: Critiques from Regulatory and Health Technology Assessment Agencies. Value Health 2022, 25, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Rojubally, A.; Pan, X.; Wolf, B.; Greenfeder, S.; Upton, A.; Gdovin Bergeson, J. A Review and Comparative Case Study Analysis of Real-World Evidence in European Regulatory and Health Technology Assessment Decision Making for Oncology Medicines. Value Health 2025, 28, 31–41. [Google Scholar] [CrossRef]
- Bloem, L.T.; Vreman, R.A.; Peeters, N.W.; Hoekman, J.; van Der Elst, M.E.; Leufkens, H.G.; Klungel, O.H.; Goettsch, W.G.; Mantel-Teeuwisse, A.K. Associations between uncertainties identified by the European Medicines Agency and national decision making on reimbursement by HTA agencies. Clin. Transl. Sci. 2021, 14, 1566–1577. [Google Scholar] [CrossRef]
- Chassany, O.; Engen, A.V.; Lai, L.; Borhade, K.; Ravi, M.; Harnett, J.; Chen, C.-I.; Quek, R.G. A call to action to harmonize patient-reported outcomes evidence requirements across key European HTA bodies in oncology. Future Oncol. 2022, 18, 3323–3334. [Google Scholar] [CrossRef]
- Jakubowski, S.; Kawalec, P.; Holko, P.; Kowalska-Bobko, I.; Kamusheva, M.; Petrova, G.; Draganić, P.; Fuksa, L.; Männik, A.; Ispán, F. Clinical aspects of reimbursement policies for orphan drugs in Central and Eastern European countries. Front. Pharmacol. 2024, 15, 1369178. [Google Scholar] [CrossRef]
- Kleijnen, S.; Leonardo Alves, T.; Meijboom, K.; Lipska, I.; De Boer, A.; Leufkens, H.G.; Goettsch, W.G. The impact of quality-of-life data in relative effectiveness assessments of new anti-cancer drugs in European countries. Qual. Life Res. 2017, 26, 2479–2488. [Google Scholar] [CrossRef]
- Nicod, E.; Meregaglia, M.; Whittal, A.; Upadhyaya, S.; Facey, K.; Drummond, M. Consideration of quality of life in the health technology assessments of rare disease treatments. Eur. J. Health Econ. 2022, 23, 645–669. [Google Scholar] [CrossRef]
- Fontrier, A.-M.; Visintin, E.; Kanavos, P. Similarities and Differences in Health Technology Assessment Systems and Implications for Coverage Decisions: Evidence from 32 Countries. Pharmacoecon. Open 2022, 6, 315–328. [Google Scholar] [CrossRef]
- Pharmaceutical Pricing and Reimbursement Information (PPRI). PPRI Pharma Profile Sweden. Available online: https://ppri.goeg.at/system/files/inline-files/PPRI_Pharma_Profile_Sweden_2023.pdf (accessed on 26 March 2025).
- Wouterse, B.; van Baal, P.; Versteegh, M.; Brouwer, W. The Value of Health in a Cost-Effectiveness Analysis: Theory Versus Practice. Pharmacoeconomics 2023, 41, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, R.; Hernandez, D.; Selberg, L.; Schlander, M. Health technology assessment (HTA) in England, France and Germany: What do matched drug pairs tell us about recommendations by national HTA agencies? J. Comp. Eff. Res. 2021, 10, 1187–1195. [Google Scholar] [CrossRef]
- Boucaud-Maitre, D.; Berdaï, D.; Salvo, F. Added therapeutic value of medicinal products for French and German health technology assessment organizations: A systematic comparison. Value Health 2021, 24, 346–352. [Google Scholar] [CrossRef]
- European Commission. Proposal for a Regulation of the European Parliament and of the Council on Health Technology Assessment and Amending Directive 2011/24/EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52018PC0051 (accessed on 26 March 2025).
- Member State Coordination Group on Health Technology Assessment (HTA CG). Guidance on the Scoping Process. V1.0. Available online: https://health.ec.europa.eu/document/download/7be11d76-9a78-426c-8e32-79d30a115a64_en?filename=hta_jca_scoping-process_en.pdf (accessed on 26 March 2025).
- van Engen, A.; Krüger, R.; Parnaby, A.; Rotaru, M.; Ryan, J.; Samaha, D.; Tzelis, D. The Impact of Additive Population(s), Intervention, Comparator(s), and Outcomes in a European Joint Clinical Health Technology Assessment. Value Health 2024, 27, 1722–1731. [Google Scholar] [CrossRef]
- Hollard, D.; Roberts, G.; Taylor, I.; Gibson, J.; Darlington, O. HTA77 PICO Consolidation in European HTA Scoping: Examining PICO Variations in Oncology Drugs in the Context of the European Joint Clinical Assessment. Value Health 2024, 27, S258. [Google Scholar] [CrossRef]
- Chirico, G.; Boland, L.; Foxon, G.; Craddy, P. HTA228 Can Just Three PICOs be Feasible for Oncology Assessments with the Joint EU HTA Framework, Whilst Considering All 27 Member States Specificities? Value Health 2023, 26, S363. [Google Scholar] [CrossRef]
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG). Allgemeine Methoden. Version 7.0 vom 19. September 2023. Available online: https://www.iqwig.de/methoden/allgemeine-methoden_version-7-0.pdf (accessed on 26 March 2025).
- Gemeinsamer Bundesausschuss (G-BA). Verfahrensordnung des Gemeinsamen Bundesausschusses. Available online: https://www.g-ba.de/richtlinien/42/ (accessed on 26 March 2025).
- Zorginstituut Nederland (ZIN). Publicaties (Engl. Publications). Available online: https://www.zorginstituutnederland.nl/publicaties (accessed on 26 March 2025).
