Analysis of the Roles of the ISLR2 Gene in Regulating the Toxicity of Zearalenone Exposure in Porcine Intestinal Epithelial Cells
Abstract
:1. Introduction
2. Results
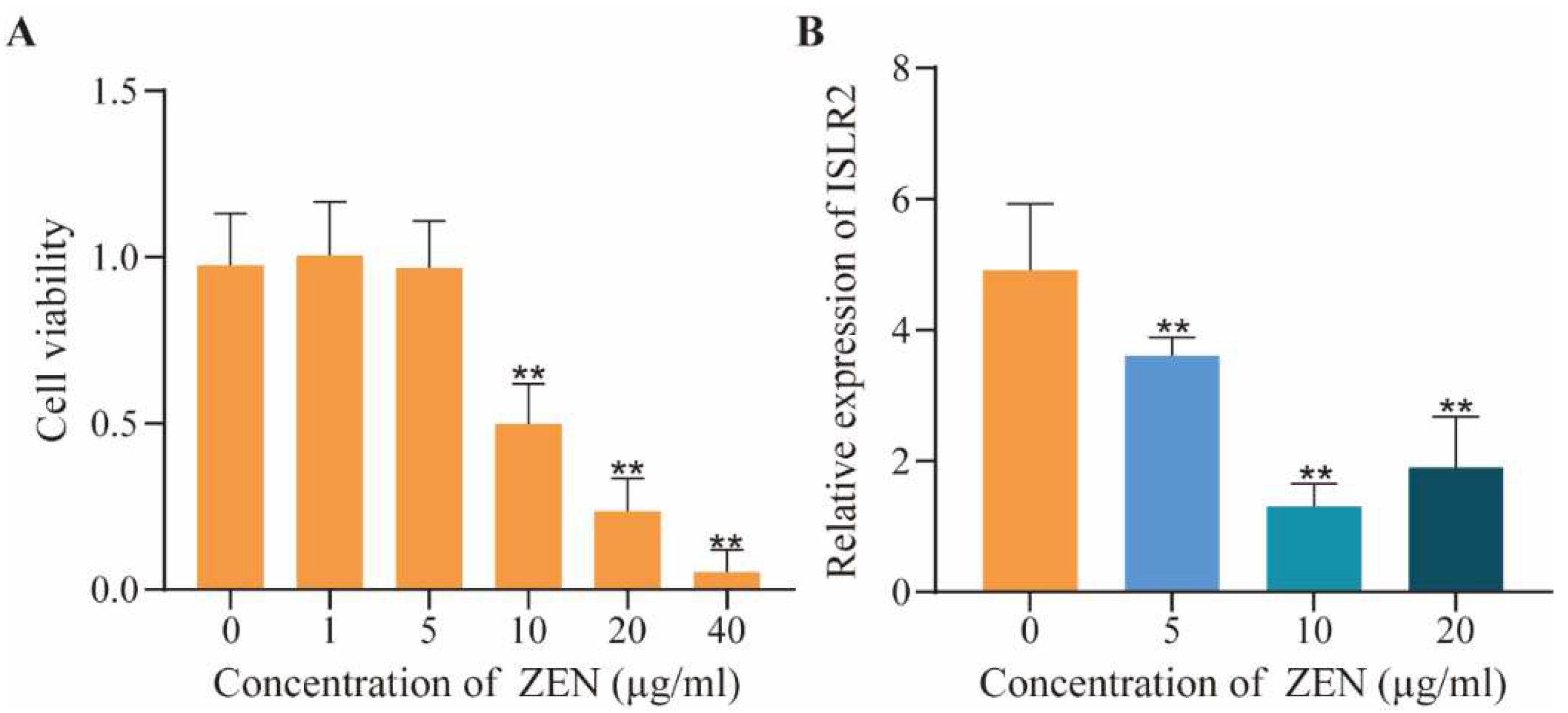
2.1. Dose-Effect of ZEN on Cell Viability and ISLR2 Expression
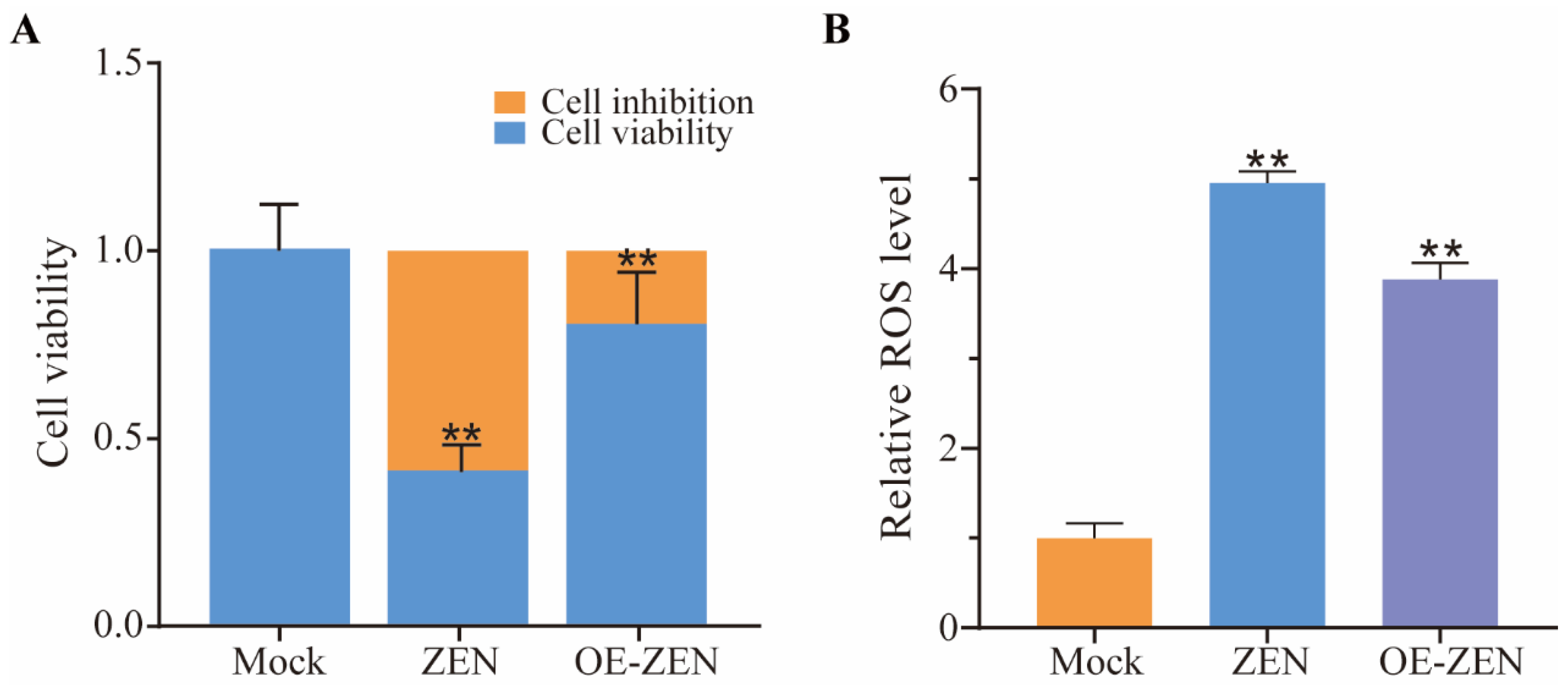
2.2. The Role of ISLR2 in ZEN Induced Cytotoxicity and Oxidative Stress
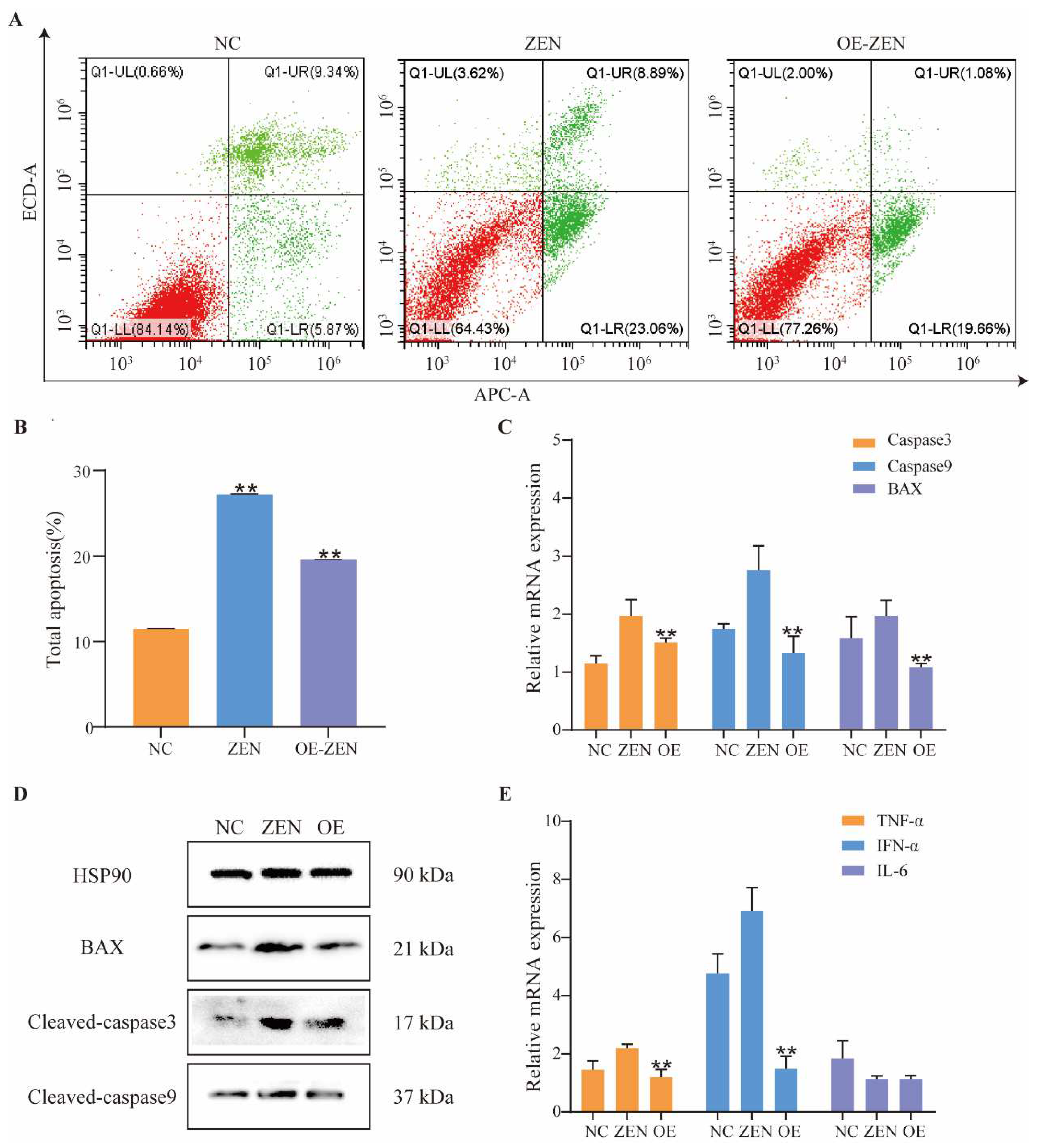
2.3. Role of ISLR2 in ZEN-Induced Cell Apoptosis and Inflammation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Construction of ISLR2 Overexpression Vector
5.2. Cell Culture
5.3. Cell Viability Assay
5.4. Cell Apoptosis Detection
5.5. Determination of Oxidative Stress Index
5.6. qRT-PCR
5.7. Western Blot Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abrunhosa, L.; Morales, H.; Soares, C.; Calado, T.; ila-Chã, A.S.; Pereira, M.; Venâncio, A. A Review of Mycotoxins in Food and Feed Products in Portugal and Estimation of Probable Daily Intakes. Crit. Rev. Food. Sci. Nutr. 2016, 56, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; Piva, A.; Ritieni, A. Dietary strategies to counteract the effects of mycotoxins: A review. J. Food. Prot. 2001, 64, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Micro. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Yumbe-Guevara, B.E.; Imoto, T.; Yoshizawa, T. Effects of heating procedures on deoxynivalenol, nivalenol and zearalenone levels in naturally contaminated barley and wheat. Food. Addit. Contam. 2003, 20, 1132–1140. [Google Scholar] [CrossRef]
