Cultivar Determines Fatty Acids and Phenolics Dynamics for Olive Fruit and Oil in Super-High-Density Orchards
Abstract
1. Introduction
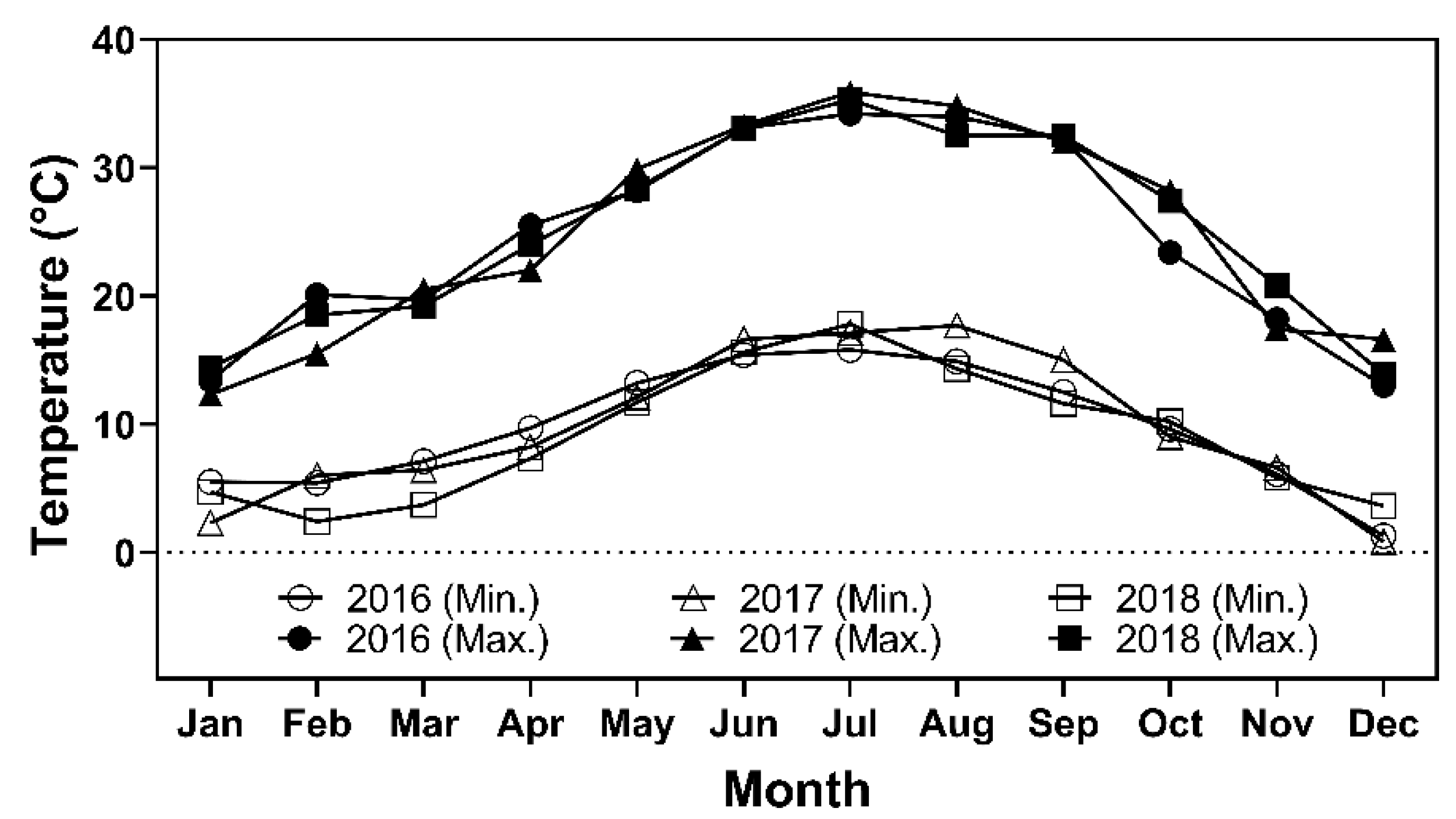
2. Materials and Methods
2.1. Olive Samples
2.2. Maturation Index
2.3. Water Concentration
2.4. Oil Concentration
2.5. Total Phenols Concentration in Olive Fruit
2.6. Olive Oil Extraction
2.7. Quality Parameters
2.8. Fatty Acid Profile
2.9. Total Phenols Concentration in Olive Oil
2.10. Statistical Analysis
3. Results
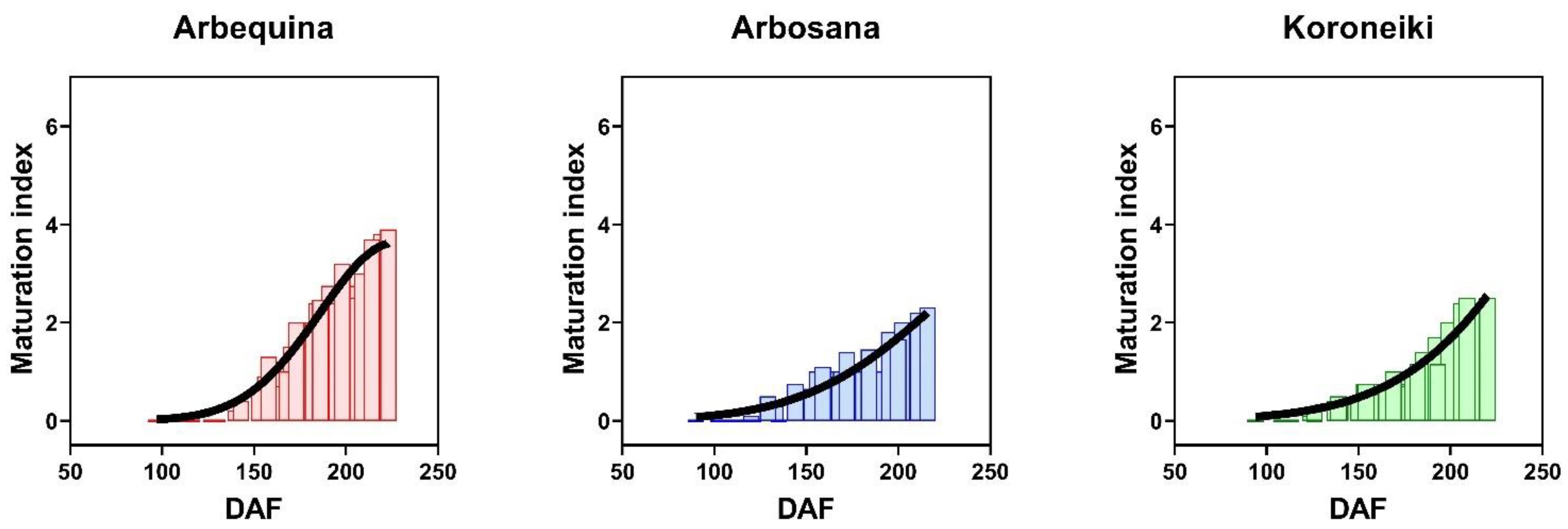
3.1. Dynamics of Maturation Index
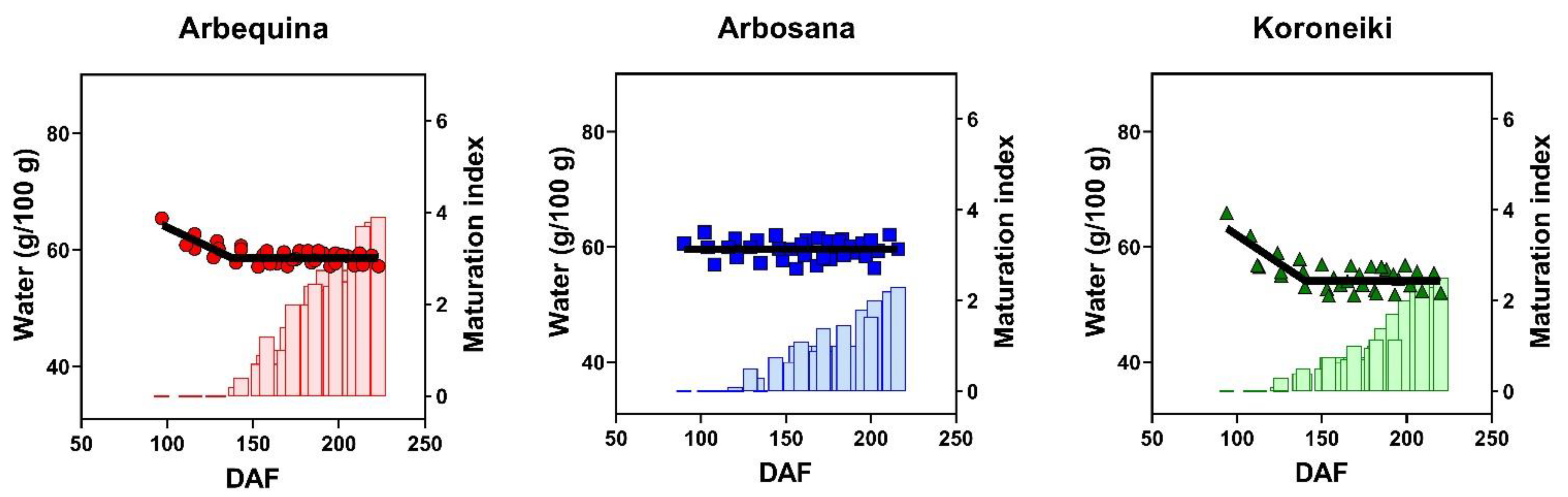
3.2. Dynamics of Water Concentration
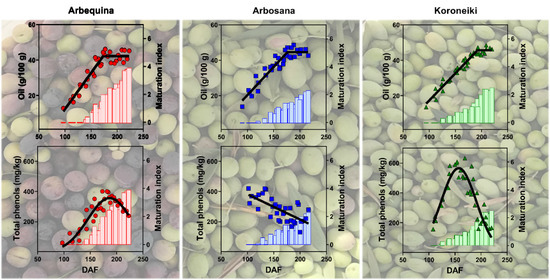
3.3. Dynamics of Oil Concentration
3.4. Dynamics of Fatty Acids Concentration
3.5. Cultivar Discrimination by Fatty Acids
3.6. Dynamics of Total Phenols Concentration in Olive Fruit and Oil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Council. Key-Figures on the World Market for Olive Oils. Available online: http://www.internationaloliveoil.org/estaticos/view/134-approved-balances (accessed on 8 December 2020).
- Vossen, P. Olive oil: History, production, and characteristics of the world’s classic oils. Hortscience 2007, 42, 1093–1100. [Google Scholar] [CrossRef]
- Connor, D.J.; Gómez-del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, management and productivity of hedgerow olive orchards: A review. Sci. Hortic. 2014, 169, 71–93. [Google Scholar] [CrossRef]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive Cultivation in the Southern Hemisphere: Flowering, Water Requirements and Oil Quality Responses to New Crop Environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef]
