Exploring the Antimicrobial Potential of Natural Substances and Their Applications in Cosmetic Formulations
Abstract
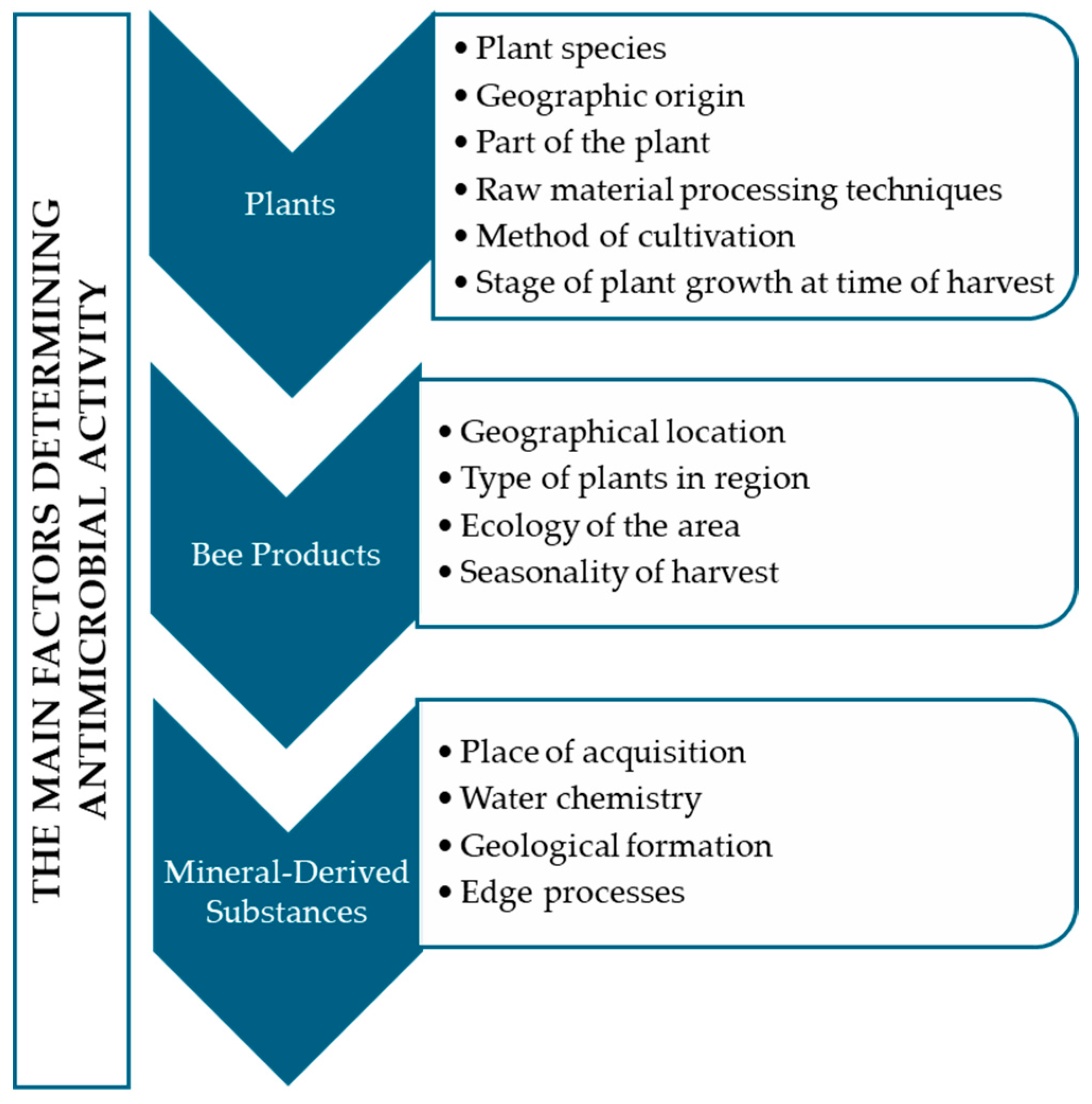
:1. Introduction
2. Materials and Methods
3. Plant-Derived Natural Substances
3.1. Garlic (Allium)
3.2. Sage (Salvia L.)
3.3. Tea Tree Oil (Melaleuca alternifolia L.)
3.4. Aloe vera (Aloe L.)
3.5. Lavender (Lavandula L.)
3.6. Fenugreek (Trigonella L.)
3.7. Oregano (Origanum L.)
3.8. Black Cumin (Nigella L.)
4. Animal-Derived Natural Substances
4.1. Bee Honey
Manuka Honey (Leptospermum scoparium)
4.2. Propolis (Propolis cera)
5. Mineral-Derived Natural Substances
5.1. Cosmetic Mud
Dead Sea Mud
5.2. Cosmetic Clays
6. Use of Natural Substances in the Cosmetics Industry
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tiwari, S.; Nanda, M.; Pattanaik, S.; Shivakumar, G.C.; Sunila, B.S.; Cicciù, M.; Minervini, G. Analytical Study on Current Trends in the Clinico-Mycological Profile among Patients with Superficial Mycoses. J. Clin. Med. 2023, 12, 3051. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms. 2021, 9, 303. [Google Scholar] [CrossRef]
- Laureano, A.C.; Schwartz, R.A.; Cohen, P.J. Facial Bacterial Infections: Folliculitis. Clin. Dermatol. 2014, 32, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Ibler, K.S.; Kromann, C.B. Recurrent Furunculosis—Challenges and Management: A Review. Clin. Cosmet. Investig. Dermatol. 2014, 7, 59–64. [Google Scholar] [CrossRef]
- Johnson, M.K. Impetigo. Adv. Emerg. Nurs. J. 2020, 42, 262–269. [Google Scholar] [CrossRef]
- Sullivan, T.; de Barra, E. Diagnosis and Management of Cellulitis. Clin. Med. 2018, 18, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Jendoubi, F.; Rohde, M.; Prinz, J.C. Intracellular Streptococcal Uptake and Persistence: A Potential Cause of Erysipelas Recurrence. Front. Med. 2019, 6, 6. [Google Scholar] [CrossRef]
- Lamb, R.C.; Dawn, G. Cutaneous Non-Tuberculous Mycobacterial Infections. Int. J. Dermatol. 2014, 53, 1197–1204. [Google Scholar] [CrossRef]
- Salemi, S.Z.; Memar, M.Y.; Kafil, H.S.; Sadeghi, J.; Ghadim, H.H.; Alamdari, H.A.; Nezhadi, J.; Ghotaslou, R. The Prevalence and Antibiotics Susceptibility Patterns of Corynebacterium Minutissimum Isolates from Skin Lesions of Patients with Suspected Erythrasma from Tabriz, Iran. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 4016173. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, H.L.; Siqueira, R.N.; Meireles, R.D.S.; Rampon, G.; de Castro, L.A.S.; e Silva, R.M. Pitted Keratolysis. An. Bras. Dermatol. 2016, 91, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Peres, N.T.A.; Bitencourt, T.A.; Martins, M.P.; Rossi, A. State-of-the-Art Dermatophyte Infections: Epidemiology Aspects, Pathophysiology, and Resistance Mechanisms. J. Fungi 2021, 7, 629. [Google Scholar] [CrossRef]
- Bae, Y.; Lee, G.M.; Sim, J.H.; Lee, S.; Lee, S.Y.; Park, Y.L. Green Nail Syndrome Treated with the Application of Tobramycin Eye Drop. Ann. Dermatol. 2014, 26, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida Albicans-the Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Devendrappa, K.; Javed, M.W. Clinical Profile of Patients with Tinea Versicolor. Int. J. Res. Dermatol. 2018, 4, 33. [Google Scholar] [CrossRef]
- Van Dissel, J.T.; Kuijper, E.J. Rapidly Growing Mycobacteria: Emerging Pathogens in Cosmetic Procedures of the Skin. Clin. Infect. Dis. 2009, 49, 1365–1368. [Google Scholar] [CrossRef]
- Kroumpouzos, G.; Harris, S.; Bhargava, S.; Wortsman, X. Complications of Fillers in the Lips and Perioral Area: Prevention, Assessment, and Management Focusing on Ultrasound Guidance. J. Plast. Reconstr. Aesthetic Surg. 2023, 84, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Yakupu, A.; Aimaier, R.; Yuan, B.; Chen, B.; Cheng, J.; Zhao, Y.; Peng, Y.; Dong, J.; Lu, S. The Burden of Skin and Subcutaneous Diseases: Findings from the Global Burden of Disease Study 2019. Front. Public Health 2023, 11, 1145513. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhou, J.; Xu, B.N.; Li, Y.; Bao, W.; Cheng, X.L.; He, Y.; Xu, C.P.; Ren, J.; Zheng, Y.R.; et al. Global Burden of Bacterial Skin Diseases: A Systematic Analysis Combined With Sociodemographic Index, 1990–2019. Front. Med. 2022, 9, 861115. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.; Jaworek, A.; Stec-Polak, M.; Radzimowska, J.; Wojas-Pelc, A. Superficial Mycoses—Analysis of Mycological Examinations from Mycology Laboratory in Krakow in Years 2010–2014. Prz. Lek. 2015, 72, 253–256. [Google Scholar]
- Abid Khan, R.M.; Dodani, S.K.; Nadeem, A.; Jamil, S.; Zafar, M.N. Bacterial Isolates and Their Antimicrobial Susceptibility Profile of Superficial and Deep-Seated Skin and Soft Tissue Infections. Asian Biomed. 2023, 17, 55–63. [Google Scholar] [CrossRef]