- Cheli, F.; Pinotti, L.; Rossi, L.; Dell’Orto, V. Effect of milling procedures on mycotoxin distribution in wheat fractions: A review. LWT-Food Sci. Technol. 2013, 54, 307–314. [Google Scholar] [CrossRef]
- Heidari, S.; Milani, J.; Nazari, S.S. Effect of the bread-making process on zearalenone levels. Food Addit. Contam. Part A 2014, 31, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, J.; Zhang, T.; Gu, A.; Li, J.; Shan, A. The Antagonistic Effect of Glutamine on Zearalenone-Induced Apoptosis via PI3K/Akt Signaling Pathway in IPEC-J2 Cells. Toxins 2021, 13, 891. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Li, J.; Shan, A. DL-Selenomethionine Alleviates Oxidative Stress Induced by Zearalenone via Nrf2/Keap1 Signaling Pathway in IPEC-J2 Cells. Toxins 2021, 13, 557. [Google Scholar] [CrossRef]
- Shen, T.; Miao, Y.; Ding, C.; Fan, W.; Liu, S.; Lv, Y.; Gao, X.; De Boevre, M.; Yan, L.; Okoth, S.; et al. Activation of the p38/MAPK pathway regulates autophagy in response to the CYPOR-dependent oxidative stress induced by zearalenone in porcine intestinal epithelial cells. Food Chem. Toxicol. 2019, 131, 110527. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, J.; Ma, L.; Chen, J.; Xu, J.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Lycopene attenuates zearalenone-induced oxidative damage of piglet sertoli cells through the nuclear factor erythroid-2 related factor 2 signaling pathway. Ecotoxicol. Environ. Saf. 2021, 225, 112737. [Google Scholar] [CrossRef]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef]
