Soybean Oil Enriched with Antioxidants Extracted from Watermelon (Citrullus colocynthis) Skin Sap and Coated in Hydrogel Beads via Ionotropic Gelation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Material
2.1.2. Materials
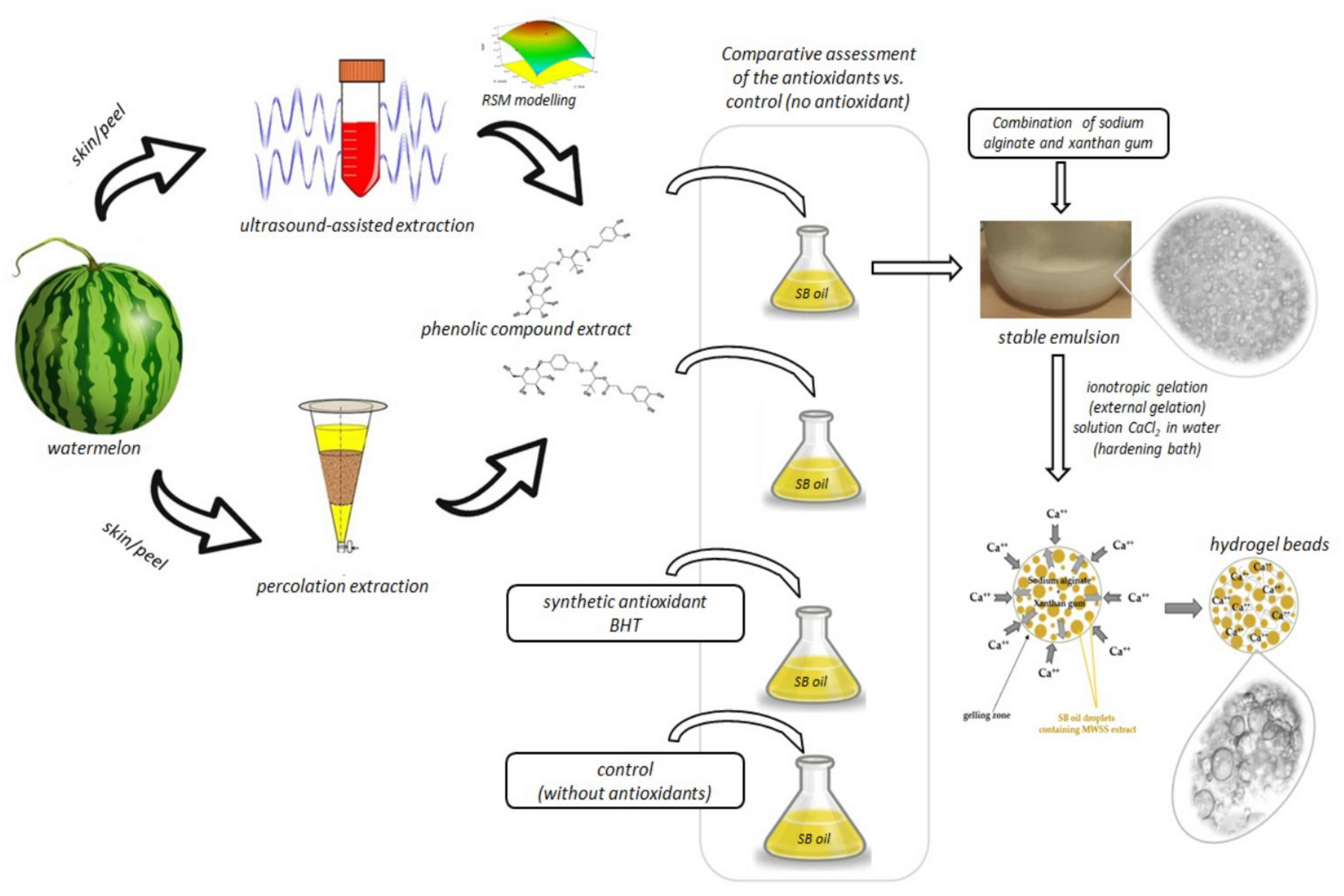
2.2. C. colocynthis Watermelon Skin Extration
2.2.1. Percolation Extraction
2.2.2. Ultrasound-Assisted Extraction (UAE)
2.3. Sample Preparation for Antioxidant Tests in Soybean Oil
2.4. Analytical Methods
2.4.1. Measuring the Amounts of Phenolic Compounds of WSS
2.4.2. Measuring DPPH Radical Scavenging Activity Assay
2.4.3. Measuring the Acid Value of Oil
- N = normality
- V = volume of sodium hydroxide titrant used (mL)
- W = weight of the fatty oil being examined (g)
2.4.4. Thiobarbituric-Acid-Reactive Substances (TBARS) Assay
2.5. Response Surface Methodology
2.6. Preparation and Evaluation of Emulsion
2.6.1. Emulsion Preparation
2.6.2. OW Emulsion Stability Evaluation
2.7. Preparation of Hydrogel Beads
2.8. Determination of Encapsulation Efficiency
2.9. Analysis of Hydrogel Beads
2.9.1. Size Analysis
2.9.2. Morphological Characterization
2.10. Statistical Analysis
Analysis of Variance (ANOVA)
3. Results and Discussions
3.1. Optimization Process Variables
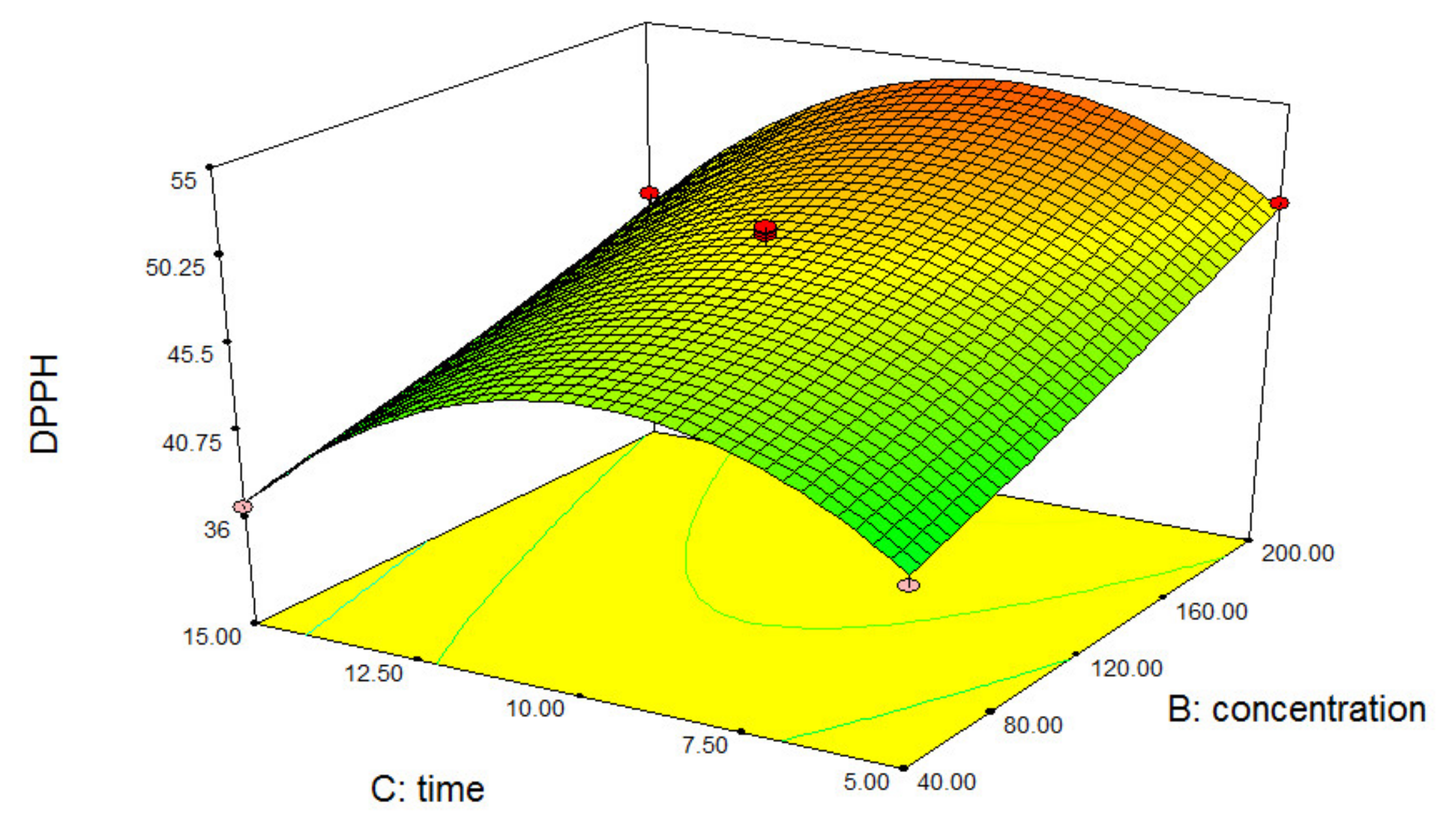
3.2. Interpretation of Free Radical Scavenging Activity of Diphenyl-1-Picrylhydrazyl (DPPH)
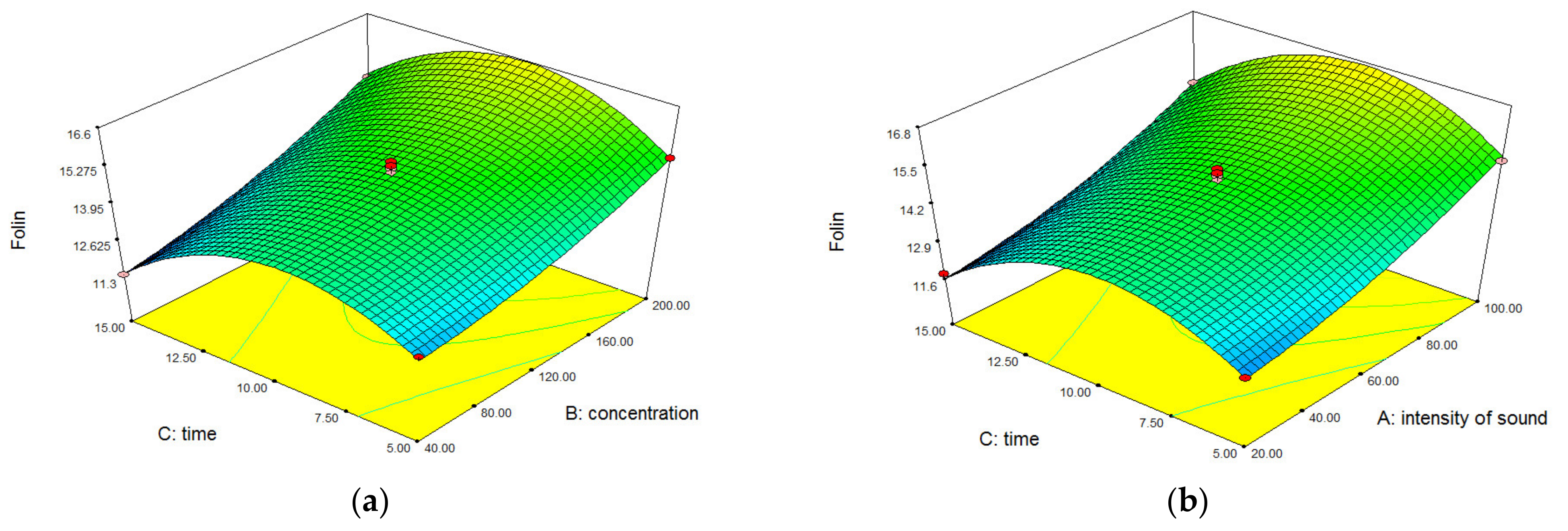
3.3. Interpretation of the Total Phenolic Content
3.4. Effect of Antioxidants on Oxidation of Soybean Oil
3.4.1. Determination of Acidity Index
3.4.2. Determination of Thiobarbituric Acid
3.5. Evaluation of Oil-in-Water Emulsions
Encapsulation Efficiency
3.6. Hydrogel Bead Characterization
Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture Foreign Agricultural Service. Oilseeds: World Markets and Trade. 2021. Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 28 September 2021).
- Galano, J.-M.; Lee, Y.Y.; Durand, T.; Lee, J.C.-Y. Special Issue on “Analytical Methods for Oxidized Biomolecules and Antioxidants” The use of isoprostanoids as biomarkers of oxidative damage, and their role in human dietary intervention studies. Free Radic. Res. 2015, 49, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Azadfar, E.; Rusu, A.; Trif, M.; Poushi, M.K.; Wang, Y. Improving the Shelf Life of Peeled Fresh Almond Kernels by Edible Coating with Mastic Gum. Coatings 2021, 11, 618. [Google Scholar] [CrossRef]
- Ravi Kiran, C.; Sasidharan, I.; Soban Kumar, D.R.; Sundaresan, A. Influence of natural and synthetic antioxidants on the degradation of Soybean oil at frying temperature. J. Food Sci. Technol. 2015, 52, 5370–5375. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Golay, P.; Acquistapace, S.; Craft, B.D. Increasing the oxidative stability of soybean oil through fortification with antioxidants. Int. J. Food Sci. Technol. 2015, 50, 666–673. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring Oxidative Stability and Changes in Key Volatile Compounds in Edible Oils during Ambient Storage through HS-SPME/GC–MS. Int. J. Food Prop. 2018, 20, S2926–S2938. [Google Scholar] [CrossRef]
