Analysis of Apple Fruit (Malus × domestica Borkh.) Quality Attributes Obtained from Organic and Integrated Production Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Managements
2.2. Climate Conditions, Flowering and Harvesting Time
2.3. Reagents and Standards
2.4. Sample Preparation
2.5. Preparation of Standard Solutions
2.6. UHPLC–DAD MS/MS Analysis of Polyphenolic Compounds
2.7. Total Phenolic Content (TPC)
2.8. Radical-Scavenging Activity (RSA)
2.9. Total Anthocyanin Content (TAC)
2.10. HPAEC/PAD Analysis of Sugars and Sugar Alcohols
2.11. Statistics
3. Results and Discussion
3.1. Determination of TPC, RSA, and TAC
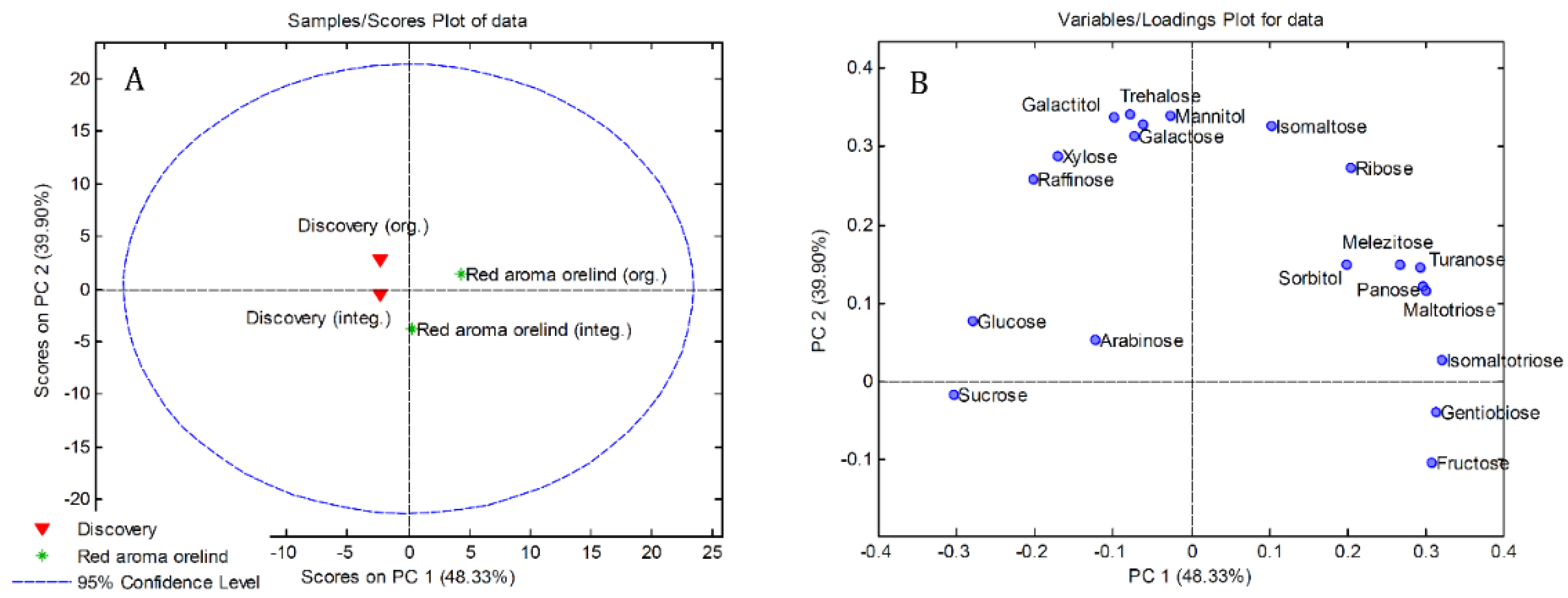
3.2. Sugars and Sugar Alcohols Profiles
3.3. Phenolic Profiles
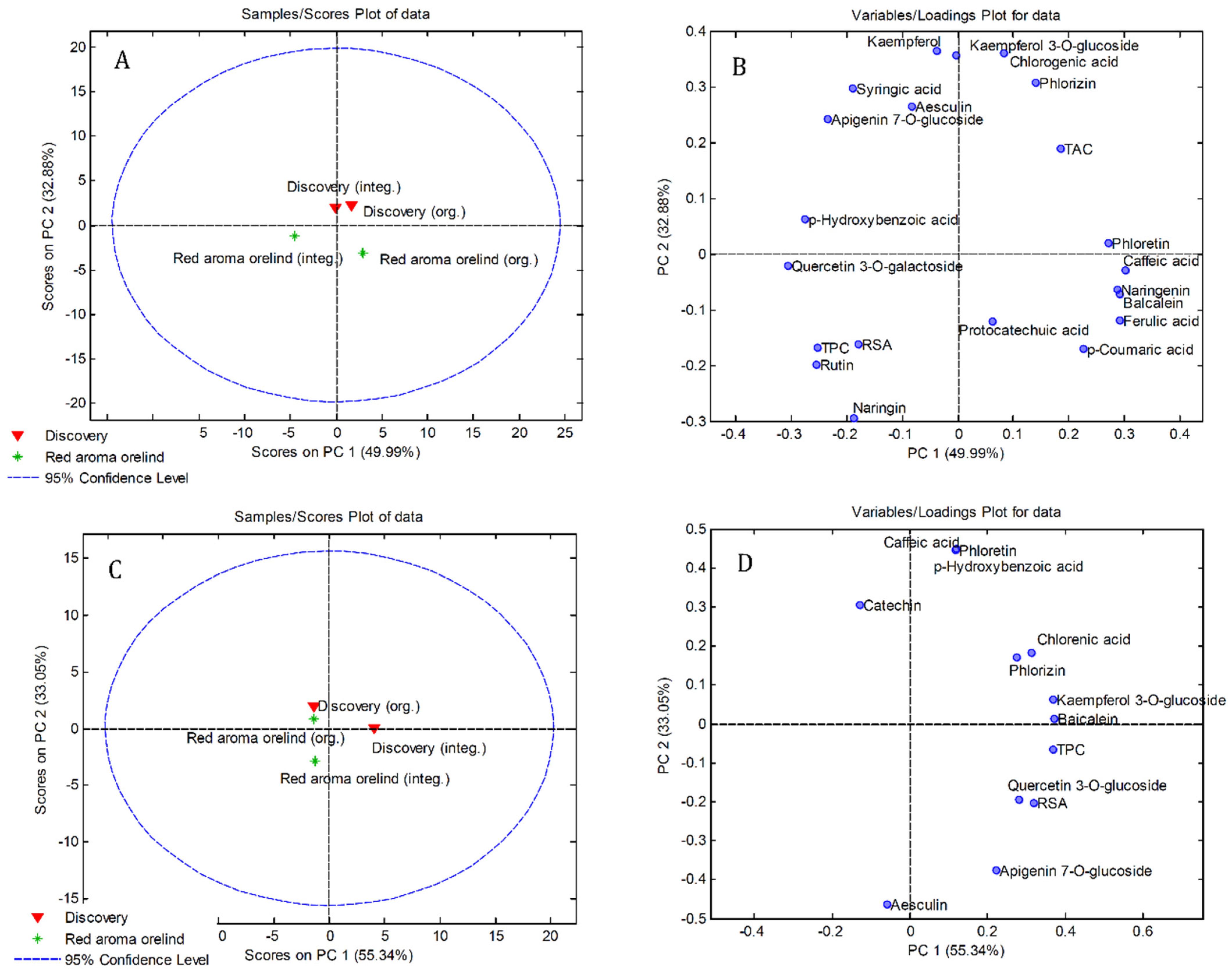
3.4. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durham, T.C.; Mizik, T. Comparative Economics of Conventional, Organic, and Alternative Agricultural Production Systems. Economies 2021, 9, 64. [Google Scholar] [CrossRef]
- Willer, H.; Trávníček, J.; Meier, C.; Schlatter, B. The World of Organic Agriculture. Statistics and Emerging Trends 2021; Research Institute of Organic Agriculture FiBL, Frick, and IFOAM—Organics International: Bonn, Germany, 2021; pp. 1–336. [Google Scholar]
- Campbell, B.L.; Mhlanga, S.; Lesschaeve, I. Perception versus reality: Canadian consumer views of local and organic. Can. J. Agric. Econ. 2013, 61, 531–558. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Parsons, R. Consumer preferences and willingness to pay for locally grown organic apples: Evidence from a conjoint study. HortScience 2010, 45, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Granatstein, D.; Kirby, E.; Ostenson, H.; Willer, H. Global situation for organic tree fruits. Sci. Hortic. 2016, 208, 3–12. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Dabić Zagorac, D.; Sredojević, M.; Milivojević, J.; Gašić, U.; Meland, M.; Natić, M. Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System. Molecules 2019, 24, 4310. [Google Scholar] [CrossRef] [Green Version]