- Mandai, K.; Guo, T.; St Hillaire, C.; Meabon, J.S.; Kanning, K.C.; Bothwell, M.; Ginty, D.D. LIG family receptor tyrosine kinase-associated proteins modulate growth factor signals during neural development. Neuron 2009, 63, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Dolan, J.; Walshe, K.; Alsbury, S.; Hokamp, K.; O’Keeffe, S.; Okafuji, T.; Miller, S.F.; Tear, G.; Mitchell, K.J. The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genom. 2007, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Nürnberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol. Rev. 2004, 198, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Berner, D.; Hoja, U.; Zenkel, M.; Ross, J.J.; Uebe, S.; Paoli, D.; Frezzotti, P.; Rautenbach, R.M.; Ziskind, A.; Williams, S.E.; et al. The protective variant rs7173049 at LOXL1 locus impacts on retinoic acid signaling pathway in pseudoexfoliation syndrome. Hum. Mol. Genet. 2019, 28, 2531–2548. [Google Scholar] [CrossRef]
- Wang, H.; Jin, J.; Wu, J.; Qu, H.; Wu, S.; Bao, W. Transcriptome and chromatin accessibility in porcine intestinal epithelial cells upon Zearalenone exposure. Sci. Data 2019, 6, 298. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, B.; Li, X.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Bai, J.; Bian, J.; et al. Zearalenone Promotes Cell Proliferation or Causes Cell Death? Toxins 2018, 10, 184. [Google Scholar] [CrossRef]
- Hassen, W.; El Golli, E.; Baudrimont, I.; Mobio, A.T.; Ladjimi, M.M.; Creppy, E.E.; Bacha, H. Cytotoxicity and Hsp70 induction in HepG2 cells in response to zearalenone and cytoprotection by sub-lethal heat shock. Toxicology 2005, 207, 293–301. [Google Scholar] [CrossRef]