- Nwankwo, I.U.; Edward, K.C.; Nwoba, C.N.; Okwudiri, C.V. Evaluation of Bacterial Species in Patients with Skin Infection and Their Antibiogram. South Asian J. Res. Microbiol. 2021, 9, 10–16. [Google Scholar] [CrossRef]
- Gadzhieva, L.; Kireeva, E.; Demchenkov, N.; Sorokovikova, T.; Morozov, A.; Bocharova, E. Nosocomial Skin and Soft Tissue Pathogens in Eastern Russia: Relevance and Antimicrobial Resistance. Arch. Euromedica 2023, 13, 1–7. [Google Scholar] [CrossRef]
- Iancu, A.V.; Maftei, N.M.; Dumitru, C.; Baroiu, L.; Gurau, G.; Elisei, A.M.; Stefan, C.S.; Tatu, A.L.; Iancu, A.F.; Arbune, M. Prevalence of Multidrug Resistance Pathogens in Dermatology: A Retrospective Study in Romania, 2018–2022. Electron. J. Gen. Med. 2024, 21, em582. [Google Scholar] [CrossRef]
- Koh, X.Q.; Pan, J.Y. Recalcitrant Cutaneous Fungal Infections—A Growing Problem. Australas. J. Dermatol. 2023, 64, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Petty, L.A.; Shorr, A.F.; Zilberberg, M.D. Current Epidemiology, Etiology, and Burden of Acute Skin Infections in the United States. Clin. Infect. Dis. 2019, 68, S193–S199. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Chen, J.; Ramsey, A.M.; Hoban, A.P.; Pham, V.; Doherty, J.A.; Mody, S.H.; Kollef, M.H. Hospitalizations with Healthcare-Associated Complicated Skin and Skin Structure Infections: Impact of Inappropriate Empiric Therapy on Outcomes. J. Hosp. Med. 2010, 5, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Filatov, V.A.; Kulyak, O.Y.; Kalenikova, E.I. Chemical Composition and Antimicrobial Potential of a Plant-Based Substance for the Treatment of Seborrheic Dermatitis. Pharmaceuticals 2023, 16, 328. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Shenoy, P.A.; Pai, V. Antimicrobial Activity of Some Essential Oils and Extracts from Natural Sources on Skin and Soft Tissue Infection Causing Microbes: An in-Vitro Study. Res. J. Pharm. Technol. 2021, 14, 3603–3609. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Allemann, I.B.; Baumann, L. Botanicals in Skin Care Products. Int. J. Dermatol. 2009, 48, 923–934. [Google Scholar] [CrossRef]
- Barbu, I.A.; Ciorîță, A.; Carpa, R.; Moț, A.C.; Butiuc-Keul, A.; Pârvu, M. Phytochemical Characterization and Antimicrobial Activity of Several Allium Extracts. Molecules 2023, 28, 3980. [Google Scholar] [CrossRef] [PubMed]
- Pyun, M.S.; Shin, S. Antifungal Effects of the Volatile Oils from Allium Plants against Trichophyton Species and Synergism of the Oils with Ketoconazole. Phytomedicine 2006, 13, 394–400. [Google Scholar] [CrossRef]
- Rahman, Z.; Afsheen, Z.; Hussain, A.; Khan, M. Antibacterial and Antifungal Activities of Garlic (Allium sativum) against Common Pathogens. BioScientific Rev. 2022, 4, 30–40. [Google Scholar] [CrossRef]
- Marpaung, Y.; Adeline, A.; Budi, A.; Alvonsine, G. Garlic (Allium sativum) Extract Effectiveness Test Against Trichophyton Rubrum and Pityrosporum Ovale Mushrooms. Jambura J. Health Sci. Res. 2022, 4, 621–631. [Google Scholar] [CrossRef]
- Aala, F.; Yusuf, U.K.; Rezaie, S.; Davari, B.; Aala, F. In Vitro Antifungal Effects of Aqueous Garlic Extract Alone and in Combination with Azoles Against Dermatophytic Fungi 1. Int. Res. J Biochem. Bioinformat. 2011, 1, 226–231. [Google Scholar]
- Mercy, K.A.; Ijeoma, I.; Emmanuel, K.J. Anti-Dermatophytic Activity of Garlic (Allium sativum) Extracts on Some Dermatophytic Fungi. Int. Lett. Nat. Sci. 2014, 24, 34–40. [Google Scholar] [CrossRef]
- Aala, F.; Yusuf, U.K.; Jamal, F.; Rezaie, S. Antimicrobial Effects of Allicin and Ketoconazole on Trichophyton Rubrum Under In Vitro Condition. Braz. J. Microbiol. 2012, 43, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.S.S. Formulations and Evaluations of Herbal Anti-Acne Gel from Coriander and Garlic. Int. J. Res. Appl. Sci. Eng. Technol. 2023, 11, 3302–3310. [Google Scholar] [CrossRef]
- Saptarini, N.M.; Herawati, I.E. Development and Evaluation of Anti-Acne Gel Containing Garlic (Allium sativum) against Propionibacterium Acnes. Asian J. Pharm. Clin. Res. 2017, 10, 260–262. [Google Scholar] [CrossRef]
- Pazyar, N.; Feily, A. Garlic in Dermatology. Dermatol. Rep. 2011, 3, e4. [Google Scholar] [CrossRef] [PubMed]
- Maluki, A.; Al-Hajjar, Q.N. Treatment of Alopecia Areata With Topical Garlic Extract. Kufa Med. J. 2009, 12, 312–318. [Google Scholar]
- Abe, K.; Yamamoto, K.; Myoda, T.; Fujii, T.; Niwa, K. Protective Effects of Volatile Components of Aged Garlic Extract against Ultraviolet B-Induced Apoptosis in Human Skin Fibroblasts. J. Food Biochem. 2022, 46, e14482. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Shin, T.W.; Lee, J.; Jeong, H.S. High Temperature and Pressure Treated Garlic: Antioxidant and Antiaging Effect on Skin. J. Korean Soc. Food Sci. Nutr. 2022, 51, 737–742. [Google Scholar] [CrossRef]
- Available online: https://incidecoder.com/products/etude-house-ac-clean-up-toner (accessed on 14 November 2024).