- Sturzenberger, N.D. Super-High-Density Olive Production in California; UC Davis Olive Center: Davis, CA, USA, 2009. [Google Scholar]
- Navas-Lopez, J.F.; León, L.; Trentacoste, E.R.; de la Rosa, R. Multi-environment evaluation of oil accumulation pattern parameters in olive. Plant Physiol. Biochem. 2019, 139, 485–494. [Google Scholar] [CrossRef]
- De la Rosa, R.; Talhaoui, N.; Rouis, H.; Velasco, L.; León, L. Fruit characteristics and fatty acid composition in advanced olive breeding selections along the ripening period. Food Res. Int. 2013, 54, 1890–1896. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Puertas, C.M.; Sadras, V.O. Effect of fruit load on oil yield components and dynamics of fruit growth and oil accumulation in olive (Olea europaea L.). Eur. J. Agron. 2010, 32, 249–254. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Puertas, C.M.; Sadras, V.O. Modelling the intraspecific variation in the dynamics of fruit growth, oil and water concentration in olive (Olea europaea L.). Eur. J. Agron. 2012, 38, 83–93. [Google Scholar] [CrossRef]
- Beltrán, G.; Del Río, C.; Sánchez, S.; Martínez, L. Seasonal changes in olive fruit characteristics and oil accumulation during ripening process. J. Sci. Food Agric. 2004, 84, 1783–1790. [Google Scholar] [CrossRef]
- Mailer, R.J.; Ayton, J.; Conlan, D. Influence of harvest timing on olive (Olea europaea) oil accumulation and fruit characteristics under Australian conditions. J. Food Agric. Environ. 2007, 5, 58–63. [Google Scholar]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Contrasting patterns of fatty acid composition and oil accumulation during fruit growth in several olive varieties and locations in a non-Mediterranean region. Eur. J. Agron. 2014, 52, 237–246. [Google Scholar] [CrossRef]
