- 2.5Impact Factor
- 5.5CiteScore
- 17 daysTime to First Decision
Novel Insight into Mechanisms of Bioactive Compounds and Its Use in the Prevention and Treatment of Thromboinflammation
This special issue belongs to the section “Applied Biosciences and Bioengineering“.
Special Issue Information
Dear Colleagues,
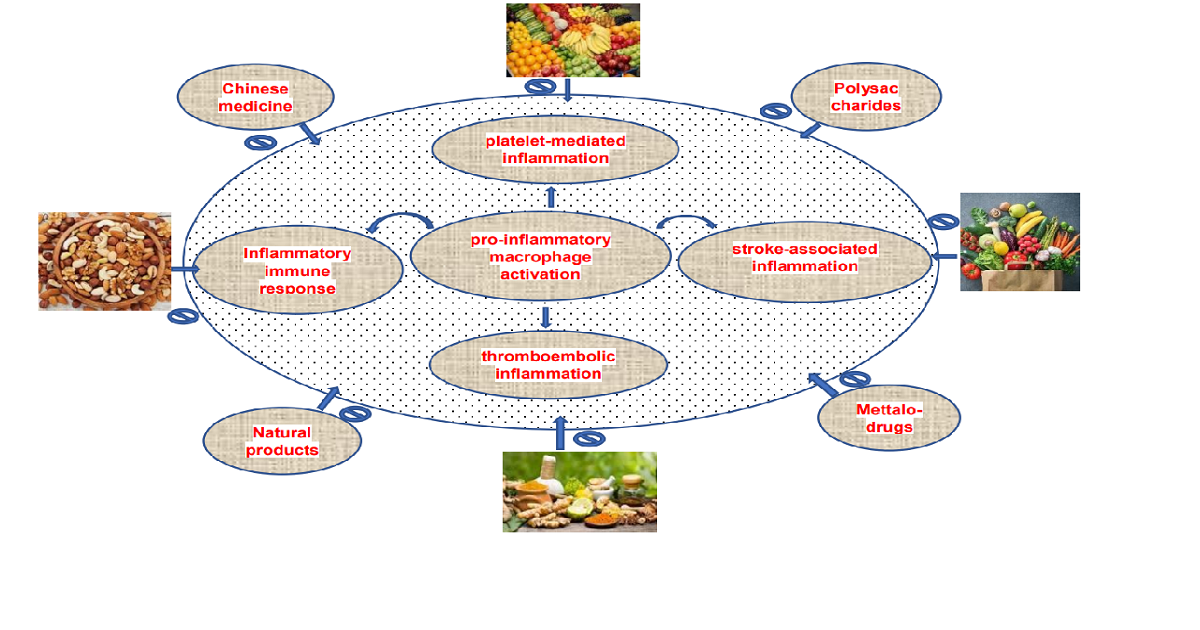
The close connection between inflammatory and thrombotic processes is presumed to have an evolutionary origin, as injuries need both an efficient hemostasis and an inflammatory immune response against entering pathogens. Behind vasoconstriction, platelets are the first immunomodulatory cells at the side of injury closing damaged blood vessels by aggregation and forming a thrombus. Thus, platelets promote inflammatory activity by an intimate crosstalk with leukocytes: in the case of vascular injury, neutrophils or monocytes are suggested to interact with endothelium-adherent platelets. Thus, platelets coordinate the inflammatory response by controlling the additional adhesion of innate immune cells to the inflamed endothelium, which is considered serious for the atherosclerotic disease process. For example, macrophage pro-inflammatory cytokine secretion is enhanced following interaction with activated platelets, proposing that the presence of activated platelets at sites of inflammation aggravates pro-inflammatory macrophage activation. The diversity of platelet receptors contributing to platelet interactions reveals various interesting targets within the context of platelet-mediated inflammation. Since a broad range of recent experimental methodologies indicate that platelets contribute to the pathogenesis of stroke-associated inflammation, the targeting of inhibiting mechanisms, which is involved in platelet activation, thromboembolism and its related inflammation is important to prevent thromboinflammatory diseases. Although many natural phytomedicine with potent anti-inflammatory properties have been noted as reasonable approaches for clinical trials, inadequate effort has been directed towards finding the molecular mechanisms underlying the therapeutic efficacy against thromboinflammatory events. Therefore, we invite authors to contribute their original research as well as review articles focused on understanding the molecular mechanisms of natural or synthetic bioactives on antithrombotic and inflammatory responses.
Potential topics include but are not limited to:
- Antiplatelet and antithrombotic mechanism of bioactive compounds;
- anti-inflammatory role and therapeutic mechanism of natural bioactives;
- the neuroprotective role of bioactive substances.
Prof. Dr. Joen-Rong Sheu
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Applied Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2400 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- bioactive compounds
- phytomedicines
- antiplatelet
- thromboinflammation
- neuroprotection
Benefits of Publishing in a Special Issue
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- Reprint: MDPI Books provides the opportunity to republish successful Special Issues in book format, both online and in print.