- Mu, H.; Mu, P.; Zhu, W.; Huang, B.; Li, H.; Yuan, L.; Deng, Y. Low doses of deoxynivalenol inhibit the cell migration mediated by H3K27me3-targeted downregulation of TEM8 expression. Biochem. Pharmacol. 2020, 175, 113897. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Gan, F.; Hu, Z.; Zhou, Y.; Huang, K. Overexpression and Low Expression of Selenoprotein S Impact Ochratoxin A-Induced Porcine Cytotoxicity and Apoptosis In Vitro. J. Agric. Food Chem. 2017, 65, 6972–6981. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, L.; Liao, Y.; Peng, Z.; Li, D.; Zhou, X.; Liu, S.; Li, Y.; Nüssler, A.K.; Liu, L.; et al. The Protective Effect of Heme Oxygenase-1 on Liver Injury Caused by DON-Induced Oxidative Stress and Cytotoxicity. Toxins 2021, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tang, Y.; Sheng, X.; Tian, Y.; Deng, M.; Du, S.; Lv, C.; Li, G.; Pan, Y.; Song, Y.; et al. Secreted stromal protein ISLR promotes intestinal regeneration by suppressing epithelial Hippo signaling. EMBO J. 2020, 39, e103255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Z.; Yang, G. Silencing of ISLR inhibits tumour progression and glycolysis by inactivating the IL6/JAK/STAT3 pathway in nonsmall cell lung cancer. Int. J. Mol. Med. 2021, 48, 222. [Google Scholar] [CrossRef]
- Cui, C.; Han, S.; Shen, X.; He, H.; Chen, Y.; Zhao, J.; Wei, Y.; Wang, Y.; Zhu, Q.; Li, D.; et al. ISLR regulates skeletal muscle atrophy via IGF1-PI3K/Akt-Foxo signaling pathway. Cell Tissue Res. 2020, 381, 479–492. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene | Sequence (5′–3′) | Product Length |
|---|---|---|
| GAPDH | F:GGTCGGAGTGAACGGATTT R:ATTTGATGTTGGCGGGAT | 245 bp |
| ISLR2 | F:GGTCCAAGGCGAGGTTGC R:CGAACTGATGCGCGTACTTG | 242 bp |