- Gemeinsamer Bundesausschuss (G-BA). Formulare und Vorgaben zum Download—Anlagen zum 5. Kapitel der Verfahrensordnung (Engl. Forms and Templates for Download—Attachments to Chapter 5 of the Rules of Procedure). Available online: https://www.g-ba.de/themen/arzneimittel/arzneimittel-richtlinie-anlagen/nutzenbewertung-35a/informationen-fuer-unternehmen/formulare-und-vorgaben/ (accessed on 26 March 2025).
- Austrian Institute for Health Technology Assessment GmbH. AIHTA—Publications. Available online: https://aihta.at/page/publikationen/en (accessed on 26 March 2025).
- Austrian Institute for Health Technology Assessment GmbH. Methodenhandbuch (Engl. Methodology Handbook). Available online: https://aihta.at/page/publikationen/en (accessed on 26 March 2025).
- Austrian Federal Office for Safety in Health Care. Austrian Medicinal Product Index—Online Search for Medicinal Products. Available online: https://aspregister.basg.gv.at/aspregister/faces/aspregister.jspx (accessed on 26 March 2025).
- Tumorzentrum Oberösterreich. Leitlinien (Engl. Guidelines). Available online: https://www.tumorzentrum.at/leitlinien (accessed on 26 March 2025).
- Belgian Health Care Knowledge Centre (KCE). All Reports. Available online: https://kce.fgov.be/en/publications/all-reports-0 (accessed on 26 March 2025).
- Rijksinstituut Voor Ziekte- en Invaliditeitsverzekering (RIZIV). Evaluatierapporten CTG en Beslissingen Minister (Engl. Evaluation Reports CTG and Ministerial Decisions). Available online: https://webappsa.riziv-inami.fgov.be/ssp/Publications (accessed on 26 March 2025).
- Irina Cleemput, M.N.; van de Sande, S.; Thiry, N. Belgian Guidelines for Economic Evaluations and Budget Impact Analyses: Second Edition. Available online: https://kce.fgov.be/sites/default/files/2021-12/KCE_183_economic_evaluations_second_edition_Report_update.pdf (accessed on 26 March 2025).
- Belgian Health Care Knowledge Centre (KCE). Methodological Approaches. Available online: https://processbook.kce.be/methodological-approaches (accessed on 26 March 2025).
- Cleemput, I.; Van Wilder, P.; Vrijens, F.; Huybrechts, M.; Ramaekers, D. Guidelines for Pharmacoeconomic Evaluations in Belgium. Available online: https://kce.fgov.be/sites/default/files/2021-12/d20081027327.pdf (accessed on 26 March 2025).
- Rijksinstituut Voor Ziekte- en Invaliditeitsverzekering (RIZIV). Farmaceutische Industrie (Engl. Pharmaceutical Industry). Available online: https://www.riziv.fgov.be/nl/thema-s/verzorging-kosten-en-terugbetaling/wat-het-ziekenfonds-terugbetaalt/geneesmiddelen/farmaceutische-industrie#richtlijnen-voor-het-indienen-van-ctg-dossiers (accessed on 26 March 2025).
- Rijksinstituut Voor Ziekte- en Invaliditeitsverzekering (RIZIV). Vergoedbare Geneesmiddelen en Radio-Farmaceutische Producten (Engl. Reimbursable Medicines and Radiopharmaceutical Products). Available online: https://webappsa.riziv-inami.fgov.be/ssp/ProductSearch (accessed on 26 March 2025).
- Belgian Board of Oncology. Clinical Guidelines. Available online: https://collegeoncologie.be/clinical-guidelines/ (accessed on 26 March 2025).
- Republic of Bulgaria—National Council On Prices and Reimbursement of Medicinal Products. Пoлoжителнo решение за Включване на Лекарствен прoдукт, Принадлежащ към Нoвo Междунарoднo Непатентнo Наименoвание (INN) (Engl. Positive Solution for the Inclusion of a Medicinal Product Belonging to a New International Non-Proprietary Name (INN)). Available online: https://www.ncpr.bg/bg/%D1%81-%D0%BF%D0%BE%D0%BB%D0%BE%D0%B6%D0%B8%D1%82%D0%B5%D0%BB%D0%BD%D0%BE-%D1%80%D0%B5%D1%88%D0%B5%D0%BD%D0%B8%D0%B5-%D0%B7%D0%B0-%D0%BD%D0%BE%D0%B2-inn.html (accessed on 26 March 2025).
- Republic of Bulgaria—National Council On Prices and Reimbursement of Medicinal Products. Отрицателнo Решение за Включване на Лекарствен Прoдукт, Принадлежащ към Нoвo Междунарoднo Непатентнo Наименoвание (INN) (Engl. Negative Decision to Include a Medicinal Product Belonging to a New International Non-Proprietary Name (INN)). Available online: https://www.ncpr.bg/bg/%D1%81-%D0%BE%D1%82%D1%80%D0%B8%D1%86%D0%B0%D1%82%D0%B5%D0%BB%D0%BD%D0%BE-%D1%80%D0%B5%D1%88%D0%B5%D0%BD%D0%B8%D0%B5-%D0%B7%D0%B0-%D0%BD%D0%BE%D0%B2-inn.html (accessed on 26 March 2025).
- Republic of Bulgaria—National Council On Prices and Reimbursement of Medicinal Products. Methodological Recommendations for Presented Documentation for Assessment of the Efficacy, Safety, and Pharmacoeconomic Parameters of Medicinal Products Applying for Inclusion in the Positive Drug List. Available online: https://ncpr.bg/images/News/Ukazania_EN.pdf (accessed on 26 March 2025).