- Kozłowska, M.; Gruczyńska, E. Comparison of the oxidative stability of soybean and sunflower oils enriched with herbal plant extracts. Chem. Zvesti. 2018, 72, 2607–2615. [Google Scholar] [CrossRef]
- Trif, M.; Socaciu, C. Evaluation of effiency, release and oxidation stability of sea buckthorn microencapsulated oil using Fourier transformed infrared spectroscopy. Chem. Listy 2008, 102, 1198–1199. [Google Scholar]
- Barden, L.; Vollmer, D.; Johnson, D.; Decker, E. Impact of iron, chelators, and free fatty acids on lipid oxidation in low-moisture crackers. J. Agric. Food Chem. 2015, 63, 1812–1818. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Chaudhary, V.; Thakur, N.; Kajla, P.; Kumar, M.; Trif, M. Natural Antimicrobials as Additives for Edible Food Packaging Applications: A Review. Foods 2021, 10, 2282. [Google Scholar] [CrossRef]
- Castelo-Branco, V.N.; Santana, I.; Di-Sarli, V.O.; Freitas, S.P.; Torres, A.G. Antioxidant capacity is a surrogate measure of the quality and stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2016, 118, 224–235. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ribeiro-Santos, R.; Ramos, F.; Conceição Castilho, M.; Sanches-Silva, A. UHPLC-DAD multi-method for determination of phenolics in aromatic plants. Food Anal. Methods 2018, 11, 440–450. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Teleky, B.-E.; Eleni, P.; Boukouvalas, C.; Krokida, M.; Kapsalis, N.; Rusu, A.V.; Socol, C.T.; Vodnar, D.C. Evaluation of the Bioactive Compounds Found in Tomato Seed Oil and Tomato Peels Influenced by Industrial Heat Treatments. Foods 2021, 10, 110. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Grossman, S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochem. Biophys. Res. Commun. 2007, 364, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, H.; Esmaeili, A.; Rad, H.S.; Delazar, A.; Behjati, M. Citrullus colocynthis as a medicinal or poisonous plant: A revised fact. J. Med. Plants Res. 2012, 6, 4922–4927. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Jawad, A.H.; Ngoh, Y.S.; Radzun, K.A. Utilization of watermelon (Citrullus lanatus) rinds as a natural low-cost biosorbent for adsorption of methylene blue: Kinetic, equilibrium and thermodynamic studies. J. Taibah Univ. Sci. 2018, 12, 371–381. [Google Scholar] [CrossRef]
- Dietrich, T.; Velasco, M.V.; Echeverría, P.; Pop, B.; Rusu, A. Crop and plant biomass as valuable material for BBB. Alternatives for valorization of green wastes. In Biotransformation of Agricultural Waste and By-Products: The Food, Feed, Fibre, Fuel (4F) Economy; Elsevier: San Diego, CA, USA, 2016. [Google Scholar]