- Peck, G.M.; Andrews, P.K.; Reganold, J.P.; Fellman, J.K. Apple orchard productivity and fruit quality under organic, conventional, and integrated management. HortScience 2006, 41, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Smith-Spangler, C.; Brandeau, M.L.; Hunter, G.E.; Clay Bavinger, J.; Pearson, M.; Eschbach, P.J.; Sundaram, V.; Liu, H.; Schirmer, P.; Stave, C.; et al. Are organic foods safer or healthier than conventional alternatives? A systematic review. Ann. Intern. Med. 2012, 157, 348–366. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant Physiol Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef]
- FaoStat. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 February 2022).
- Vasylieva, N.; James, H. Production and trade patterns in the world apple market. Innov. Mark. 2021, 17, 16–25. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Mutić, J.; Tešić, Ž.; Meland, M. Evaluation of fruit mineral contents of two apple cultivars grown in organic and integrated production systems. Acta Hortic. 2020, 1281, 59–66. [Google Scholar] [CrossRef]
- Wicklund, T.; Guyot, S.; Le Quéré, J.-M. Chemical Composition of Apples Cultivated in Norway. Crops 2021, 1, 3. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, J.; Qin, S.; Niu, Z.; Qiao, X.; Yang, B. Influence of genetic background, growth latitude and bagging treatment on phenolic compounds in fruits of commercial cultivars and wild types of apples (Malus sp.). Eur. Food Res. Technol. 2021, 247, 1149–1165. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of phytochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food. Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Alberti, A.; dos Santos, T.P.M.; Zielinski, A.A.F.; dos Santos, C.M.E.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. Lebensm. Wiss. Technol. 2016, 65, 436–443. [Google Scholar] [CrossRef]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a Source of Dietary Phytonutrients: Bioavailability and Evidence of Protective Effects against Human Cardiovascular Disease. Food Nutr. Sci. 2014, 5, 1234–1246. [Google Scholar] [CrossRef] [Green Version]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Coelho, E.; Pinto, M.; Bastos, R.; Cruz, M.; Nunes, C.; Rocha, S.M.; Coimbra, M.A. Concentrate Apple Juice Industry: Aroma and Pomace Valuation as Food Ingredients. Appl. Sci. 2021, 11, 2443. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Segliņa, D. Lipophilic composition of eleven apple seed oils: A promising source of unconventional oil from industry by-products. Ind. Crop. Prod. 2014, 60, 86–91. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Lazarević, K.; Šegan, S.; Natić, M.; Tosti, T.; Ćirić, I.; Meland, M. Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Seeds from Apple Cultivars (Malus domestica Borkh.) Grown in Norway. Foods 2021, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- FiBL Statistics 2019. Available online: https://statistics.fibl.org/world/selected-crops-world.html (accessed on 25 February 2021).
- Revdal, E.; Meland, M.; Sønsteby, A.; Martinussen, I.; Korsæth, A. Nasjonalt system for uttesting av frukt-og bærsorter i Norge —en utredning. In NIBIO Rapport; NIBIO: Oslo, Norway, 2021; Volume 7, pp. 1–28. Available online: https://hdl.handle.net/11250/2739214 (accessed on 30 March 2022).