- Gómez-González, S.; Ruiz-Jiménez, J.; De Castro, M.D.L. Oil content and fatty acid profile of Spanish cultivars during olive fruit ripening. JAOCS J. Am. Oil Chem. Soc. 2011, 88, 1737–1745. [Google Scholar] [CrossRef]
- De La Rosa, R.; León, L.; Moreno, I.; Barranco, D.; Rallo, L. Ripening time and fruit characteristics of advanced olive selections for oil production. Aust. J. Agric. Res. 2008, 59, 46–51. [Google Scholar] [CrossRef]
- Li, X.; Flynn, J.D.; Wang, S.C. The Effects of Variety, Growing Region, and Drought Stress on Fatty Acid and Sterol Compositions of California Olive Oil. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 215–230. [Google Scholar] [CrossRef]
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G.F. Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. J. Am. Oil Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.H.C.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-ligstroside Aglycon as a Key Contributor to Pungency. J. Agric. Food Chem. 2003. [Google Scholar] [CrossRef]
- Crawford, L.M.; Holstege, D.M.; Wang, S.C. High-throughput extraction method for phenolic compounds in olive fruit (Olea europaea). J. Food Compos. Anal. 2018, 66, 136–144. [Google Scholar] [CrossRef]
- Romero-Segura, C.; García-Rodríguez, R.; Sánchez-Ortiz, A.; Sanz, C.; Pérez, A.G. The role of olive β-glucosidase in shaping the phenolic profile of virgin olive oil. Food Res. Int. 2012, 45, 191–196. [Google Scholar] [CrossRef]