- Varnham, A. Seed Oil: Biological Properties, Health Benefits and Commercial Applications; Nova Science Publishers: New York, NY, USA, 2014. [Google Scholar]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs with Special Emphasis on Self-Emulsifying Systems. Int. Sch. Res. Not. Pharm. 2013, 2013, 848043. [Google Scholar] [CrossRef]
- Jurj, A.; Pop, L.A.; Zanoaga, O.; Ciocan-Cârtiţă, C.A.; Cojocneanu, R.; Moldovan, C.; Raduly, L.; Pop-Bica, C.; Trif, M.; Irimie, A.; et al. New Insights in Gene Expression Alteration as Effect of Paclitaxel Drug Resistance in Triple Negative Breast Cancer Cells. Cell Physiol. Biochem. 2020, 54, 648–664. [Google Scholar] [PubMed]
- Corstens, M.N.; Troost, F.J.; Alleleyn, A.M.E.; Klaassen, T.; Berton-Carabin, C.C.; Schroën, K.; Masclee, A.A.M. Encapsulation of lipids as emulsion-alginate beads reduces food intake: A randomized placebo-controlled cross-over human trial in overweight adults. Nutr. Res. 2019, 63, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray Drying for the Encapsulation of Oils—A Review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef]
- Yingngam, B.; Kacha, W.; Rungseevijitprapa, W.; Sudta, P.; Prasitpuriprecha, C.; Brantner, A. Response Surface Optimization of Spray-Dried Citronella Oil Microcapsules with Reduced Volatility and Irritation for Cosmetic Textile Uses. Powder Technol. 2019, 355, 372–385. [Google Scholar] [CrossRef]
- Bangar, S.P.; Siroha, A.K.; Nehra, M.; Trif, M.; Ganwal, V.; Kumar, S. Structural and Film-Forming Properties of Millet Starches: A Comparative Study. Coatings 2021, 11, 954. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Ori-Jesu, M. The use of antioxidants in vegetable oils—A review. Afr. J. Biotechnol. 2008, 7, 4836–4842. [Google Scholar]
- Zhang, G.; Chen, Y.; Tariq, K.; An, Z.; Wang, S.; Qumar Memon, F.; Si, H. Optimization of ultrasound assisted extraction method for polyphenols from Desmodium triquetrum (L.) DC. with response surface methodology (RSM) and in vitro determination of its antioxidant properties. Czech J. Food Sci. 2020, 38, 115–122. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q.; Wu, Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013, 20, 846–854. [Google Scholar] [CrossRef]
- Mas’ud, F.; Mahendradatta, M.; Laga, A.; Zainal, Z. Optimization of mango seed kernel oil extraction using response surface methodology. OCL 2017, 24, D503. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Bartolomé, B.; Aguilera, Y.; Martin-Cabrejas, M.A. Extraction of phenolic compounds from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Adesanya, A.O.; Olaseinde, O.O.; Oguntayo, O.D.; Otulana, J.O.; Adefule, A.K. Effects of Methanolic Extract of Citrullus lanatus Seed on Experimentally induced prostatic Hyperplasia. Eur. J. Med. Plants 2011, 1, 171–179. [Google Scholar]