- Ján, M.; Davide, S. Selected Quantitative Parameters Comparison of Apples from Bio- and Conventional Production. Athens J. Sci. 2018, 5, 343–354. [Google Scholar] [CrossRef]
- Róth, E.; Berna, A.; Beullens, K.; Yarramraju, S.; Lammertyn, J.; Schenk, A.; Nicolaï, B. Postharvest quality of integrated and organically produced apple fruit. Postharvest Biol. Technol. 2007, 45, 11–19. [Google Scholar] [CrossRef]
- Holb, I.J.; Dremak, P.; Bitskey, K.; Gonda, I. Yield response, pest damage and fruit quality parameters of scab-resistant and scab-susceptible apple cultivars in integrated and organic production systems. Sci. Hortic. 2012, 145, 109–117. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Baránski, M.; Kazimierczak, R.; Ponder, A.; Kopczyńska, K.; Hallmann, E. Selected Antioxidants in Organic vs. Conventionally Grown Apple Fruits. Appl. Sci. 2020, 10, 2997. [Google Scholar] [CrossRef]
- Woese, K.; Lange, D.; Boess, C.; Bögl, K.W. A comparison of organically and conventionally grown foods—Results of a review of the relevant literature. J. Sci. Food Agric. 1997, 74, 281–293. [Google Scholar] [CrossRef]
- Vanzo, A.; Jenko, M.; Vrhovsek, U.; Stopar, M. Metabolomic Profiling and Sensorial Quality of ‘Golden Delicious’, ‘Liberty’, ‘Santana’, and ‘Topaz’ Apples Grown Using Organic and Integrated Production Systems. J. Agric. Food Chem. 2013, 61, 6580–6587. [Google Scholar] [CrossRef] [PubMed]
- Lamperi, L.; Chiuminatto, U.; Cincinelli, A.; Galvan, P.; Giordani, E.; Lepri, L.; Del Bubba, M. Polyphenol levels and free radical scavenging ac-tivities of four apple cultivars from integrated and organic farming in different Italian areas. J. Agric. Food Chem. 2008, 56, 6536–6546. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Psomas, A.; Zovoili, A.; Siatis, V. Polyphenolic profile and antioxidant activity of five apple cultivars grown under organic and conventional agricultural practices. Int. J. Food Sci. Technol. 2009, 44, 1167–1175. [Google Scholar] [CrossRef]
- Santarelli, V.; Neri, L.; Sacchetti, G.; Di Mattia, C.D.; Mastrocola, D.; Pittia, P. Response of organic and conventional apples to freezing and freezing pre-treatments: Focus on polyphenols content and antioxidant activity. Food Chem. 2020, 308, 125570. [Google Scholar] [CrossRef]
- Adamczyk, M.J.; Kostyra, E.; Wasiak-Zys, G.; Hallmann, E.; Batorska, D.; Rembiałkowska, E. Sensory and Instrumental Analysis of Selected Cultivars of Apples from Organic and Conventional Production; Fordergemeinschaft Okologischer Obstbau e.V. (FOKO): Weinsberg, Germany, 2010; pp. 264–273. [Google Scholar]
- Maas, F.; Krogstad, T.; Fotirić Akšić, M.; Meland, M. Survey of nutrient levels in apple trees and soil in four fruit growing regions in Norway. In NIBIO Rapport; NIBIO: Oslo, Norway, 2022; Volume 8, pp. 1–39. Available online: https://hdl.handle.net/11250/2987555 (accessed on 30 March 2022).