- Republic of Bulgaria—National Council on Prices And Reimbursement of Medicinal Products. Ordinance on Terms, Rules and Procedure for Regulation and Registration of Prices for Medicinal Products. Available online: https://ncpr.bg/images/REGULATIONS/NUPRRRCLP_EN.pdf (accessed on 26 March 2025).
- National Health Insurance Fund. Списъци с лекарствени прoдукти (Engl. List of Medicinal Products). Available online: https://www.nhif.bg/bg/medicine_food/medical-list/2024 (accessed on 26 March 2025).
- Фoндация МОРЕ—ДАРЗАЛАС. Всички (Engl. the Guides). Available online: https://conference.more-darzalas.com/dokumenti/ (accessed on 26 March 2025).
- MOPE e-Guidelines. МОРЕ Ръкoвoдства (Engl. SEA Guides). Available online: https://app-eguidelines.more-darzalas.com/home (accessed on 26 March 2025).
- Republika Hrvatska Ministarstvo Zdravstva. Baza Procjena Zdravstvenih Tehnologija (Health Technology Assessment Database). Available online: https://zdravlje.gov.hr/o-ministarstvu/djelokrug-1297/kvaliteta-zdravstvene-zastite-6579/procjena-zdravstvenih-tehnologija-6580/baza-procjena-zdravstvenih-tehnologija-6583/6583 (accessed on 26 March 2025).
- Halmed Agency for Medicinal Products and Medical Devices of Croatia. Medicinal Products Database. Available online: https://www.halmed.hr/en/Lijekovi/Baza-lijekova/ (accessed on 26 March 2025).
- Pharmaceutical Services Ministry of Health. Medicinal Products Price List. Available online: https://www.moh.gov.cy/Moh/phs/phs.nsf/All/A20C974C631B250AC2258B43001F0BB7?OpenDocument (accessed on 26 March 2025).
- State Institute for Drug Control (SULK). Postup Klinického Hodnocení Léčivých Přípravků/PZLÚ pro Účely Úhradové Regulace—Obecné Principy (Engl. Clinical Evaluation Procedure for Medicinal Products/PZLÚ for the Purpose of Reimbursement Regulation—General Principles). Available online: https://sukl.gov.cz/pokyny-stanoveni-cen-a-uhrad-leciv/cau-13/ (accessed on 26 March 2025).
- Ministerstvo Zdravotnictví České Republiky. Manuál pro Žadatele (Engl. Manual for Applicants). Available online: https://www.google.com/url?client=internal-element-cse&cx=b631493ef79544c36&q=https://mzd.gov.cz/wp-content/uploads/wepub/7981/18162/P%25C5%2599%25C3%25ADloha%2520%25C4%258D.%25202%2520Manu%25C3%25A1l%2520pro%2520%25C5%25BDADATELE_listopad.doc&sa=U&ved=2ahUKEwiI9aWzk_CIAxVW8bsIHQVGA-oQFnoECAcQAQ&usg=AOvVaw0wMgSMixt6PsOj2lSK5VMX&arm=e (accessed on 26 March 2025).
- State Institute for Drug Control (SULK). Database of Medicinal Products. Available online: https://prehledy.sukl.cz/prehled_leciv.html#/ (accessed on 26 March 2025).
- Czech Society for Oncology. Modrá Kniha (Engl. Blue Book). Available online: https://www.linkos.cz/lekar-a-multidisciplinarni-tym/personalizovana-onkologie/modra-kniha-cos/aktualni-vydani-modre-knihy/ (accessed on 26 March 2025).
- Česká Hematologická Spolecnost ČLS JEP. Červená Kniha—Léčebné Postupy v Hematologii (Engl. Red Book—Therapeutic Procedures in Haematology). Available online: https://www.hematology.cz/cervena-kniha-lecebne-postupy-v-hematologii/ (accessed on 26 March 2025).
- Medicinrådet. Anbefalinger og Vejledninger (Engl. Recommendations and Guidelines). Available online: https://medicinraadet.dk/anbefalinger-og-vejledninger?page=&order=&take=¤tpageid=1095&database=1095&secondary=&q=&period=0 (accessed on 26 March 2025).
- Medicinrådet. Ansøgningsproces for Nye Lægemidler og Indikationsudvidelser (Engl. Application Process for New Medicinal Products and Indication Extensions). Available online: https://medicinraadet.dk/ansogning (accessed on 26 March 2025).
- Behandlingsrådet. About the Evaluations. Available online: https://behandlingsraadet.dk/in-english/about-the-evaluations (accessed on 26 March 2025).
- Retsinformation. Guidance on the Preparation of Health Economic Analyses of Medicinal Products. Available online: https://www.retsinformation.dk/eli/retsinfo/2018/9153 (accessed on 26 March 2025).
- Medicinrådet. Ansøgningsskema (Engl. Application). Available online: https://medicinraadet.dk/ansogning/ansogningsskema (accessed on 26 March 2025).
- Lægemiddelstyrelsen Danish Medicines Agency. Medicinpriser.dk. Available online: https://www.medicinpriser.dk/default.aspx?lng=2 (accessed on 26 March 2025).
- Sundhedsstyrelsen. Om Nationale Kliniske Anbefalinger og Retningslinjer (Engl. About National Clinical Recommendations and Guidelines). Available online: https://www.sst.dk/da/Fagperson/Retningslinjer-og-procedurer/NKA-og-NKR/Om-NKA-og-NKR (accessed on 26 March 2025).
- University of Tartu Institute of Family Medicine and Public Health. HTA Reports. Available online: https://tervis.ut.ee/en/node/137759 (accessed on 26 March 2025).