- Available online: https://incidecoder.com/products/gelcatriz-gel-body-face (accessed on 14 November 2024).
- Available online: https://Www.Farmasi.Pl/Farmasi/Product/Detail/Dr-c-Tuna-Reviving-Regeneruj%C4%85cy-Olejek-Do-W%C5%82os%C3%B3w-30-Ml?Pid=1000311&srsltid=AfmBOoqS3cWhTNFKS77WQrZHIcWDfA64vOST5D6HQ0wSIVvJ7WuTXRx2 (accessed on 14 November 2024).
- Djaković Sekulić, T.; Božin, B.; Smoliński, A. Chemometric Study of Biological Activities of 10 Aromatic Lamiaceae Species’ Essential Oils. J. Chemom. 2016, 30, 188–196. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Maccioni, D.; Cocco, E.; Gonçalves, M.J.; Porcedda, S.; Piras, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Advances in the Phytochemical Characterisation and Bioactivities of Salvia Aurea L. Essential Oil. Plants 2023, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial Activity of the Essential Oils of Salvia officinalis L. and Salvia triloba L. Cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Stanciu, G.; Lupsor, S.; Oancea, E.; Mititelu, M. Biological Activity of Essential Sage Oil. J. Sci. Arts 2022, 22, 211–218. [Google Scholar] [CrossRef]
- Silvestin Celi Garcia, C.; Roesch Ely, M.; Adelfo Wasum, R.; Catarina de Antoni Zoppa, B.; Wollheim, C.; Ângela Neves, G.; Weiss Angeli, V.; Cristhinia Borges de Souza, K. Assessment of Salvia officinalis (L.) Hydroalcoholic Extract for Possible Use in Cosmetic Formulation as Inhibitor of Pathogens in the Skin. J. Basic Appl. Pharm. Sci. Rev. Ciências Farm. Básica Apl. 2012, 33, 509–514. [Google Scholar]
- Levaya, Y.K.; Zholdasbaev, M.E.; Atazhanova, G.A.; Akhmetova, S.B. Antibacterial Activity of Ultrasonic Extracts of Salvia Stepposa Growing in Kazakhstan. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2021, 101, 45–49. [Google Scholar] [CrossRef]
- Available online: https://novaclear.eu/produkt/mydlo-pielegnacyjne-o-dzialaniu-antybakteryjnym/ (accessed on 14 November 2024).
- Available online: https://incidecoder.com/products/nutribiotic-super-shower-gel (accessed on 14 November 2024).
- Available online: https://ecospa.pl/olejek-szalwii-muszkatolowej (accessed on 14 November 2024).
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca Alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Tejedor, F.; González-García, P.; Mayordomo, R. Solubilization in Vitro of Tea Tree Oil and First Results of Antifungal Effect in Onychomycosis. Enfermedades Infecc. Y Microbiol. Clin. 2021, 39, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Kabir Mumu, S.; Mahboob Hossain, M. Antimicrobial Activity of Tea Tree Oil against Pathogenic Bacteria and Comparison of Its Effectiveness with Eucalyptus Oil, Lemongrass Oil and Conventional Antibiotics. Am. J. Microbiol. Res. 2018, 6, 73–78. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Nguyen, L.A.; DeVico, B.; Mannan, M.; Chang, M.; Rada Santacruz, C.; Siragusa, C.; Everhart, S.; Fazen, C.H. Tea Tree Essential Oil Kills Escherichia Coli and Staphylococcus Epidermidis Persisters. Biomolecules 2023, 13, 1404. [Google Scholar] [CrossRef]
- Esmael, A.; Hassan, M.G.; Amer, M.M.; Abdelrahman, S.; Hamed, A.M.; Abd-raboh, H.A.; Foda, M.F. Antimicrobial Activity of Certain Natural-Based Plant Oils against the Antibiotic-Resistant Acne Bacteria. Saudi J. Biol. Sci. 2020, 27, 448–455. [Google Scholar] [CrossRef]
- Mazzarello, V.; Donadu, M.G.; Ferrari, M.; Piga, G.; Usai, D.; Zanetti, S.; Sotgiu, M.A. Treatment of Acne with a Combination of Propolis, Tea Tree Oil, and Aloe vera Compared to Erythromycin Cream: Two Double-Blind Investigations. Clin. Pharmacol. 2018, 10, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://incidecoder.com/products/aromatica-tea-tree-calming-gel (accessed on 15 November 2024).
- Available online: https://aarkada.com/produkt/serum-aarkada-tc16-11-ml/ (accessed on 15 November 2024).
- Available online: https://teatreetherapy.com/antiseptic-ointment (accessed on 15 November 2024).