- Kolawole, T.; Dapper, D.V.; Oluwatayo, O.B.; Wali, C. Effects of Methanolic Extract of the Rind of Lanatus (Watermelon) in Aspirin Induced Gastric Ulceration in Male Wistar Rats. Merit Res. J. Med. Med Sci. 2016, 4, 344–350. [Google Scholar]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Way, M.L.; Jones, J.E.; Nichols, D.S.; Dambergs, R.G.; Swarts, N.D. A Comparison of Laboratory Analysis Methods for Total Phenolic Content of Cider. Beverages 2020, 6, 55. [Google Scholar] [CrossRef]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2018, 101, 1466–1472. [Google Scholar] [CrossRef]
- Refinery, N.P.; Braimah, M.N. Utilization of response surface methodology (RSM) in the optimization of crude oil refinery. J. Multidiscip. Eng. Sci. Technol. 2016, 3, 4361–4369. [Google Scholar]
- Gangurde, A.B.; Amin, P.D. Microencapsulation by Spray Drying of Vitamin A Palmitate from Oil to Powder and Its Application in Topical Delivery System. J. Encapsulation Adsorpt. Sci. 2017, 7, 10–39. [Google Scholar] [CrossRef]
- Rusu, A.V.; Criste, F.L.; Mierliţă, D.; Socol, C.T.; Trif, M. Formulation of Lipoprotein Microencapsulated Beadlets by Ionic Complexes in Algae-Based Carbohydrates. Coatings 2020, 10, 302. [Google Scholar] [CrossRef]
- UTHSCSA. Available online: https://www.uthscsa.edu/academics/dental/departments/comprehensive-dentistry?/dig/itdesc.html (accessed on 26 September 2021).
- Easy Freeware. Available online: https://www.easyfreeware.com/imagetool-191478-freeware.html (accessed on 26 September 2021).
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.P.; Mason, T.J. Potential for the use of Ultrasound in the Extraction of Antioxidants from Rosmarinus officinalis for the Food and Pharmaceutical Indus. J. Ultrason. Sonochem. 2004, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy is flavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, X.; Hao, Y.; Xu, G.; Xu, G.; Liu, D. Ultrasound assisted extraction of hesperidin from Penggan (Citrus reticulata) peel. Ultrason. Sonochem. 2008, 15, 227–232. [Google Scholar] [CrossRef]
- Zou, T.-B.; Wang, M.; Gan, R.-Y.; Ling, W.-H. Optimization of Ultrasound-Assisted Extraction of Anthocyanins from Mulberry, Using Response Surface Methodology. Int. J. Mol. Sci. 2011, 12, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Kamran Khan, M.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimization of ultrasound assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Y.Q.; Basavanagoud, B.; Jamil, M.K. Characteristics studies of molecular structures in drugs. Saudi Pharm. J. 2017, 25, 580–586. [Google Scholar] [CrossRef]
- Qadir, R.; Anwar, F.; Gilani, M.A.; Zahoor, S.; Misbah ur Rehman, M.; Mustaqeem, M. RSM/ANN based optimized recovery of phenolics from mulberry leaves by enzyme-assisted extraction. Czech J. Food Sci. 2019, 37, 99–105. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Monzón, L.; Becerra, G.; Aguirre, E.; Rodríguez, G.; Villanueva, E. Ultrasound-assisted extraction of polyphenols from avocado residues: Modeling and optimization using response surface methodology and artificial neural networks. Sci. Agropecu. 2021, 12, 33–40. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Sepúlveda, L.; Verma, D.K.; Luna-García, H.A.; Rodríguez-Durán, L.V.; Ilina, A.; Aguilar, C.N. Conventional and Emerging Extraction Processes of Flavonoids. Processes 2020, 8, 434. [Google Scholar] [CrossRef]