- Pantelić, M.; Zagorac, D.D.; Davidović, S.; Todić, S.; Bešlić, Z.; Gašić, U.; Tešić, Ž.; Natić, M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Gašić, U.; Natić, M.; Mišić, D.; Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Lušić, D. Chemical markers for the authentication of unifloral Salvia officinalis L. honey. J. Food Compos. Anal. 2015, 44, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Natić, M.; Dabić, D.; Papetti, A.; Fotirić Akšić, M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž. Analysis and characterization of phytochemicals in Mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- McGhie, T.K.; Hunt, M.; Barnett, L.E. Cultivar and growing region determine the antioxidant polyphenolic concentration and composition of apples grown in New Zealand. J. Agric. Food Chem. 2005, 53, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Mikulič Petkovšek, M.; Štampar, F.; Veberič, R. Accumulation of phenolic compounds in apple in response to infection by the scab pathogen, Venturia inaequalis. Physiol. Mol. 2009, 74, 60–67. [Google Scholar] [CrossRef]
- Piagentini, A.M.; Pirovani, M.E. Total Phenolics Content, Antioxidant Capacity, Physicochemical attributes, and browning susceptibility of different apple cultivars for minimal processing. Int. J. Fruit Sci. 2017, 17, 102–116. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liang, D.; Zou, Y.; Li, P.; Ma, F. Phenolic compounds and antioxidant activity in red-fleshed apples. J. Funct. Foods 2015, 18, 1086–1094. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Yuri, J.; Maldonado, F.; Razmilic, I.; Neira, A.; Quilodran, A.; Palomo, I. Concentrations of total phenols and antioxidant activity in apple do not differ between conventional and organic orchard Management. J. Food Agric. Environ. 2012, 10, 207–216. [Google Scholar]
- Kim, H.-Y.; Farcuh, M.; Cohen, Y.; Crisosto, C.; Sadka, A.; Blumwald, E. Non-climacteric ripening and sorbitol homeostasis in plum fruits. Plant Sci. 2015, 231, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Tosti, T.; Nedić, N.; Marković, M.; Ličina, V.; Milojković-Opsenica, D.; Tešić, Ž. Influence of frost damage on the sugars and sugar alcohol composition in quince (Cydonia oblonga Mill.) Floral nectar. Acta Physiol. Plant. 2015, 37, 1701. [Google Scholar] [CrossRef]
- Jakopič, J.; Slatnar, A.; Štampar, F.; Veberič, R.; Simoncic, A. Analysis of selected primary metabolites and phenolic profile of ‘Golden Delicious’ apples from four production systems. Fruits 2012, 64, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Tappy, L.; Le, K.A.; Tran, C.; Paquot, N. Fructose and metabolic diseases: New findings, new questions. Nutrition 2010, 26, 1044–1104. [Google Scholar] [CrossRef] [PubMed]
- Bertazza, G.; Cristoferi, G.; Bignami, C. Fruit Composition and Quality of Organically and Conventionally Grown Apple, Apricot and Pear in the Veneto Region (Northern Italy). Acta Hortic. 2010, 873, 309–316. [Google Scholar] [CrossRef]
- Kouřimská, L.; Kubaschová, K.; Sus, J.; Nový, P.; Dvořáková, B.; Koudela, M. Comparison of the carbohydrate content in apples and carrots grown in organic and integrated farming systems. Potravinarstvo 2014, 8, 178–183. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Hecke, K.; Herbinger, K.; Veberič, R.; Trobec, M.; Toplak, H.; Štampar, F.; Keppel, H.; Grill, D. Sugar-, acid- and phenol contents in apple cultivars from organic and integrated fruit cultivation. Eur. J. Clin. Nutr. 2006, 60, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, V.H.; Suprasanna, P. Prospects of Halophytes in Understanding and Managing Abiotic Stress Tolerance. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 29–56. [Google Scholar]
- Meyer, T.S.M.; Miguel, Â.S.M.; Fernández, D.R.; Ortiz, G.M.D. Biotechnological Production of Oligosaccharides—Applications in the Food Industry. In Food Production and Industry; Eissa, A.H.A., Ed.; IntechOpen: London, UK, 2015; Available online: https://www.intechopen.com/chapters/48909 (accessed on 13 March 2022).