| Caspase3 | F:GGAATGGCATGTCGATCTGGT R:ACTGTCCGTCTCAATCCCAC | 351 bp |
| Caspase9 | F:TGGAACTCAAGCCAGAGGAG R:CTGCATTCAGGACGTAAGCC | 195 bp |
| BAX | F:TGCCTCAGGATGCATCTACC R:AAGTAGAAAAGCGCGACCAC | 199 bp |
| TNF-a | F:TTCCAGCTGGCCCCTTGAGC R:GAGGGCATTGGCATACCCAC | 146 bp |
| IL-6 | F:TTCACCTCTCCGGACAAAAC R:TCTGCCAGTACCTCCTTGCT | 122 bp |
| OE-ISLR2 | F:ttAAGCTTgccaccATGGGCTCCAGCCCAGAC R:caGAATTCTCAGCCTGCTGTCTGCCTATAG | 2431 bp |
| ISLR2-SNP | F:CTGGGTTTAGGTATGTTAGA R:ACACTGGCTCAGGACTC | 419 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Z.; Gu, X.; Xiao, Y.; Zhou, Y.; Bao, W.; Wu, S.; Wang, H. Analysis of the Roles of the ISLR2 Gene in Regulating the Toxicity of Zearalenone Exposure in Porcine Intestinal Epithelial Cells. Toxins 2022, 14, 639. https://doi.org/10.3390/toxins14090639
Bi Z, Gu X, Xiao Y, Zhou Y, Bao W, Wu S, Wang H. Analysis of the Roles of the ISLR2 Gene in Regulating the Toxicity of Zearalenone Exposure in Porcine Intestinal Epithelial Cells. Toxins. 2022; 14(9):639. https://doi.org/10.3390/toxins14090639
Chicago/Turabian StyleBi, Zhenbin, Xuezhu Gu, Yeyi Xiao, Yajing Zhou, Wenbin Bao, Shenglong Wu, and Haifei Wang. 2022. "Analysis of the Roles of the ISLR2 Gene in Regulating the Toxicity of Zearalenone Exposure in Porcine Intestinal Epithelial Cells" Toxins 14, no. 9: 639. https://doi.org/10.3390/toxins14090639
APA StyleBi, Z., Gu, X., Xiao, Y., Zhou, Y., Bao, W., Wu, S., & Wang, H. (2022). Analysis of the Roles of the ISLR2 Gene in Regulating the Toxicity of Zearalenone Exposure in Porcine Intestinal Epithelial Cells. Toxins, 14(9), 639. https://doi.org/10.3390/toxins14090639