- Kv, S.; Thejasri Br, K.; Gds, S.; Sivaji, G. Optimization of ultrasound-assisted extraction of watermelon seed oil using response surface methodology. Pharma Innov. J. 2018, 7, 546–549. [Google Scholar]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Adzahan, N.M.; Sarker, M.Z.I.; Ganjloo, A. Optimization of Ultrasound-Assisted Extraction of Crude Oil from Winter Melon (Benincasa hispida) Seed Using Response Surface Methodology and Evaluation of Its Antioxidant Activity, Total Phenolic Content and Fatty Acid Composition. Molecules 2012, 17, 11748–11762. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Fahad, A.J.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. Food Meas. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Özbek, Z.A.; Günç Ergönül, P. Chapter 51—Cold pressed soybean oil. In Cold Pressed Oils; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 575–585. ISBN 9780128181881. [Google Scholar]
- Trif, M.; Vodnar, D.C.; Mitrea, L.; Rusu, A.V.; Socol, C.T. Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads. Coatings 2019, 9, 235. [Google Scholar] [CrossRef]
- Lim, H.W.; Jang, H.M.; Ha, S.M.; Chai, Y.G.; Yoo, S.I.; Zhang, B.T. A Lab-on-a-Chip Module for Bead Separation in DNA-Based Concept Learning. In DNA Computing. DNA 2003. Lecture Notes in Computer Science; Chen, J., Reif, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 2943. [Google Scholar]
- Ruger, C.W.; Klinker, E.J.; Hammond, E.G. Abilities of Some Antioxidants to Stabilize Soybean Oil in Industrial use Conditions. J. Am. Oil Chem. Soc. 2002, 79, 733–736. [Google Scholar] [CrossRef]
- Yang, C.Y.; Mandal, P.K.; Han, K.H.; Fukushima, M.; Choi, K.; Kim, C.J.; Lee, C.H. Capsaicin and tocopherol in red pepper seed oil enhances the thermal oxidative stability during frying. J. Food Sci. Technol. 2010, 47, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Taghvaei, M.; Jafari, S.M.; Sadeghi-Mahoonak, A.; Mehregan-Nikoo, A.; Rahmanian, N.; Hajitabar, J.; Meshginfar, N. The effect of natural antioxidants extracted from plant and animal resources on the oxidative stability of soybean oil. LWT Food Sci. Technol. 2014, 56, 124–130. [Google Scholar] [CrossRef]
- Suja, K.P.; Abraham, J.T.; Thamizh, S.N.; Jayalekshmy, A.; Arumughan, C. Antioxidant efficacy of sesame cake extract in vegetable oil protection. Food Chem. 2004, 84, 393–400. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Trif, M.; Csutak, E.; Perez-Moral, N.; Gagyi, T.; Pintori, D.; Bethke, M.; Wilde, P.J. Terifiq Eu Project: Multiple Gel in Oil in Water Emulsions as Fat Replacers in Sauces and Ready Prepared Foods. Bull. Univ. Agric. Sci. Veter- Med. Cluj-Napoca. Food Sci. Technol. 2016, 73, 47–48. [Google Scholar] [CrossRef]