- Bashir, A.; Saeed, B.; Mujahid, T.Y.; Jehan, N. Comparative Study of Antimicrobial Activities of Aloe vera Extracts and Antibiotics against Isolates from Skin Infections. Afr. J. Biotechnol. 2011, 10, 3835–3840. [Google Scholar]
- Pradhan, B. Phytochemistry, Pharmacology and Toxicity of Aloe vera: A Versatile Plant with Extensive Therapeutic Potential. Plant Arch. 2023, 23, 327–333. [Google Scholar] [CrossRef]
- Ghuman, S.; Ncube, B.; Finnie, J.F.; McGaw, L.J.; Coopoosamy, R.M.; Van Staden, J. Antimicrobial Activity, Phenolic Content, and Cytotoxicity of Medicinal Plant Extracts Used for Treating Dermatological Diseases and Wound Healing in KwaZulu-Natal, South Africa. Front. Pharmacol. 2016, 7, 320. [Google Scholar] [CrossRef] [PubMed]
- Danish, P.; Ali, Q.; Hafeez, M.; Malik, A. Antifungal and Antibacterial Activity of Aloe vera Plant Extract. Biol. Clin. Sci. Res. J. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Pouyafard, A.; Jabbaripour, N.; Jafari, A.A.; Owlia, F. Investigating the Anti-Fungal Activity of Different Concentrations of Aloe vera in Candida Albicans Infection under In Vitro Conditions. J. Adv. Med. Biomed. Res. 2023, 31, 268–274. [Google Scholar] [CrossRef]
- Amalia, R.; Sari, R.; Kedokteran, F.; Tanjungpura, U.; Hadari Nawawi Pontianak, J.H. Penentuan Nilai FICI Kombinasi Ekstrak Kulit Daun Lidah Buaya (Aloe vera (L.) Burm.f.) Dan Gentamisin Sulfat Terhadap Bakteri Staphylococcus Aureus Determination of FICI of Ethanolic Extract of Aloe vera Skin Leaves (Aloe vera (L.) Burm.f.) and Gentamicin Sulphate Againts Staphylococcus Aureus. Tradit. Med. J. 2017, 22, 175–181. [Google Scholar]
- Choi, S.H.; Shin, H.S. Anti-Inflammatory and Anti-Bacterial Effects of Aloe vera MAP against Multidrug-Resistant Bacteria. Nat. Product. Sci. 2017, 23, 286–290. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kim, K. Anti-Propionibacterium Acnes and the Anti-Inflammatory Effect of Aloe Ferox Miller Components. J. Herb. Med. 2017, 9, 53–59. [Google Scholar] [CrossRef]
- Bilal, M.; Lubis, M.S.; Yuniarti, R.; Nasution, H.M. Formulation Of Anti-Acne Extract Aloe vera (Aloe vera (L.) Burm.f.) In Hibiting The Activity Of Propionibacterium Acnes. Int. J. Health Pharm. 2022, 3, 241–248. [Google Scholar] [CrossRef]
- Firmansyah, F.; Vajrika, S.A.; Muhtadi, W.K. Effect of Combination of Carbopol-940 Base and HPMC Gel Extract of Aloe vera Flesh on Physical Properties and Antibacterial Activity of Propionibacterium Acnes. Malahayati Nurs. J. 2022, 4, 3347–3357. [Google Scholar] [CrossRef]
- Available online: https://www.esi.it/en/aloe-vera-gel-pure/ (accessed on 15 November 2024).
- Available online: https://nacomi-shop.pl/pl/p/serum-zelowe-aloesowe-do-twarzy-50-ml/126 (accessed on 15 November 2024).
- Available online: https://incidecoder.com/products/aloe-vera-australia-aloe-skin-hair-gel (accessed on 15 November 2024).
- Białon, M.; Krzysko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef]
- da Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; da Silva Melo, D.A.; Donadio, M.V.F.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.B.; et al. Antioxidant, Analgesic and Anti-Inflammatory Effects of Lavender Essential Oil. An. Acad. Bras. Cienc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef]
- De Rapper, S.; Viljoen, A.; Van Vuuren, S. The in Vitro Antimicrobial Effects of Lavandula Angustifolia Essential Oil in Combination with Conventional Antimicrobial Agents. Evid.-Based Complement. Altern. Med. 2016, 2016, 2752739. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielínska-Bliźniewska, H.; Dołegowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and in Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules 2019, 25, 95. [Google Scholar] [CrossRef]
- Tkachenko, H.; Opryshko, M.; Gyrenko, O.; Maryniuk, M.; Buyun, L.; Kurhaluk, N. Antibacterial Properties of Commercial Lavender Essential Oil against Some Gram-Positive and Gram-Negative Bacteria. Agrobiodivers. Improv. Nutr. Health Life Qual. 2022, 6, 220–228. [Google Scholar] [CrossRef]
- Zu, Y.; Yu, H.; Liang, L.; Fu, Y.; Efferth, T.; Liu, X.; Wu, N. Activities of Ten Essential Oils towards Propionibacterium Acnes and PC-3, A-549 and MCF-7 Cancer Cells. Molecules 2010, 15, 3200–3210. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Swarcewicz, M.; Dobrowolska, A. The Potential of Use Lavender from Vegetable Waste as Effective Antibacterial and Sedative Agents. Med. Chem. 2014, 4, 734–737. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Ahmad, K. Antimicrobial Properties of Some Plant Essential Oils against Two Human Pathogens. Int. J. Pharm. Chem. Anal. 2023, 9, 184–187. [Google Scholar] [CrossRef]
- Zuzarte, M.; Vale-Silva, L.; Gonçalves, M.J.; Cavaleiro, C.; Vaz, S.; Canhoto, J.; Pinto, E.; Salgueiro, L. Antifungal Activity of Phenolic-Rich Lavandula Multifida L. Essential Oil. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1359–1366. [Google Scholar] [CrossRef]
- Available online: https://inaessentials.pl/products/naturalny-szampon-lawendowy-przeciw-lupiezowi-do-przetluszczajacych-sie-wlosow-250-ml-z-olejkiem-lawendowym-1 (accessed on 15 November 2024).
- Available online: https://heritagestore.com/products/lavender-water-w-atomizer?_pos=4&_psq=lave&_ss=e&_v=1.0 (accessed on 15 November 2024).
- Available online: https://Www.Illavandetodiassisi.Com/Catalogo-Vendita-Online-d/Productidn/1866171/Lawendowy-Krem-Przeciwtrdzikowy-Do-Twarzy (accessed on 15 November 2024).
- Available online: https://incidecoder.com/products/avalon-organics-intense-defense-cleansing-gel (accessed on 15 November 2024).
- Ramya Premanath, J.; Sudisha, N.; Lakshmi Devi, S.M. Aradhya Antibacterial and Anti-Oxidant Activities of Fenugreek (Trigonella foenum graecum L.) Leaves. Res. J. Med. Plants 2011, 5, 695–705. [Google Scholar]
- Kulkarni, M.; Hastak, V.; Jadhav, V.; Date, A.A. Fenugreek Leaf Extract and Its Gel Formulation Show Activity against Malassezia Furfur. Assay. Drug Dev. Technol. 2020, 18, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Alluri, N.; Majumdar, M. Phytochemical Analysis and in Vitro Antimicrobial Activity of Calotropis Gigantea, Lawsonia Inermis and Trigonella Foecum-Graecum. Int. J. Pharm. Pharm. Sci. 2014, 6, 524–527. [Google Scholar]
- Subramaniam, G.; Cootee, R.R.P.; Han, C.C.; Sivasamugham, L.A. Anti-Bacterial Activity of Trigonella Foenum-Graecum against Skin Pathogens. J. Exp. Biol. Agric. Sci. 2021, 9, S110–S115. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Shahinuzzaman, M.; Haque, A.; Khatun, R.; Yaakob, Z. Gas Chromatography Mass Spectrometry Analysis and in Vitro Antibacterial Activity of Essential Oil from Trigonella Foenum-Graecum. Asian Pac. J. Trop. Biomed. 2015, 5, 1033–1036. [Google Scholar] [CrossRef]
- Available online: https://incidecoder.com/products/reequil-anti-recurrence-dandruff-lotion (accessed on 18 November 2024).