- University of Tartu Institute of Family Medicine and Public Health. Metoodika (Engl. Methods). Available online: https://tervis.ut.ee/et/tervisetehnoloogiate-hindamine/metoodika (accessed on 26 March 2025).
- Riigi Teataja. Procedure for Drafting and Amendment of a List of Medicinal Products of the Estonian Health Insurance Fund and the Content of Criteria for Establishing the List and Evaluators of Compliance with the Criteria, and Establishment and Rules of Procedure of a Medicinal Products Committee. Annex. Available online: https://www.riigiteataja.ee/en/eli/501032018001/consolide (accessed on 26 March 2025).
- Republic of Estonia Agency of Medicines. Register of Medicinal Products. Available online: https://ravimiregister.ee/en/default.aspx?pv=HumRavimid.Otsing (accessed on 26 March 2025).
- Finnish Medicines Agency (Fimea). Arviointiraportit (Assessment Reports). Available online: https://fimea.fi/kehittaminen/hoidollinen_ja_taloudellinen_arvo/arvioinnit (accessed on 26 March 2025).
- Pharmaceuticals Pricing Board Finland. Application Forms and Instructions. Available online: https://www.hila.fi/en/applying-and-notifications/application-forms-and-instructions-2/ (accessed on 26 March 2025).
- Finnish Medicines Agency (Fimea). Assessment of New Hospital-Only Medicinal Products. Available online: https://fimea.fi/documents/147152901/159465524/Sairaalal%C3%A4%C3%A4kkeiden+arviointiprosessi+2024_EN.pdf/47dd5089-b1e3-cc35-fb42-77b0bf05c6a7/Sairaalal%C3%A4%C3%A4kkeiden+arviointiprosessi+2024_EN.pdf?t=1724759445588 (accessed on 26 March 2025).
- Kansaneläkelaitos Kela (The Social Insurance Institution). Medicinal Products Database. Available online: https://asiointi.kela.fi/laakekys_app/LaakekysApplication?kieli=fi (accessed on 26 March 2025).
- The Finnish Medical Society Duodecim. Suositukset (Engl. Recommendations). Available online: https://www.kaypahoito.fi/suositukset (accessed on 26 March 2025).
- Haute Autorité de Santé (HAS). Avis et Décisions sur les Médicaments (Engl. Opinions and Decisions on Medicines). Available online: https://www.has-sante.fr/jcms/ (accessed on 26 March 2025).
- Haute autorité de santé (HAS). Soumission d’une Demande Auprès de la Commission de la Transparence (Engl. Submission of an Application to the Transparency Commission). Available online: https://www.has-sante.fr/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=c_1280596 (accessed on 26 March 2025).
- Haute Autorité de Santé (HAS). Doctrine de la Commission de la Transparence (Engl. Transparency Committee Doctrine). Available online: https://www.has-sante.fr/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=p_3243812 (accessed on 26 March 2025).
- Haute Autorité de Santé (HAS). Matrice de Dossier Type (Engl. Template). Available online: https://www.has-sante.fr/jcms/c_1280594/ (accessed on 26 March 2025).
- Haute Autorité de Santé (HAS). Actualités (Engl. News). Available online: https://www.has-sante.fr/jcms/fc_2874902/en/actualites (accessed on 26 March 2025).
- Haute Autorité de Santé (HAS). Toutes nos Publications par Thèmes (Engl. All Our Publications by Theme). Available online: https://www.has-sante.fr/jcms/fc_2875208/fr/rechercher-une-recommandation-un-avis?histstate=1 (accessed on 26 March 2025).
- Gemeinsamer Bundesausschuss (G-BA). Beschlüsse des Gemeinsamen Bundesausschusses. Available online: https://www.g-ba.de/beschluesse/ (accessed on 26 March 2025).
- Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Suche (Engl. Search). Available online: https://portal.dimdi.de/amguifree/am/search.xhtml (accessed on 26 March 2025).
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. (AWMF) (Engl. Association of the Scientific Medical Societies in Germany). Publikation Medizinischer Leitlinien. Offizielle Leitlinien der AWMF (Engl. Publication of Medical Guidelines. Official Guidelines of the AWMF). Available online: https://www.awmf.org/leitlinien (accessed on 26 March 2025).
- Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e.V. (DGHO) (Engl. German Society for Hematology and Medical Oncology). Onkopedia Leitlinien (Engl. Onkopedia Guidelines). Available online: https://www.onkopedia.com/de/onkopedia/guidelines (accessed on 26 March 2025).
- ΕΦHΜΕΡΙ∆A ΤHΣ ΚΥΒΕΡΝHΣΕΩΣ ΤHΣ ΕΛΛHΝΙΚHΣ ∆HΜOΚΡAΤΙA (Engl. Gazette of the Government of the Hellenic Republic). AΠOΦAΣΕΙΣ Aριθμ. οικ. 52029 (Engl. Decisions No. Int. 52029). Available online: https://www.kodiko.gr/nomologia/download_fek?f=fek/2018/b/fek_b_2768_2018.pdf&t=f918dd306ce8231eba387cd1d2b17451 (accessed on 26 March 2025).
- National Organization for Medicines. Search Human Product. Available online: https://services.eof.gr/human-search/home.xhtml?lang=en (accessed on 26 March 2025).
- Főosztály NNéGKT-é. Ajánlások (Engl. Recommendations). Available online: https://ogyei.gov.hu/ajanlasok/ (accessed on 26 March 2025).
- Nemzeti Népegészségügyi és Gyógyszerészeti Központ Technológia-értékelő Főosztály (Engl. National Center for Public Health and Pharmacy Technology Assessment Department). Strukturált KérelmezőiSablon (Engl. Structured Request Template). Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fogyei.gov.hu%2Fdynamic%2Fgyogyszer_markaneve_indikacio_rovidites_datum.docx (accessed on 26 March 2025).