- Jose Motilva, M.; Jess Tovar, M.; Paz Romero, M.; Alegre, S.; Girona, J. Influence of regulated deficit irrigation strategies applied to olive trees (Arbequina cultivar) on oil yield and oil composition during the fruit ripening period. J. Sci. Food Agric. 2000, 80, 2037–2043. [Google Scholar] [CrossRef]
- Polari, J.J.; Mori, M.; Wang, S.C. Virgin Olive Oils from Super-High-Density Orchards in California: Impact of Cultivar, Harvest time, and Crop Season on Quality and Chemical Composition. Eur. J. Lipid Sci. Technol. 2020, 2000180. [Google Scholar] [CrossRef]
- Mailer, R.J.; Ayton, J.; Graham, K. The influence of growing region, cultivar and harvest timing on the diversity of australian olive oil. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 877–884. [Google Scholar] [CrossRef]
- Polari, J.J.; Zhang, L.; Ferguson, L.; Maness, N.O.; Wang, S.C. Impact of Microclimate on Fatty Acids and Volatile Terpenes in “Kerman” and “Golden Hills” Pistachio (Pistacia vera) Kernels. J. Food Sci. 2019, 84, 1937–1942. [Google Scholar] [CrossRef]
- Sánchez, J.; Harwood, J.L. Biosynthesis of triacylglycerols and volatiles in olives. Eur. J. Lipid Sci. Technol. 2002, 104, 564–573. [Google Scholar] [CrossRef]
- Morelló, J.R.; Romero, M.P.; Motilva, M.J. Effect of the maturation of the olive fruit on the phenolic fraction of drupes and oils from Arbequina, Farga, and Morrut cultivars. J. Agric. Food Chem. 2004, 52, 6002–6009. [Google Scholar] [CrossRef]
- USDA. United States Standards for Grades of Olive Oil and Olive-Pomace Oil; USDA: Washington, DC, USA, 2010.