- Available online: https://www.bandi.pl/sklep/ekstrakty/tricho-wcierka-ekstrakt-przeciw-przetluszczaniu-sie-skory-glowy-i-wlosow-957/ (accessed on 18 November 2024).
- Available online: https://incidecoder.com/products/idraet-purifying-gel-cleanser (accessed on 18 November 2024).
- Available online: https://incidecoder.com/products/ilcsi-fenugreek-eye-contour-cream (accessed on 18 November 2024).
- Vale-Silva, L.; Silva, M.J.; Oliveira, D.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L.; Pinto, E. Correlation of the Chemical Composition of Essential Oils from Origanum Vulgare Subsp. Virens with Their in Vitro Activity against Pathogenic Yeasts and Filamentous Fungi. J. Med. Microbiol. 2012, 61, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum Vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and in Vivo Study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.; Souza, B.; De Oliveira Lima, E.; Nunes Guedes, D.; De Oliveira Pereira, F.; Leite De Souza, E.; Barbosa De Sousa, F. Efficacy of Origanum Essential Oils for Inhibition of Potentially Pathogenic Fungi. Braz. J. Pharm. Sci. 2010, 46, 499–508. [Google Scholar]
- Angiolella, L.; Rojas, F.; Mussin, J.; Giusiano, G. Modulatory Effect of Origanum Vulgare Essential Oil and Carvacrol on Malassezia Spp. Virulence Factors. Med. Mycol. 2023, 61, myad026. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.; Plessas, S.; Kimbaris, A.; Varvatou, M.; Mantzourani, I.; Fournomiti, M.; Tzouti, V.; Nerantzaki, A.; Bezirtzoglou, E. Mode of Antimicrobial Action of Origanum Vulgare Essential Oil against Clinical Pathogens. Curr. Res. Nutr. Food Sci. 2017, 5, 109–115. [Google Scholar] [CrossRef]
- Amri, I.A.; Ramadani, N.F.; Hamidah, F.; Dameanti, F.N.A.E.P.; Adrenalin, S.L. Potential Antibacterial Effects of Ethanol Extract and Essential Oil of Origanum Vulgare on Klebsiella Pneumonia and Staphylococcus Aureus. World’s Vet. J. 2023, 13, 486–491. [Google Scholar] [CrossRef]
- Available online: https://bewit.love/gb/produkt/esencialni-voda-z-oregana?variant=4747 (accessed on 18 November 2024).
- Available online: https://incidecoder.com/products/fungisol-antibacterial-soap (accessed on 18 November 2024).
- Available online: https://incidecoder.com/products/margaret-dabbs-london-nail-cuticle-treatment (accessed on 20 November 2024).
- Shafodino, F.S.; Lusilao, J.M.; Mwapagha, L.M. Phytochemical Characterization and Antimicrobial Activity of Nigella sativa Seeds. PLoS ONE 2022, 17, e0272457. [Google Scholar] [CrossRef]
- Rahman, A.U.; Abdullah, A.; Faisal, S.; Mansour, B.; Yahya, G. Unlocking the Therapeutic Potential of Nigella sativa Extract: Phytochemical Analysis and Revealing Antimicrobial and Antioxidant Marvels. BMC Complement. Med. Ther. 2024, 24, 266. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Upadhyay, B.; Buragohain, J.; Rai, M. Phytochemical Analysis, Antioxidant, Antimicrobial and Anticancer Activity of Nigella sativa and Oroxylum Indicum. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2024, 94, 1059–1065. [Google Scholar] [CrossRef]
- Sharikh, M.; Udayalaxmi, J.; Rao, P.; Student, M. A Study of Antibacterial Effect of Nigella sativa Seed Extract on Clinical Isolates of Methicillin-Resistant Staphylococcus Aureus (MRSA). Indian J. Public Health Res. Dev. 2020, 11, 1516–1520. [Google Scholar]
- Nawarathne, N.W.; Wijesekera, K.; Wijayaratne, W.M.D.G.B.; Napagoda, M. Development of Novel Topical Cosmeceutical Formulations from Nigella sativa L. with Antimicrobial Activity against Acne-Causing Microorganisms. Sci. World J. 2019, 2019, 5985207. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Zargaran, A.; Farzaei, M.H.; Iranpanah, A.; Heydarpour, F.; Najafi, F.; Rahimi, R. The Effect of a Hydrogel Made by Nigella sativa L. on Acne Vulgaris: A Randomized Double-Blind Clinical Trial. Phytother. Res. 2020, 34, 3052–3062. [Google Scholar] [CrossRef] [PubMed]
- Aljabre, S.H.M.; Randhawa, M.A.; Akhtar, N.; Alakloby, O.M.; Alqurashi, A.M.; Aldossary, A. Antidermatophyte Activity of Ether Extract of Nigella sativa and Its Active Principle, Thymoquinone. J. Ethnopharmacol. 2005, 101, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://incidecoder.com/products/blume-meltdown-acne-oil (accessed on 20 November 2024).
- Available online: https://arganove.pl/products/naturalne-mydlo-z-czarnuszka-antybakteryjne?_pos=8&_sid=edbf64ae7&_ss=r (accessed on 20 November 2024).