- Pullicin, A.J.; Penner, M.H.; Lim, J. The Sweet Taste of Acarbose and Maltotriose: Relative Detection and Underlying Mechanism. Chem. Senses 2019, 44, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic-Malinovska, R.; Kuzmanova, S.; Winkelhausen, E. Oligosaccharide Profile in Fruits and Vegetables as Sources of Prebiotics and Functional Foods. Int. J. Food Prop. 2014, 17, 949–965. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Tanou, G.; Scossa, F.; Sarrou, E.; Stamatakis, G.; Samiotaki, M.; Martens, S.; Fernie, A.R.; Molassiotis, A. Decoding altitude-activated regulatory mechanisms occurring during apple peel ripening. Hortic. Res. 2020, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Bureau, S.; Renard, C.M.G.C.; Plenet, D.; Gautier, H.; Touloumet, L.; Girard, T.; Simon, S. Cultivar and Year Rather than Agricultural Practices Affect Primary and Secondary Metabolites in Apple Fruit. PLoS ONE 2015, 10, e0141916. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Paluszczak, J.; Baer-Dubowska, W. DNA Methylation as a Target of Cancer Chemoprevention by Dietary Polyphenols. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 1385–1392. [Google Scholar]
- Veberic, R.; Trobec, M.; Herbinger, K.; Hofer, M.; Grill, D.; Stampar, F. Phenolic compounds in some apple (Malus domestica Borkh) cultivars of organic and integrated production. J. Sci. Food Agric. 2005, 85, 1687–1694. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Xie, X.B.; Li, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant Compounds for Plant Defence and for a Healthy Human Diet. Not. Bot. Horti. Agrobot. 2018, 46, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, R.; Yang, R.; Young, C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Marks, S.C.; Mullen, W.; Crozier, A. Flavonoid and chlorogenic acid profiles of English cider apples. J. Sci. Food Agric. 2007, 87, 719–728. [Google Scholar] [CrossRef]
- Wold, H. Soft Modelling by Latent Variables: The Non-Linear Iterative Partial Least Squares (NIPALS) Approach. J. Appl. Probab. 1975, 12, 117–142. [Google Scholar] [CrossRef]
- Francini, A.; Romeo, S.; Cifelli, M.; Gori, D.; Domenici, V.; Sebastiani, L. 1H NMR and PCA-based analysis revealed variety dependent changes in phenolic contents of apple fruit after drying. Food Chem. 2017, 221, 1206–1213. [Google Scholar] [CrossRef]
- Mureşan, A.E.; Sestras, A.F.; Militaru, M.; Păucean, A.; Tanislav, A.E.; Puscas, A.; Mateescu, M.; Mureşan, V.; Marc (Vlaic), R.A.; Sestras, R.E. Chemometric Comparison and Classification of 22 Apple Genotypes Based on Texture Analysis and Physico-Chemical Quality Attributes. Horticulturae 2022, 8, 64. [Google Scholar] [CrossRef]
- Gabriel, L.S.; Prestes, R.A.; Pinheiro, L.A.; Barison, A.; Wosiacki, G. Multivariate analysis of the spectroscopic profile of the sugar fraction of apple pomace. Braz. Arch. Biol. Technol. 2013, 56, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Włodarska, K.; Pawlak-Lemańska, K.; Górecki, T.; Sikorska, E. Classification of commercial apple juices based on multivariate analysis of their chemical profiles. Int. J. Food Prop. 2017, 20, 1773–1785. [Google Scholar] [CrossRef] [Green Version]