- Alapkezelő, N.E. Data for International Price Comparisons. Available online: https://www.neak.gov.hu/felso_menu/szakmai_oldalak/gyogyszer_segedeszkoz_gyogyfurdo_tamogatas/egeszsegugyi_vallalkozasoknak/gyartok_forgalomba_hozok/dipc (accessed on 26 March 2025).
- Nemzeti Egészségbiztosítási Alapkezelő. A Szakmai Irányelvek Nyilvántartása (Engl. The Register of Professional Guidelines). Available online: http://www.neak.gov.hu/felso_menu/szakmai_oldalak/szakmai_iranyelvek/szakmai_iranyelvek (accessed on 26 March 2025).
- Health Information and Quality Authority. Health Technology Assessments. Available online: https://www.hiqa.ie/reports-and-publications/health-technology-assessments (accessed on 26 March 2025).
- National Centre for Pharmacoeconomics, Ireland. Drugs. Available online: https://www.ncpe.ie/category/drugs/ (accessed on 26 March 2025).[Green Version]
- Health Information and Quality Authority. Health Technology Assessments-Guidelines/Guidance. Available online: https://www.hiqa.ie/reports-and-publications/health-technology-assessments?tid_1=All&field_hta_topics_target_id=66&field_covid_19_topics_target_id=All&keys= (accessed on 26 March 2025).
- National Centre for Pharmacoeconomics Ireland. Submission Templates. Available online: https://www.ncpe.ie/submission-process/submission-templates/ (accessed on 26 March 2025).
- Health Service Executive. Reimbursable Items—Medicines and Aids Provided. Available online: https://www.hse.ie/eng/staff/pcrs/items/ (accessed on 26 March 2025).
- Health Service Executive. National Clinical Guidelines. Available online: https://www.hse.ie/eng/services/list/5/cancer/profinfo/guidelines/ (accessed on 26 March 2025).
- Serlyfjaskrá. Sérlyfjaskrá Inniheldur Upplýsingar um Öll Lyf Sem Eru Markaðssett á Íslandi (Engl. The Icelandic Medicines Registry Contains Information on All Medicines Marketed in Iceland). Available online: https://www.serlyfjaskra.is/ (accessed on 26 March 2025).
- Island.is. Clinical Guidelines. Available online: https://island.is/en/clinical-guidelines (accessed on 26 March 2025).
- Italian Medicines Agency (AIFA). Report Tecnico-Scientifici per Specialità Medicinale (Engl. Technical Scientific Reports for Medicinal Product). Available online: https://www.aifa.gov.it/en/report-tecnico-scientifici (accessed on 26 March 2025).
- Italian Medicines Agency (AIFA). Domanda di Rimborsabilità e Prezzo (Engl. Application for Reimbursement and Pricing). Available online: https://www.aifa.gov.it/en/domanda-rimborsabilita-e-prezzo (accessed on 26 March 2025).
- Cabina di Regia/Tavolo Innovazione—Programma Nazionale di HTA Dispositivi Medici. Sotto Gruppo 1 (SG1)—GDL2 “Metodi, Formazione e Comunicazione” (Engl. Sub-Group 1 (SG1)—GDL2 “Methods, Training and Communication”). Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2855_ulterioriallegati_ulterioreallegato_0_alleg.pdf (accessed on 26 March 2025).
- Italian Medicines Agency (AIFA). Lists of Class A and Class H Medicinal Products. Available online: https://www.aifa.gov.it/liste-farmaci-a-h (accessed on 26 March 2025).
- Associazione Italiana di Oncologia Medica (AIOM). Lineee Guida (Engl. Guidelines). Available online: https://www.aiom.it/linee-guida-aiom/ (accessed on 26 March 2025).
- State Agency of Medicines Republic of Latvia. Zāļu Izmaksu Efektivitātes Novērtēšana (Engl. Evaluation of the Cost-Effectiveness of the Drug). Available online: https://www.zva.gov.lv/lv/industrijai/zalu-registracijas-apliecibu-ipasnieki/zalu-izmaksu-efektivitates-novertesana (accessed on 26 March 2025).
- State Agency of Medicines Republic of Latvia. State Agency of Medicines Republic of Latvia. Available online: https://dati.zva.gov.lv/zalu-registrs/en (accessed on 26 March 2025).
- Nacionālais Veselības Dienests. Meklēšanas Rezultāts—Vēža Vadlīnijas (Engl. Search Results—Caner Guidelines). Available online: https://www.vmnvd.gov.lv/lv/search?q=v%C4%93%C5%BEa%20vadl%C4%Abnijas (accessed on 26 March 2025).
- State Medicines Control Agency of Lithuania. Information to the Applicants. Available online: https://vvkt.lrv.lt/en/health-technology-assessment/information-to-the-applicants/ (accessed on 26 March 2025).
- Valstybinė Vaistų Kontrolės Tarnyba. Medicines Search. Available online: https://vapris.vvkt.lt/vvkt-web/public/medications?lang=en# (accessed on 26 March 2025).
- Caisse Nationale de Santé (CNS). Médicaments (Engl. Pharmaceuticals). Available online: https://cns.public.lu/fr/professionnels-sante/dossiers-thematiques/medicaments-et-dispositifs/medicaments.html (accessed on 26 March 2025).
- Institut National du Cancer. Guidelines & Position Papers. Available online: https://institutnationalducancer.lu/events-publications/#guidelines (accessed on 26 March 2025).
- Directorate for Pharmaceutical Affairs (DPA). Application to the Superintendent of Public Health for the Consideration of a Medicinal Product to Be Covered by the Government Formulary List as per the Government Health Services (Medicinal Products) Regulations. 2009. Available online: https://pharmaceuticalaffairs.gov.mt/wp-content/uploads/2024/03/T01_form.docx (accessed on 26 March 2025).