- Bangar, S.P.; Purewal, S.S.; Trif, M.; Maqsood, S.; Kumar, M.; Manjunatha, V.; Rusu, A.V. Functionality and Applicability of Starch-Based Films: An Eco-Friendly Approach. Foods 2021, 10, 2181. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, H.; Benmoussa, H.; Saadaoui, E.; Saanoun, I.; Negri, N.; Elfalleh, W. Optimization of ultrasound and microwave-assisted extraction of phenolic compounds from olive leaves by response surface methodology. Res. J. Biotechnol. 2019, 14, 28–37. [Google Scholar]
- Pan, G.Y.; Yu, G.Y.; Zhu, C.H.; Qiao, J.L. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS). Ultrason. Sonochem. 2012, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Dobrinčić, A.; Repajić, M.; Garofulić, I.E.; Tuđen, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Liu, S. Power ultrasound and its applications: A state-of-the-art review. Ultrason. Sonochem. 2020, 62, 104722. [Google Scholar] [CrossRef]
- Mitrea, L.; Ranga, F.; Fetea, F.; Dulf, F.V.; Rusu, A.; Trif, M.; Vodnar, D.C. Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms 2019, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Fäldt, P.; Bergenståhl, B. Spray-dried whey protein/lactose/soybean oil emulsions. 1. Surface composition and particle structure. Food Hydrocoll. 1996, 10, 421–429. [Google Scholar] [CrossRef]
- Maurer, S.; Ghebremedhin, M.; Zielbauer, B.I.; Knorr, D.; Vilgis, T.A. Microencapsulation of soybean oil by spray drying using oleosomes. J. Phys. D Appl. Phys. 2016, 49, 054001. [Google Scholar] [CrossRef]
- Mori, C.; Kadota, K.; Tozuka, Y.; Shimosaka, A.; Yoshida, M.; Shirakawa, Y. Application of nozzleless electrostatic atomization to encapsulate soybean oil with solid substances. J. Food Eng. 2019, 246, 25–32. [Google Scholar] [CrossRef]
- Cimino, R.; Bhangu, S.K.; Baral, A.; Ashokkumar, M.; Cavalieri, F. Ultrasound-Assisted Microencapsulation of Soybean Oil and Vitamin D Using Bare Glycogen Nanoparticles. Molecules 2021, 26, 5157. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, S.S.; Chetan, B.P.; Pramod, P.M. Preparation and Characterization of Microcapsules Containing Soybean Oil and Their Application in Self-Healing Anticorrosive Coatings. Polym. Plast. Technol. Eng. 2008, 57, 1334–1343. [Google Scholar]
- Ding, J.; Xu, Z.; Qi, B.; Cui, S.; Wang, T.; Jiang, L.; Zhang, Y.; Sui, X. Fabrication and characterization of soybean oil bodies encapsulated in maltodextrin and chitosan-EGCG conjugates: An in vitro digestibility study. Food Hydrocoll. 2019, 94, 519–527. [Google Scholar] [CrossRef]