- Salatino, A. Perspectives for Uses of Propolis in Therapy against Infectious Diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef] [PubMed]
- Ajobiewe, P.T.; Malann, Y.D.; Ajobiewe, H.F.; Ajobiewe, J.O.; Udefuna, P.A.; Ogundeji, A.A.; Yashim, A.N.; Alau, K.K.; Ibrahim, A.E.; Abioye, J.O.K.; et al. Critical Appraisal of the Action of Honey on Skin Infection, a Case Study of Honeys from Four Different Locations in Nigeria. Sch. J. Appl. Med. Sci. 2022, 10, 389–392. [Google Scholar] [CrossRef]
- Ariani, Y.; Aliyatur, T.; Wicaksono, B. Literature Review: The Effect of Honey in Pressure Ulcer Wound Healing Acceleration. J. Rekonstr. Dan Estet. 2022, 7, 37–42. [Google Scholar] [CrossRef]
- Castro, M.L.; Cury, J.A.; Rosalen, P.L.; Alencar, S.M.; Duarte, S.; Koo, H. Própolis do Sudeste e Nordeste do Brasil: Influência da Sazonalidade na Atividade Antibacteriana e Composição Fenólica. Química Nova 2007, 30, 1512–1516. [Google Scholar] [CrossRef]
- Wahyuningtyas, E.S.; Rizkiyani, A.D.; Handayani, E. Madu Manuka Sebagai Terapi Penyembuhan Luka Pada Pasien Ulkus Diabetik: Literature Review. J. Keperawatan Widya Gantari Indones. 2024, 8, 63–74. [Google Scholar] [CrossRef]
- Bazaid, A.S.; Alamri, A.; Almashjary, M.N.; Qanash, H.; Almishaal, A.A.; Amin, J.; Binsaleh, N.K.; Kraiem, J.; Aldarhami, A.; Alafnan, A. Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey. Appl. Sci. 2022, 12, 9928. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Karwowski, B.T. The Antioxidant Potential of Commercial Manuka Honey from New Zealand—Biochemical and Cellular Studies. Curr. Issues Mol. Biol. 2024, 46, 6366–6376. [Google Scholar] [CrossRef] [PubMed]
- El-Senduny, F.F.; Hegazi, N.M.; Abd Elghani, G.E.; Farag, M.A. Manuka Honey, a Unique Mono-Floral Honey. A Comprehensive Review of Its Bioactives, Metabolism, Action Mechanisms, and Therapeutic Merits. Food Biosci. 2021, 42, 101038. [Google Scholar] [CrossRef]
- Girma, A.; Seo, W.; SheI, R.C. Antibacterial Activity of Varying UMF-Graded Manuka Honeys. PLoS ONE 2019, 14, e0224495. [Google Scholar] [CrossRef]
- Bouacha, M.; Besnaci, S.; Boudiar, I. Comparative Study of the Antibacterial Activity of Algerian Honeys and Manuka Honey Toward Pathogenic Bacteria from Burn Wound Infections. Mikrobiol. Zh 2023, 85, 26–36. [Google Scholar] [CrossRef]
- Chhawchharia, A.; Haines, R.R.; Green, K.J.; Barnett, T.C.; Bowen, A.C.; Hammer, K.A. In Vitro Antibacterial Activity of Western Australian Honeys, and Manuka Honey, against Bacteria Implicated in Impetigo. Complement. Ther. Clin. Pract. 2022, 49, 101640. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.R.; Afegbua, S.L. Single and Joint Antibacterial Activity of Aqueous Garlic Extract and Manuka Honey on Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 472–478. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Manuka Honey in Combination with Azithromycin Shows Potential for Improved Activity against Mycobacterium Abscessus. Cell Surf. 2022, 8, 100090. [Google Scholar] [CrossRef]
- Liang, J.; Adeleye, M.; Onyango, L.A. Combinatorial Efficacy of Manuka Honey and Antibiotics in the in Vitro Control of Staphylococci and Their Small Colony Variants. Front. Cell Infect. Microbiol. 2023, 13, 1219984. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.F.; Molan, P.C.; Harfoot, C.G. The Sensitivity of Dermatophytes to the Antimicrobial Activity of Manuka Honey and Other Honey. Pharm. Pharmacol. Commun. 1996, 2, 471–473. [Google Scholar]
- Available online: https://Kikgel.Com.Pl/Produkty/Manuka/#manuka-Ig (accessed on 20 November 2024).
- Available online: https://manukahealth.shop/en/products/manuka-blemish-spot-gel (accessed on 20 November 2024).
- Available online: https://incidecoder.com/products/la-bella-figura-purifying-manuka-mask (accessed on 20 November 2024).
- Available online: https://incidecoder.com/products/arata-anti-dandruff-hair-tonic (accessed on 20 November 2024).
- da Silva, J.F.M.; de Souza, M.C.; Matta, S.R.; de Andrade, M.R.; Vidal, F.V.N. Correlation Analysis between Phenolic Levels of Brazilian Propolis Extracts and Their Antimicrobial and Antioxidant Activities. Food Chem. 2006, 99, 431–435. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abd, F.K.; Hadyb, E.; Abd, F.A.M. Chemical Composition and Antimicrobial Activity of European Propolis. Z. Naturforschung C 2000, 55, 70–75. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; Barreto, G.D.A.; Costa, S.S.; Da Silva, D.F.; Brandão, H.N.; Da Rocha, J.L.C.; Dellagostin, O.A.; Henriques, J.A.P.; Umsza-Guez, M.A.; et al. Chemical Composition and Biological Activity of Extracts Obtained by Supercritical Extraction and Ethanolic Extraction of Brown, Green and Red Propolis Derived from Different Geographic Regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Uzel, A.; Sorkun, K.; Önçaǧ, Ö.; Çoǧulu, D.; Gençay, Ö.; Salih, B. Chemical Compositions and Antimicrobial Activities of Four Different Anatolian Propolis Samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kalaba, V.; Golić, B.; Tanja, I.L.I.Ć.; Kalaba, D.; Zrnić, N. Antibacterial Action of Propolis on Selected Bacterial Reference Strains. Vet. J. Repub. Srp. 2020, 20, 173–182. [Google Scholar] [CrossRef]
- Boyanova, L.; Kolarov, R.; Gergova, G.; Mitov, I. In Vitro Activity of Bulgarian Propolis against 94 Clinical Isolates of Anaerobic Bacteria. Anaerobe 2006, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kusumaningtyas, E.; Endrawati, D.; Siswandi, R. The Use of Propolis in Ointment Ingredient for the Treatment of Dermatophytosis Infection. IOP Conf. Ser. Earth Environ. Sci. 2023, 1271, 012073. [Google Scholar] [CrossRef]
- Hamdan, I.A.M.; Khalaf, T.M. The Effectiveness of Propolis as a Natural Antibiotic against Some Skin Fungi Pathogenic to Humans. Int. J. Pharm. Bio Med. Sci. 2024, 4, 115–121. [Google Scholar] [CrossRef]
- Konsila, K.; Assavalapsakul, W.; Phuwapraisirisan, P.; Chanchao, C. Anti-Malassezia Globosa (MYA-4889, ATCC) Activity of Thai Propolis from the Stingless Bee Geniotrigona Thoracica. Heliyon 2024, 10, e29421. [Google Scholar] [CrossRef] [PubMed]
- Güneş, Ü.Y.; Eşer, I. Effectiveness of a Honey Dressing for Healing Pressure Ulcers. J. Wound Ostomy Cont. Nurs. 2007, 34, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Moolenaar, M.; Louwrens Poorter, R.; Van Der Toorn, P.P.G.; Willem Lenderink, A.; Poortmans, P.; Cornelis Gerardus Egberts, A. The Effect of Honey Compared to Conventional Treatment on Healing of Radiotherapy-Induced Skin Toxicity in Breast Cancer Patients. Acta Oncol. 2006, 45, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://Apipol.Com.Pl/Produkt/Masc-Propolisowa-3/ (accessed on 21 November 2024).
- Available online: https://Kerpro.Pl/Remmeles-Propolis-Spray-Do-Dezynfekcji-Stop-o-Dzialaniu-Odswiezajacym-500-Ml/ (accessed on 21 November 2024).
- Available online: https://incidecoder.com/products/ample-n-acne-shot-ampoule (accessed on 21 November 2024).
- Spilioti, E.; Vargiami, M.; Letsiou, S.; Gardikis, K.; Sygouni, V.; Koutsoukos, P.; Chinou, I.; Kassi, E.; Moutsatsou, P. Biological Properties of Mud Extracts Derived from Various Spa Resorts. Environ. Geochem. Health 2017, 39, 821–833. [Google Scholar] [CrossRef]
- Khlaifat, A.; Al-Khashman, O.; Qutob, H. Physical and Chemical Characterization of Dead Sea Mud. Mater. Charact. 2010, 61, 564–568. [Google Scholar] [CrossRef]
- Ma’or, Z.; Henis, Y.; Alon, Y.; Orlov, E.; Sørensen, K.B.; Oren, A. Antimicrobial Properties of Dead Sea Black Mineral Mud. Int. J. Dermatol. 2006, 45, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Almalty, A.M.; Alkhatib, H.S. The Cutaneous Effects of Long-Term Use of Dead Sea Mud on Healthy Skin: A 4-Week Study. Int. J. Dermatol. 2021, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://bingospa.eu/pl/p/TRADZIK-LOJOTOK-Redual-Bloto-Morze-Martwe-100-BINGOSPA/35 (accessed on 21 November 2024).