- Government of Malta. The Government Formulary List. Available online: https://pharmaceuticalaffairs.gov.mt/en/resources/the-government-formulary-list/ (accessed on 26 March 2025).
- Direktoratet for Medisinske Produkter. Fullførte Metodevurderinger for Legemidler (Engl. Completed Health Technology Assessments for Medicinal Products). Available online: https://www.dmp.no/offentlig-finansiering/metodevurdering-av-medisinske-produkter/metodevurdering-av-legemidler/fullforte-metodevurderinger-for-legemidler (accessed on 26 March 2025).
- Direktoratet for Medisinske Produkter. Template for Submission of Documentation for the Single Technology Assessment of Pharmaceuticals. Available online: https://www.dmp.no/en/public-funding-and-pricing/health-technology-assessments/medicines/submission-of-documentation-for-single-technology-assessment-of-pharmaceuticals/template-for-submission-of-documentation-for-the-single-technology-assessment-of-pharmaceuticals (accessed on 26 March 2025).
- Direktoratet for Medisinske Produkter. Legemiddelsøk (Engl. Drug Search). Available online: https://www.legemiddelsok.no/ (accessed on 26 March 2025).
- Helsedirektoratet. Nasjonale Anbefalinger, råd, Pakkeforløp og Pasientforløp (Engl. National Recommendations, Advice, Care Pathways, and Patient Pathways). Available online: https://www.helsedirektoratet.no/produkter?tema=retningslinje (accessed on 26 March 2025).
- Agency for Health Technology Assessment and Tariff System (AOTMiT). News. Available online: https://www.aotm.gov.pl/en/aktualnosci/ (accessed on 26 March 2025).
- Agency for Health Technology Assessment and Tariff System (AOTMiT). Guidelines. Available online: https://www.aotm.gov.pl/en/guidelines/ (accessed on 26 March 2025).
- Rejestr Prduktów Leczniczych (RPL). Znajdź Produkt Leczniczy (Engl. Find a Medicinal Product). Available online: https://rejestry.ezdrowie.gov.pl/rpl/search/public (accessed on 26 March 2025).
- Polskie Towarzystwo Onkologii Klinicznej. Aktualne Zalecenia i Standardy (Engl. Current Recommendations and Standards). Available online: https://ptok.pl/aktualne-zalecenia-i-standardy (accessed on 26 March 2025).
- Infarmed. Lista de Novas DCI/Indicações Terapêuticas com Financiamento Público (Engl. List of New DCI/Therapeutic Indications with Public Funding). Available online: https://www.infarmed.pt/web/infarmed/relatorios-de-avaliacao-de-financiamento-publico (accessed on 26 March 2025).
- Vinhas, J.; Dias, S.; Gouveia, A.M.; Correia, A.; Dias, C.V.; Sousa, D.; Oliveira, J.; Perelman, J.; Azevedo, L.; Marques, N.; et al. Alex Correia, Sara Couto Methodology for Pharmacotherapeutic Assessment of Health Technologies. Available online: https://www.infarmed.pt/documents/15786/1963929/METOD_AFT_v3.0_ENvf_fev2023/b0cb1c54-adca-721a-6466-75ba04cdd542 (accessed on 26 March 2025).
- Infomed. Human Medicinal Products Database. Available online: https://extranet.infarmed.pt/INFOMED-fo/index.xhtml (accessed on 26 March 2025).
- National Agency of Medicines and Medical Devices (NAMMDR). Rapoarte de evaluare a tehnologiilor medicale (Engl. Health Technology Assessment Reports). Available online: https://www.anm.ro/medicamente-de-uz-uman/evaluare-tehnologii-medicale/rapoarte-de-evaluare-a-tehnologiilor-medicale/ (accessed on 26 March 2025).
- National Agency of Medicines and Medical Devices (NAMMDR). Orders of the Minister of Health—Medicines for Human Use. Available online: https://www.anm.ro/en/medicamente-de-uz-uman/legislatie/ordine-de-ministru/ (accessed on 26 March 2025).
- National Agency of Medicines and Medical Devices (NAMMDR). Forms and Tariffs—Medicines for Human Use. Available online: https://www.anm.ro/en/medicamente-de-uz-uman/formulare-si-tarife/ (accessed on 26 March 2025).
- National Agency of Medicines and Medical Devices (NAMMDR). Lista Medicamentelor din Nomenclator (Engl. List of Medicines in the Nomenclature). Available online: https://nomenclator.anm.ro/medicamente (accessed on 26 March 2025).
- National Institute for Value and Technologies in Healthcare (NIHO). Published Projects. Available online: https://niho.sk/en/publikovane-projekty/ (accessed on 26 March 2025).
- Ministerstvo Zdravotníctva Slovenskej Republiky. Dokumenty—Kategorizácia Liekov (Engl. Documents—Categorization of Medicinal Products). Available online: https://www.health.gov.sk/?kategorizacia-liekov-1 (accessed on 26 March 2025).
- Ministerstvo Zdravotníctva Slovenskej Republiky. Zoznam Kategorizovaných Liekov (Engl. List of Categorized Drugs). Available online: https://www.health.gov.sk/?zoznam-kategorizovanych-liekov (accessed on 26 March 2025).
- State Institute for Drug Control (SUKL). Medicine Search. Available online: https://www.sukl.sk/hlavna-stranka/english-version?page_id=256 (accessed on 26 March 2025).
- Centralna Baza Zdravil. Iskanje Podatkov (Engl. Search for Data). Available online: http://www.cbz.si/cbz/bazazdr2.nsf/Search/$searchForm?SearchView (accessed on 26 March 2025).