- Chen, B.; McClements, D.J.; Gray, D.A.; Decker, E.A. Stabilization of soybean oil bodies by enzyme (laccase) cross-linking of adsorbed beet pectin coatings. J. Agric. Food Chem. 2010, 58, 9259–9265. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Huang, X.; Yang, X.; Guo, J.; Yin, S.; He, X.; Wang, L.-J.; Zhu, J.-H.; Qi, J.-R.; Zheng, E.-L. In vitro assessment of the bioaccessibility of fatty acids and tocopherol from soybean oil body emulsions stabilized with ι-carrageenan. J. Agric. Food Chem. 2012, 60, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Huang, X.; Yang, X.; Guo, J.; Zheng, E.; Yin, S.; Zhu, J.-H.; Qi, J.-R.; He, X.-T.; Zhang, J.-B. Stabilization of soybean oil body emulsions using ι-carrageenan: Effects of salt, thermal treatment and freeze-thaw cycling. Food Hydrocoll. 2012, 28, 110–120. [Google Scholar] [CrossRef]
- Wu, N.; Yang, X.; Teng, Z.; Yin, S.; Zhu, J.; Qi, J. Stabilization of soybean oil body emulsions using κ, ι, λ-carrageenan at different pH values. Food Res. Int. 2011, 44, 1059–1068. [Google Scholar] [CrossRef]
- Liu, C.; Wang, R.; He, S.; Cheng, C.; Ma, Y. The stability and gastro-intestinal digestion of curcumin emulsion stabilized with soybean oil bodies. LWT Food Sci. Technol. 2020, 131, 109663. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ferenczi, L.J.; Ștefănescu, B.E.; Vodnar, D.C. Inhibitory potential of Lactobacillus plantarum on Escherichia coli. Bull. UASVM Food Sci. Technol. 2017, 74, 99–101. [Google Scholar] [CrossRef][Green Version]
- Hao, J.; Xu, D.; Cao, Y. Recent developments and prospects in the composition, extraction, stability, delivery system, digestion and food applications of plant oil bodies. Authorea 2021. [Google Scholar] [CrossRef]



| Levels | Factors | ||
|---|---|---|---|
| Upper | Center | Lower | |
| 100 | 60 | 20 | (X1) (kHz) (Intensity of sound) |
| 150 | 100 | 50 | (X2) (ppm) (Concentration of sap) |
| 15 | 10 | 5 | (X3) (min) (Extraction time) |
| Intensity of Sound (kHz) | Concentration (ppm) | Time (minutes) | DPPH (%) | Folin–Ciocalteau (Milligrams of Gallic Acid per Gram of Extract) |
|---|---|---|---|---|
| 100 | 200 | 10 | 56.80 ± 0.2 | 18.25 ± 0.2 |
| 60 | 200 | 5 | 49.60 ± 0.3 | 14.85 ± 0.1 |
| 20 | 200 | 10 | 39.14 ± 0.25 | 15.85 ± 0.25 |
| 60 | 200 | 15 | 44.79 ± 0.4 | 14.37 ± 0.5 |
| 60 | 120 | 10 | 49.54 ± 0.5 | 14.88 ± 0.2 |
| 20 | 120 | 15 | 25.39 ± 0.2 | 11.80 ± 0.3 |
| 100 | 120 | 5 | 49.2 ± 0.25 | 15.00 ± 0.15 |
| 100 | 120 | 15 | 43.17 ± 0.4 | 14.37 ± 0.4 |
| 100 | 40 | 10 | 50.52 ± 0.2 | 16.07 ± 0.1 |
| 60 | 40 | 5 | 39.65 ± 0.1 | 12.80 ± 0.5 |
| 20 | 40 | 10 | 30.79 ± 0.3 | 12.57 ± 0.3 |
| 60 | 40 | 15 | 36.50 ± 0.2 | 11.39 ± 0.1 |
| Extraction Method | Power Inhibitory Free Radical (%) | Phenolic Compounds (Grams) |
|---|---|---|
| Optimized extraction | 57.084 a | 18.248 a |
| Percolation | 41.19 b | 15.32 b |
| Source | S. Squares | df | M. Square | F. Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 47.42 | 9 | 5.27 | 194.54 | <0.0001 | Sig |
| A-intensity of sound | 15.12 | 1 | 15.12 | 558.41 | <0.0001 | |
| B-concentration | 13.76 | 1 | 13.76 | 508.03 | <0.0001 | |
| C-time | 1.27 | 1 | 1.27 | 47.02 | <0.0001 | |
| AB | 0.3 | 1 | 0.3 | 11.17 | 0.0075 | |
| AC | 4 × 10−4 | 1 | 4 × 10−4 | 0.015 | 0.9057 | |