| Sample | Peel | Pulp | |||
|---|---|---|---|---|---|
| TPC * | RSA ** | TAC *** | TPC * | RSA ** | |
| Discovery (organic p.) | 11.33 ± 0.21 d | 120.88 ± 0.00 b | 0.54 ± 0.02 a | 0.95 ± 0.02 c | 25.58 ± 0.42 c |
| Discovery (integrated p.) | 17.75 ± 0.05 b | 103.21 ± 1.14 d | 0.25 ± 0.00 c | 1.60 ± 0.01 a | 32.04 ± 0.53 a |
| Red Aroma Orelind (organic p.) | 16.45 ± 0.18 c | 118.21 ± 1.89 c | 0.29 ± 0.01 b | 0.91 ± 0.02 c | 28.18 ± 0.11 b |
| Red Aroma Orelind (integrated p.) | 22.89 ± 0.51 a | 136.21 ± 0.94 a | 0.18 ± 0.00 d | 1.05 ± 0.00 b | 28.92 ± 0.11 b |
| Sugars | ‘Discovery’ | ‘Red Aroma Orelind’ | Cultivars | Production Systems | ||||
|---|---|---|---|---|---|---|---|---|
| Integrated | Organic | Integrated | Organic | ‘Red Aroma O.’ | ‘Discovery’ | Integrated | Organic | |
| Sorbitol | 0.235 c | 0.257 b | 0.278 a,* | 0.227 d | 0.253 a | 0.246 a | 0.257 a | 0.242 a |
| Trehalose | 0.505 a | 0.415 c | 0.432 b | 0.337 d | 0.385 a | 0.460 a | 0.469 a | 0.376 b |
| Arabinose | 0.301 c | 0.513 a | 0.321 b | 0.241 d | 0.281 a | 0.407 a | 0.311 a | 0.377 a |
| Glucose | 37.410 b | 40.833 a | 30.586 d | 31.918 c | 31.252 b | 39.122 a | 33.998 b | 36.376 a |
| Sucrose | 16.078 b | 17.560 a | 12.968 d | 14.887 c | 13.928 b | 16.819 a | 14.523 a | 16.224 a |
| Fructose | 45.862 d | 46.780 c | 55.926 a | 52.914 b | 54.92 a | 46.321 b | 50.894 a | 50.347 a |
| Isomaltose | 1.296 b | 1.214 c | 1.363 a | 1.049 d | 1.206 a | 1.255 a | 1.330 a | 1.132 b |
| Melezitose | 0.063 c | 0.073 b | 0.127 a | 0.056 d | 0.092 a | 0.068 a | 0.096 a | 0.064 a |
| Gentiobiose | 0.019 c | 0.005 d | 0.096 a | 0.061 b | 0.078 a | 0.012 b | 0.014 a | 0.005 a |
| Turanose | 0.184 b | 0.080 d | 0.434 a | 0.152 c | 0.292 a | 0.132 a | 0.309 a | 0.115 b |
| Raffinose | 0.581 a | 0.281 b | 0.162 c | 0.104 d | 0.133 b | 0.431 a | 0.371 a | 0.193 a |
| Isomaltotriose | 0.033 c | 0.027 d | 0.264 a | 0.104 b | 0.184 a | 0.030 b | 0.149 a | 0.065 a |
| Maltose | 2.372 a | 1.330 c | 1.660 b | 0.987 d | 1.324 a | 1.851 a | 2.016 a | 1.158 b |
| Panose | 0.015 b | 0.014 b | 0.555 a | 0.016 b | 0.286 a | 0.014 b | 0.285 a | 0.015 a |
| Maltotriose | 0.008 c | 0.007 c | 0.521 a | 0.020 b | 0.270 a | 0.007 b | 0.264 a | 0.013 a |
| Galactose | 0.907 a | 0.661 c | 0.731 b | 0.607 d | 0.669 a | 0.784 a | 0.819 a | 0.634 b |
| Galactitol | 1.041 a | 0.882 b | 0.884 b | 0.688 c | 0.786 a | 0.961 a | 0.962 a | 0.785 b |
| Ribose | 0.411 b | 0.337 c | 0.506 a | 0.296 d | 0.401 a | 0.374 a | 0.459 a | 0.317 b |
| Mannitol | 0.813 a | 0.614 c | 0.703 b | 0.537 d | 0.620 a | 0.714 a | 0.758 a | 0.576 b |
| Xylose | 2.226 a | 2.059 b | 1.738 c | 1.322 d | 1.530 b | 2.143 a | 1.982 a | 1.690 a |
| TSI | 113.30 a | 118.76 b,c | 120.10 c | 118.52 b,c | 120.06 c | 116.03 b | 116.70 b | 119.39 b,c |