- Institute of Oncology Ljubljana. Priporočila in Klinične Poti (Engl. Recommendations and Clinical Pathways). Available online: https://www.onko-i.si/priporocila (accessed on 26 March 2025).
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Informes de Posicionamiento Terapéutico (Engl. Therapeutic Positioning Reports). Available online: https://www.aemps.gob.es/medicamentos-de-uso-humano/informes-de-posicionamiento-terapeutico/# (accessed on 26 March 2025).
- Ministerio de Sanidad. Guía de Evaluación Económica de Medicamentos (Engl. Economic Evaluation Guide of Medicines). Available online: https://www.sanidad.gob.es/areas/farmacia/comitesAdscritos/prestacionFarmaceutica/docs/20240227_CAPF_Guia_EE_definitiva.pdf (accessed on 26 March 2025).
- Ministerio de Sanidad. BIFIMED: Buscador de la Información Sobre la Situación de Financiación de los Medicamentos (Engl. BIFIMED: Search Engine for Information on the Financing Situation of Medicines). Available online: https://www.sanidad.gob.es/profesionales/medicamentos.do (accessed on 26 March 2025).
- Sociedad Española de Oncología Médica (SEOM). Guías Clínicas SEOM (Engl. SEOM Clinical Guidelines). Available online: https://seom.org/publicaciones/guias-clinicas/105418-guias-clinicas-seom (accessed on 26 March 2025).
- Tandvårds- och Läkemedelsförmånsverket. Beslut Läkemedel (Engl. Decisions on Medicines). Available online: https://www.tlv.se/beslut/beslut-lakemedel.html (accessed on 26 March 2025).
- Tandvårds- och Läkemedelsförmånsverket. Ny Handbok för Företag som Ansöker om Pris och Subvention för Förbrukningsartiklar (Engl. New Handbook for Companies When Applying for Reimbursement and Price of Medicines). Available online: https://www.tlv.se/press/nyheter/arkiv/2023-10-31-ny-handbok-for-foretag-som-ansoker-om-pris-och-subvention-for-forbrukningsartiklar.html (accessed on 26 March 2025).
- Tandvårds- och Läkemedelsförmånsverket. Allmänna råd (Engl. General Advice). Available online: https://www.tlv.se/om-tlv/regelverk/allmanna-rad.html (accessed on 26 March 2025).
- Tandvårds- och Läkemedelsförmånsverket (TLV). Apply for Reimbursement. Available online: https://www.tlv.se/in-english/medicines/apply-for-reimbursement.html (accessed on 26 March 2025).
- Tandvårds- och Läkemedelsförmånsverket. Sök Priser och Beslut i Databasen (Engl. Search for Prices and Decisions in the Database). Available online: https://www.tlv.se/beslut/sok-priser-och-beslut-i-databasen.html (accessed on 26 March 2025).
- cancercentrum.se. Nationella Vårdprogram (Engl. National Care Program). Available online: https://kunskapsbanken.cancercentrum.se/vardprogram/ (accessed on 26 March 2025).
- Socialstyrelsen. Sök Nationella Riktlinjer (Engl. Search National Guidelines). Available online: https://www.socialstyrelsen.se/kunskapsstod-och-regler/regler-och-riktlinjer/nationella-riktlinjer/riktlinjer-och-utvarderingar/ (accessed on 26 March 2025).
- Janusinfo Region Stockholm. Kloka Listan 2024 (Engl. The Wise List 2024). Available online: https://klokalistan.se/ (accessed on 26 March 2025).
- Zorginstituut Nederland (ZIN). Guideline for Economic Evaluations in Healthcare (2024 Version). Available online: https://english.zorginstituutnederland.nl/publications/reports/2024/01/16/guideline-for-economic-evaluations-in-healthcare (accessed on 26 March 2025).
- Zorginstituut Nederland (ZIN). Specialist Medicinal Products Assessment Procedure. Available online: https://english.zorginstituutnederland.nl/publications/reports/2020/05/11/specialist-medicinal-products-assessment-procedure (accessed on 26 March 2025).
- Zorginstituut Nederland (ZIN). Format Farmaco-Economisch Dossier (Volgens Richtlijn 2024) (Engl. Format Pharmaco-Economic Dossier (According to Guideline 2024)). Available online: https://www.zorginstituutnederland.nl/publicaties/publicatie/2025/02/14/format-farmaco-economisch-dossier (accessed on 26 March 2025).
- Zorginstituut Nederland (ZIN). Medicijnkosten.nl (Engl. Costs for Medicines). Available online: https://www.medicijnkosten.nl/ (accessed on 26 March 2025).
- CIBG Ministerie van Volksgezonheid Welzijn en Sport. Actueel Overzicht Sluismiddelen 2024 (Engl. Current Overview of Medicines Under the Lock System 2024). Available online: https://www.farmatec.nl/documenten/publicaties/2024/12/30/actueel-overzicht-sluismiddelen-2024 (accessed on 26 March 2025).
- Federatie Medisch Specialisten. Richtlijnen A-Z (Engl. Guidelines A-Z). Available online: https://richtlijnendatabase.nl (accessed on 26 March 2025).
- Joint Nordic HTA-Bodies. Welcome to the Nordic Collaboration JNHB. Available online: https://jnhtabodies.org/ (accessed on 26 March 2025).
- Beneluxa Initiative on Pharmaceutical Policy. Beneluxa Initiative. Available online: https://beneluxa.org/ (accessed on 26 March 2025).