- Available online: https://incidecoder.com/products/mg217-psoriasis-dead-sea-soap (accessed on 21 November 2024).
- Available online: https://Puremineral.ca/Product/Mud-Hair-Shampoo/ (accessed on 21 November 2024).
- Sarruf, F.D.; Contreras, V.J.P.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. The Scenario of Clays and Clay Minerals Use in Cosmetics/Dermocosmetics. Cosmetics 2024, 11, 7. [Google Scholar] [CrossRef]
- Daneluz, J.; da Silva Favero, J.; dos Santos, V.; Weiss-Angeli, V.; Gomes, L.B.; Mexias, A.S.; Bergmann, C.P. The Influence of Different Concentrations of a Natural Clay Material as Active Principle in Cosmetic Formulations. Mater. Res. 2020, 23, e20190572. [Google Scholar] [CrossRef]
- Behroozian, S.; Svensson, S.L.; Li, L.Y.; Davies, J.E. Broad-Spectrum Antimicrobial and Antibiofilm Activity of a Natural Clay Mineral from British Columbia, Canada. mBio 2020, 11, e02350-20. [Google Scholar] [CrossRef]
- Gomes, C.F.; Gomes, J.H.; da Silva, E.F. Bacteriostatic and Bactericidal Clays: An Overview. Environ. Geochem. Health 2020, 42, 3507–3527. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Holland, M.; Eberl, D.D.; Brunet, T.; Burnet de Courrsou, L. Killer clays! Natural antibacterial clay minerals. Mineral. Soc. Bull. 2004, 139, 3–8. [Google Scholar]
- Adusumilli, S.; Haydel, S.E. In Vitro Antibacterial Activity and in Vivo Efficacy of Hydrated Clays on Mycobacterium ulcerans Growth. BMC Complement. Altern. Med. 2016, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Haydel, S.E.; Remenih, C.M.; Williams, L.B. Broad-Spectrum in Vitro Antibacterial Activities of Clay Minerals against Antibiotic-Susceptible and Antibiotic-Resistant Bacterial Pathogens. J. Antimicrob. Chemother. 2008, 61, 353–361. [Google Scholar] [CrossRef]
- Williams, L.B.; Haydel, S.E.; Giese, R.F.; Eberl, D.D. Chemical and Mineralogical Characteristics of French Green Clays Used for Healing. Clays Clay Miner. 2008, 56, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Behroozian, S.; Svensson, S.L.; Davies, J. Kisameet Clay Exhibits Potent Antibacterial Activity against the ESKAPE Pathogens. mBio 2016, 7, e01842-15. [Google Scholar] [CrossRef]
- Meier, L.; Stange, R.; Michalsen, A.; Uehleke, B. Clay Jojoba Oil Facial Mask for Lesioned Skin and Mild Acne-Results of a Prospective, Observational Pilot Study. Forsch. Komplementarmed 2012, 19, 75–79. [Google Scholar] [CrossRef]
- Available online: https://ecospa.pl/francuska-glinka-zielona-montmorillonite (accessed on 22 November 2024).
- Available online: https://www.cattier-paris.com/en/visage/masque-visage/mask-a-clay-green-new-formula.html (accessed on 22 November 2024).
- Available online: https://incidecoder.com/products/hema-hydrating-face-wash-with-green-clay (accessed on 22 November 2024).
- Hussain, F.; Pathan, S.; Sahu, K.; Gupta, B. Herbs as Cosmetics for Natural Care: A Review. GSC Biol. Pharm. Sci. 2022, 19, 316–322. [Google Scholar] [CrossRef]
- Klaschka, U. Naturally Toxic: Natural Substances Used in Personal Care Products. Environ. Sci. Eur. 2015, 27, 1. [Google Scholar] [CrossRef]
- Warke, S.P.; Patil, P.R.; Sarode, P.; Sachdev, S.; Ingale, K.S.; Bhirud, M.R. Design and Evaluation of Natural Face Pack. Int. J. Multidiscip. Res. 2024, 6, 1–8. [Google Scholar] [CrossRef]
- Yuwaniyom, P. Legal Problem Arising from Organic Cosmetic. Ph.D. Thesis, Faculty of Law, Thammasat University, Bangkok, Thailand, 2015. [Google Scholar]
- Lauriola, M.M.; Corazza, M. The Wild Market of Natural Cosmetics of Obscure Safety. Dermatology 2019, 235, 527–528. [Google Scholar] [CrossRef]
- Available online: https://incidecoder.com/products/yari-100-pure-natural-garlic-oil-for-body-and-hair (accessed on 28 November 2024).
- Available online: https://incidecoder.com/products/meideme-green-salvia-multi-soothing-gel (accessed on 28 November 2024).
- Available online: https://incidecoder.com/products/butuh-wetless-deodorant-antiperspirant (accessed on 28 November 2024).