| BC | 0.22 | 1 | 0.22 | 8.02 | 0.0178 | |
| A^2 | 0.85 | 1 | 0.85 | 31.24 | 0.0002 | |
| B^2 | 0.63 | 1 | 0.63 | 23.39 | 0.0007 | |
| C^2 | 16.55 | 1 | 16.55 | 611.05 | <0.0001 | |
| Residual | 0.27 | 10 | 0.027 | |||
| Lack of Fit | 0.15 | 3 | 0.049 | 2.76 | 0.1215 | N.Sig |
| Pure Error | 0.12 | 7 | 0.018 | |||
| Cor Total | 47.69 | 19 |
| Source | S. Squares | df | M. Square | F. Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1353.53 | 9 | 150.39 | 157.82 | <0.0001 | Sig |
| A-intensity of sound | 713.1 | 1 | 713.1 | 748.32 | <0.0001 | |
| B-concentration | 135.05 | 1 | 135.05 | 141.73 | <0.0001 | |
| C-time | 38.02 | 1 | 38.02 | 39.9 | <0.0001 | |
| AB | 1.07 | 1 | 1.07 | 1.12 | 0.314 | |
| AC | 1.66 | 1 | 1.66 | 1.75 | 0.2158 | |
| BC | 0.69 | 1 | 0.69 | 0.72 | 0.4151 | |
| A^2 | 143.74 | 1 | 143.74 | 150.84 | <0.0001 | |
| B^2 | 0.65 | 1 | 0.65 | 0.68 | 0.4276 | |
| C^2 | 242.61 | 1 | 242.61 | 254.6 | <0.0001 | |
| Residual | 9.53 | 10 | 0.95 | |||
| Lack of Fit | 1.99 | 3 | 0.66 | 0.62 | 0.6267 | N-Sig |
| Pure Error | 7.54 | 7 | 1.08 | |||
| Cor Total | 1363.06 | 19 |
| R-Squared | 0.9943 |
| Adj R-Squared | 0.9892 |
| Pred R-Squared | 0.9474 |
| R-Squared | 0.9837 |
| Adj R-Squared | 0.9711 |
| Pred R-Squared | 0.9303 |
| Beads | Mean Area (mm2) | Mean Roundness | Mean Diameter (mm) |
|---|---|---|---|
| Hydrogel beads obtained | 3.48 ± 0.01 | 0.84 ± 0.04 | 2.1 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, M.; Azadfar, E.; Trif, M.; Jabaleh, R.A.; Rusu, A.; Bahrami, Z.; Sharifi, M.; Bangar, S.P.; Ilyas, N.; Ștefănescu, B.E.; et al. Soybean Oil Enriched with Antioxidants Extracted from Watermelon (Citrullus colocynthis) Skin Sap and Coated in Hydrogel Beads via Ionotropic Gelation. Coatings 2021, 11, 1370. https://doi.org/10.3390/coatings11111370
Farooq M, Azadfar E, Trif M, Jabaleh RA, Rusu A, Bahrami Z, Sharifi M, Bangar SP, Ilyas N, Ștefănescu BE, et al. Soybean Oil Enriched with Antioxidants Extracted from Watermelon (Citrullus colocynthis) Skin Sap and Coated in Hydrogel Beads via Ionotropic Gelation. Coatings. 2021; 11(11):1370. https://doi.org/10.3390/coatings11111370
Chicago/Turabian StyleFarooq, Muhammad, Elham Azadfar, Monica Trif, Ramezan Ali Jabaleh, Alexandru Rusu, Zohre Bahrami, Mahniya Sharifi, Sneh Punia Bangar, Naila Ilyas, Bianca Eugenia Ștefănescu, and et al. 2021. "Soybean Oil Enriched with Antioxidants Extracted from Watermelon (Citrullus colocynthis) Skin Sap and Coated in Hydrogel Beads via Ionotropic Gelation" Coatings 11, no. 11: 1370. https://doi.org/10.3390/coatings11111370
APA StyleFarooq, M., Azadfar, E., Trif, M., Jabaleh, R. A., Rusu, A., Bahrami, Z., Sharifi, M., Bangar, S. P., Ilyas, N., Ștefănescu, B. E., & Wang, Y. (2021). Soybean Oil Enriched with Antioxidants Extracted from Watermelon (Citrullus colocynthis) Skin Sap and Coated in Hydrogel Beads via Ionotropic Gelation. Coatings, 11(11), 1370. https://doi.org/10.3390/coatings11111370