- Kalf, R.R.; Vreman, R.A.; Delnoij, D.M.; Bouvy, M.L.; Goettsch, W.G. Bridging the gap: Can International Consortium of Health Outcomes Measurement standard sets align outcomes accepted for regulatory and health technology assessment decision-making of oncology medicines. Pharmacol. Res. Perspect. 2021, 9, e00742. [Google Scholar] [CrossRef]
- National Agency of Medicines and Medical Devices (NAMMDR). Order no. 1353 of 30 July 2020 on Amendment and Supplementation of Order of the Minister of Health no. 861/2014 on Ap-proval of Criteria and Methodology for Assessment of Health Technologies, of Documentation to Be Submitted by Applicants, Methodological Means Used in the Assessment for Inclusion, Extension of Indications, Non-Inclusion into or Exclusion from the List of International Non-Proprietary Names of on-Prescription Medicinal Products as Provided to Insurants, Irrespective of Personal Contribution, in the Frame of the Health Insurance System, as well as of International Non-Proprietary Names of Medicinal Products Provided in National Health Insurance Programs, as well as the Means for Appeal Thereof. Available online: https://www.anm.ro/en/_/ORDINE/Order%20of%20the%20Minister%20of%20Health%20no.%201353_30%20july%202020.pdf (accessed on 26 March 2025).
- Pharmaceuticals pricing board Finland. Preparing a Health Economic Evaluation to Be Attached to the Application for Reimbursement Status and Wholesale Price for a Medical Product. Available online: https://www.hila.fi/content/uploads/2024/02/Instructions_TTS_280325.pdf (accessed on 10 June 2025).
- Angelis, A.; Lange, A.; Kanavos, P. Using health technology assessment to assess the value of new medicines: Results of a systematic review and expert consultation across eight European countries. Eur. J. Health Econ. 2018, 19, 123–152. [Google Scholar] [CrossRef]
- Wolters, S.; de Jong, L.A.; Jansen, C.; Jansman, F.G.; Postma, M.J. Differences in evidentiary requirements for oncology drug effectiveness assessments among six European health technology assessment bodies—Can alignment be improved? Expert Rev. Pharmacoecon. Outcomes Res. 2024, 24, 251–265. [Google Scholar] [CrossRef]
- Wolters, S.; Jansman, F.G.; Postma, M.J. Differences in evidentiary requirements between European Medicines Agency and European health technology assessment of oncology drugs—Can alignment be enhanced? Value Health 2022, 25, 1958–1966. [Google Scholar] [CrossRef]
- Zamora, B.; Maignen, F.; O’Neill, P.; Mestre-Ferrandiz, J.; Garau, M. Comparing access to orphan medicinal products in Europe. Orphanet J. Rare Dis. 2019, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Armoiry, X.; Späth, H.-M.; Henaine, A.-M.; Dussart, C.; Counsell, C.; Connock, M. Ocrelizumab not recommended in France for patients with primary progressive multiple sclerosis while recommended in England: A review comparing the assessment by HAS and NICE. Expert Opin. Biol. Ther. 2021, 21, 741–747. [Google Scholar] [CrossRef] [PubMed]
- de Pouvourville, G.; Cunningham, D.; Fricke, F.-U.; Lindgren, P.; Mantovani, L.; Murphy, L.A.; Solà-Morales, O.; Mestre-Ferrandiz, J.; Akehurst, R. Across-country variations of real-world data and evidence for drugs: A 5-European-country study. Value Health 2023, 26, 3–10. [Google Scholar] [CrossRef]
- Dóczy, V.; Sódar, B.W.; Hölgyesi, Á.; Merész, G.; Gaál, P. Development, testing, and implementation of a new procedure to assess the clinical added benefit of pharmaceuticals. Int. J. Technol. Assess. Health Care 2022, 38, e58. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, I.; Dintsios, C.-M. Confirmatory versus explorative endpoint analysis: Decision-making on the basis of evidence available from market authorization and early benefit assessment for oncology drugs. Health Policy 2018, 122, 599–606. [Google Scholar] [CrossRef]
- Ruof, J.; Knoerzer, D.; Dünne, A.-A.; Dintsios, C.-M.; Staab, T.; Schwartz, F.W. Analysis of endpoints used in marketing authorisations versus value assessments of oncology medicines in Germany. Health Policy 2014, 118, 242–254. [Google Scholar] [CrossRef][Green Version]
- Lipska, I.; Hoekman, J.; McAuslane, N.; Leufkens, H.G.; Hövels, A.M. Does conditional approval for new oncology drugs in Europe lead to differences in health technology assessment decisions? Clin. Pharmacol. Ther. 2015, 98, 489–491. [Google Scholar] [CrossRef]
- Tafuri, G.; Pagnini, M.; Moseley, J.; Massari, M.; Petavy, F.; Behring, A.; Catalan, A.; Gajraj, E.; Hedberg, N.; Obach, M.; et al. How aligned are the perspectives of EU regulators and HTA bodies? A comparative analysis of regulatory-HTA parallel scientific advice. Br. J. Clin. Pharmacol. 2016, 82, 965–973. [Google Scholar] [CrossRef]
- Medicinrådet. The Danish Medicines Council’s Process Guide for Assessing New Medicines. Available online: https://medicinraadet-classic.azureedge.net/media/pktfmij5/the-danish-medicines-council-s-process-guide-for-assessing-new-medicines-version-2-0.pdf (accessed on 26 March 2025).
- Ministerio de Sanidad. Real Decreto XXXXXXX/2024, de X de XXXXXX, por el que se Regula la Evaluación de Tecnologías Sanitarias (Royal Decree for Health Technology Assessment) (Draft). Available online: https://www.sanidad.gob.es/normativa/audiencia/docs/DG_54_24_Solicitud_informacion_publica_RD_EVALUACION_TECNOLOGIAS_SANITARIAS.pdf (accessed on 26 March 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Market Access Society. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).