| Main Skin and Subcutaneous Tissue Diseases | Pathogen | Ref |
|---|---|---|
| Acne vulgaris | Cutibacterium acnes, Propionibacterium acnes | [2] |
| Folliculitis | Staphylococcus aureus | [3] |
| Furuncles (or Carbuncles) | Staphylococcus aureus | [4] |
| Impetigo | Staphylococcus aureus, Streptococcus pyogenes | [5] |
| Cellulitis | Streptococcus spp., Staphylococcus aureus | [6] |
| Erysipelas | Streptococcus pyogenes | [7] |
| Cutaneous mycobacterial infections | Mycobacterium | [8] |
| Erythematosquamous dermatitis | Corynebacterium minutissimum | [9] |
| Pitted keratolysis | Corynebacterium sp., Micrococcus sedentarius, Dermatophilus congolensis | [10] |
| Dermatophytosis (glabrous skin/hairy skin/nails) | Trichophyton spp., Epidermophyton spp., Microsporum spp. | [11] |
| Green nail syndrome | Pseudomonas aeruginosa | [12] |
| Cutaneous candidiasis | Candida spp. | [13] |
| Pityriasis versicolor | Malassezia furfur | [14] |
| Infections after aesthetic medical procedures | Mycobacterium, Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli | [15,16] |
| Raw Materials | Producer | Country of Origin | Trade Name | Cosmetic Form | Type of Substance in the Cosmetic Composition (INCI) | Properties of the Cosmetic According to the Producer | Ref. | Ref. Antimicrobial Activity |
|---|---|---|---|---|---|---|---|---|
| Allium L. | Farmasi Dr.C. Tuna | Turkey | Reviving Hair Oil Hair and Scalp | Serum | Allium Sativum Bulb Oil |
| [46] | [31,32,33,34,35,36,37,38,39] |
| Yari | Netherlands | 100% Pure Natural Garlic Oil for Body and Hair | Oil | Allium Sativum Bulb Extract |
| [177] | ||
| Etude House | Korean | Ac Clean Up Toner | Toner | Allium Sativum Bulb Extract |
| [44] | ||
| Salvia L. | NutriBiotic | USA | Super Shower Gel | Gel | Salvia Officinalis (Sage) |
| [54] | [47,48,49,50,51,52] |
| Diagnosis | Poland | Novaclear Handsclear Antibacterial Care Soap | Soap | Salvia Officinalis Flower/Leaf/Stem Extract |
| [53] | ||
| Medume | Korea | Green Salvia Multi Soothing Gel | Gel | Salvia Plebeia Extract |
| [178] | ||
| Melaleuca alternifolia L. | AROMATICA | Korea | Tea Tree Calming Gel | Gel | Melaleuca Alternifolia Leaf Extract |
| [63] | [56,57,58,59,60,61,62] |
| Arrkada | Poland | Serum TC16 Arkada for Skin and Nail Regeneration | Serum | Melaleuca Alternifolia Leaf Oil |
| [64] | ||
| Tree Tea Therapy | United States | Antiseptic Ointment | Ointment | (Tea Tree) leaf oil Melaleuca alternifolia |
| [65] | ||
| Aloe L. | ESI Aloe Vera Gel | Italy | Pure Aloe Vera Gel | Gel | Aloe Barbadensis Leaf Juice |
| [76] | [66,67,68,69,71,72,73,74,75] |
| Aloe Vera Australia | Australia | Aloe Skin and Hair Gel | Gel | Aloe Vera Juice |
| [78] | ||
| Nacomi | Poland | Soothing Gel Formula | Serum | Aloe Barbadensis Leaf Juice, Aloe Barbadensis Leaf Extract |
| [77] | ||
| Lavendula L. | InEssentials | Poland | Natural Lavender Shampoo | Shampoo | Lavandula Angustifolia Flower Water |
| [88] | [79,81,83,84,85,86,87] |
| Heritage Store | United States | Lavender Water | Mist | Lavandula Angustifolia (Lavender) Oil |
| [89] | ||
| Il Lavandeto Di Assisi | Italy | Lavender Anti-Acne Face Cream | Cream | Lavandula Angustifolia Distillate Water |
| [90] | ||
| Trigonella L. | Idraet | Brazil | Purifying Gel Cleanser | Gel | Trigonella Foenum-Graecum |
| [99] | [92,93,94,96] |
| Bandi | Poland | Bandi Tricho-Esthetic | Serum | Trigonella Foenum - Graecum Seed Extract |
| [98] | ||
| Re’equil | India | Anti-Recurrence Dandruff Lotion | Lotion | Trigonella Foenum-Graecum Seed Oil |
| [97] | ||
| Origanum L. | Fungisol | Philippines | Fun.G Antibacterial Soap | Soap | Origanum Vulgare (Oregano) Extract |
| [108] | [101,102,103,104,105,106] |
| BEWIT | Bulgaria | Oregano Hydrolate | Hydrolat | Origanum Vulgare Flower Water |
| [107] | ||
| Margaret Dabbs London | Great Britain | Nail and Cuticle Treatment | Oil | Origanum Vulgare (Oregano) Leaf Oil |
| [109] | ||
| Nigella L. | Butuh | Indonesia | Weightless Deodorant Antiperspirant | Antiperspirant | Nigella Sativa Seed Extract |
| [179] | [110,111,112,113,114,115,116] |
| Arganove | Poland | Antibacterial Soap | Soap | Nigella Sativa Seed Oil, Nigella Sativa Powder |
| [118] | ||
| Blume | Canada | Meltdown Acne Oil | Oil | Black Cumin Seed Oil |
| [117] | ||
| Leptospermum scoparium | Manuka Health | New Zealand | Manuka Blemish Spot Gel | Gel | Leptospermum scoparium Branch/Leaf Oil |
| [135] | [127,128,129,130,132,133] |
| Arata | India | Anti-dandruff Hair Tonic | Tonic | Leptospermum scoparium (Manuka) Branch/Leaf Oil |
| [137] | ||
| La Bella Figura | United States | Purifying Manuka Mask | Mask | Organic Leptospermum Scoparium (Raw Manuka Honey) |
| [136] | ||
| Propolis cera | AMPLE:N | Korea | Acne Shot Ampoule | Serum | Propolis Extract |
| [151] | [122,140,141,142,143,144,145,146,147,148] |
| Farmina | Poland | Propolis Ointment 3% | Ointment | Propolis Extract |
| [149] | ||
| Remmele’s Propolis | Germany | Propolis Balsam Spray | Spray | Propolis Cera |
| [150] | ||
| Dead Sea Mud | BINGOSPA | Poland | Redual+ | Mask | Maris Limus |
| [156] | [154,155] |
| MG217 | Israel | Psoriasis Dead Sea Soap | Soap | Maris Sal (Dead Sea Salt), Maris Limus (Dead Sea Mud) |
| [157] | ||
| Pure Mineral | Izrael | Mud Hair Shampoo | Shampoo | Maris Limus |
| [158] | ||
| French Green Clay | ECOSPA | Poland | 100% Natural French Green Clay | Powder | Montmorillonite, Illite French Green Clay |
| [169] | [161,162,165,166,167,168] |
| CATTIER PARIS | France | Green Clay Mask | Mask | Kaolin, Illite, Montmorillonite |
| [170] | ||
| Hema | Netherlands | Hydrating Face Wash with Green Clay | Gel | Illite, Kaolin, Montmorillonite |
| [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulik-Siarek, K.; Klimek-Szczykutowicz, M.; Błońska-Sikora, E.; Zarembska, E.; Wrzosek, M. Exploring the Antimicrobial Potential of Natural Substances and Their Applications in Cosmetic Formulations. Cosmetics 2025, 12, 1. https://doi.org/10.3390/cosmetics12010001
Kulik-Siarek K, Klimek-Szczykutowicz M, Błońska-Sikora E, Zarembska E, Wrzosek M. Exploring the Antimicrobial Potential of Natural Substances and Their Applications in Cosmetic Formulations. Cosmetics. 2025; 12(1):1. https://doi.org/10.3390/cosmetics12010001
Chicago/Turabian StyleKulik-Siarek, Katarzyna, Marta Klimek-Szczykutowicz, Ewelina Błońska-Sikora, Emilia Zarembska, and Małgorzata Wrzosek. 2025. "Exploring the Antimicrobial Potential of Natural Substances and Their Applications in Cosmetic Formulations" Cosmetics 12, no. 1: 1. https://doi.org/10.3390/cosmetics12010001
APA StyleKulik-Siarek, K., Klimek-Szczykutowicz, M., Błońska-Sikora, E., Zarembska, E., & Wrzosek, M. (2025). Exploring the Antimicrobial Potential of Natural Substances and Their Applications in Cosmetic Formulations. Cosmetics, 12(1), 1. https://doi.org/10.3390/cosmetics12010001