| Phenolic Compounds | ‘Discovery’ (Org.) | ‘Discovery’ (Integ.) | ‘Red Aroma Orelind’ (Org.) | ‘Red Aroma Orelind’ (Integ.) | ‘Discovery’ (Org.) | ‘Discovery’ (Integ.) | ‘Red Aroma Orelind’ (Org.) | ‘Red Aroma Orelind’ (Integ.) |
|---|---|---|---|---|---|---|---|---|
| Peel | Pulp | |||||||
| Protocatechuic acid | 0.53 c,* | -- | 0.43 b | 0.37 a | -- | -- | -- | -- |
| Aesculin | 11.09 d | 10.03 c | 9.42 b | 10.44 c | 1.74 a | 1.77 a | 1.78 a | 1.83 a |
| Chlorogenic acid | 62.09 d,e | 64.27 e | 24.30 b | 25.33 b | 42.49 c | 60.56 d | 21.39 a | 21.51 a |
| p-Hydroxybenzoic acid | 1.05 c | 0.91 c | 0.76 b | 1.34 d | -- | 0.32 a | -- | -- |
| Catechin | -- | -- | -- | -- | 11.36 | -- | -- | -- |
| Caffeic acid | 8.47 b | 8.46 b | 8.61 b | 8.17 b | 3.30 a | 3.30 a | 3.24 a | -- |
| Syringic acid | 1.49 a | 1.84 b | -- | 1.66 ab | -- | -- | -- | -- |
| Rutin | -- | -- | 11.60 a | 46.90 b | -- | -- | -- | -- |
| p-Coumaric acid | 0.77 a | 1.33 b | 1.94 c | 0.51 a | -- | -- | -- | -- |
| Quercetin 3-O-galactoside | 88.64 c | 96.85 d | 76.81 b | 146.21 e | 0.87 a | 0.98 a | 0.79 a | 0.92 a |
| Ferulic acid | 0.33 a | 0.26 a | 0.60 b | -- | -- | -- | -- | -- |
| Naringin | -- | -- | 0.08 a | 0.14 b | -- | -- | -- | -- |
| Kaempferol 3-O-glucoside | 11.72 e | 14.33 f | 6.19 c | 8.30 d | 0.27 a | 0.42 b | 0.23 a | 0.23 a |
| Apigenin 7-O-glucoside | 0.41 b | 0.45 b,c | -- | 0.54 c | -- | 0.12 a | -- | 0.12 a |
| Phlorizin | 67.27 f | 46.53 e | 33.27 d | 29.18 c | 1.18 a | 2.39 b | 2.00 b | 0.93 a |
| Phloretin | 6.91 c | 5.94 b | 6.68 c | 5.62 b | 2.00 a | 2.01 a | 2.01 a | -- |
| Baicalein | 1.21 c | 1.08 b | 1.26 c | 1.00 b | 0.23 a | 0.24 a | 0.24 a | 0.23 a |
| Naringenin | 2.17 c | 1.81 a | 2.25 d | 1.65 b | -- | -- | -- | -- |
| Kaempferol | 17.68 b,c | 18.70 c | 14.40 a | 16.08 b | -- | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotirić Akšić, M.; Dabić Zagorac, D.; Gašić, U.; Tosti, T.; Natić, M.; Meland, M. Analysis of Apple Fruit (Malus × domestica Borkh.) Quality Attributes Obtained from Organic and Integrated Production Systems. Sustainability 2022, 14, 5300. https://doi.org/10.3390/su14095300
Fotirić Akšić M, Dabić Zagorac D, Gašić U, Tosti T, Natić M, Meland M. Analysis of Apple Fruit (Malus × domestica Borkh.) Quality Attributes Obtained from Organic and Integrated Production Systems. Sustainability. 2022; 14(9):5300. https://doi.org/10.3390/su14095300
Chicago/Turabian StyleFotirić Akšić, Milica, Dragana Dabić Zagorac, Uroš Gašić, Tomislav Tosti, Maja Natić, and Mekjell Meland. 2022. "Analysis of Apple Fruit (Malus × domestica Borkh.) Quality Attributes Obtained from Organic and Integrated Production Systems" Sustainability 14, no. 9: 5300. https://doi.org/10.3390/su14095300
APA StyleFotirić Akšić, M., Dabić Zagorac, D., Gašić, U., Tosti, T., Natić, M., & Meland, M. (2022). Analysis of Apple Fruit (Malus × domestica Borkh.) Quality Attributes Obtained from Organic and Integrated Production Systems. Sustainability, 14(9), 5300. https://doi.org/10.3390/su14095300