- Lee, C.; Polari, J.J.; Kramer, K.E.; Wang, S.C. Near-Infrared (NIR) Spectrometry as a Fast and Reliable Tool for Fat and Moisture Analyses in Olives. ACS Omega 2018, 3, 16081–16088. [Google Scholar] [CrossRef]
- Ramli, U.S.; Salas, J.J.; Quant, P.A.; Harwood, J.L. Metabolic control analysis reveals an important role for diacylglycerol acyltransferase in olive but not in oil palm lipid accumulation. FEBS J. 2005, 272, 5764–5770. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef] [PubMed]
- Morelló, J.R.; Vuorela, S.; Romero, M.P.; Motilva, M.J.; Heinonen, M. Antioxidant Activity of Olive Pulp and Olive Oil Phenolic Compounds of the Arbequina Cultivar. J. Agric. Food Chem. 2005, 53, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Garcia, F.; Blanco, S.; Peinado, M.A.; Peragon, J. Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. “Picual” trees during fruit ripening. Tree Physiol. 2008, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
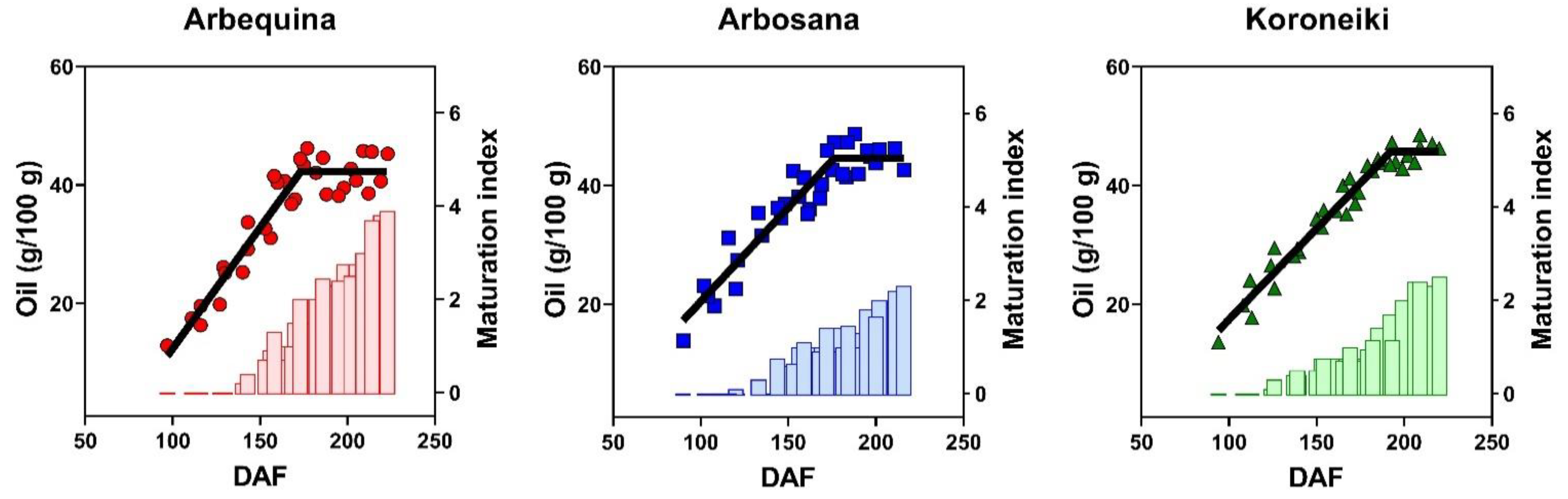
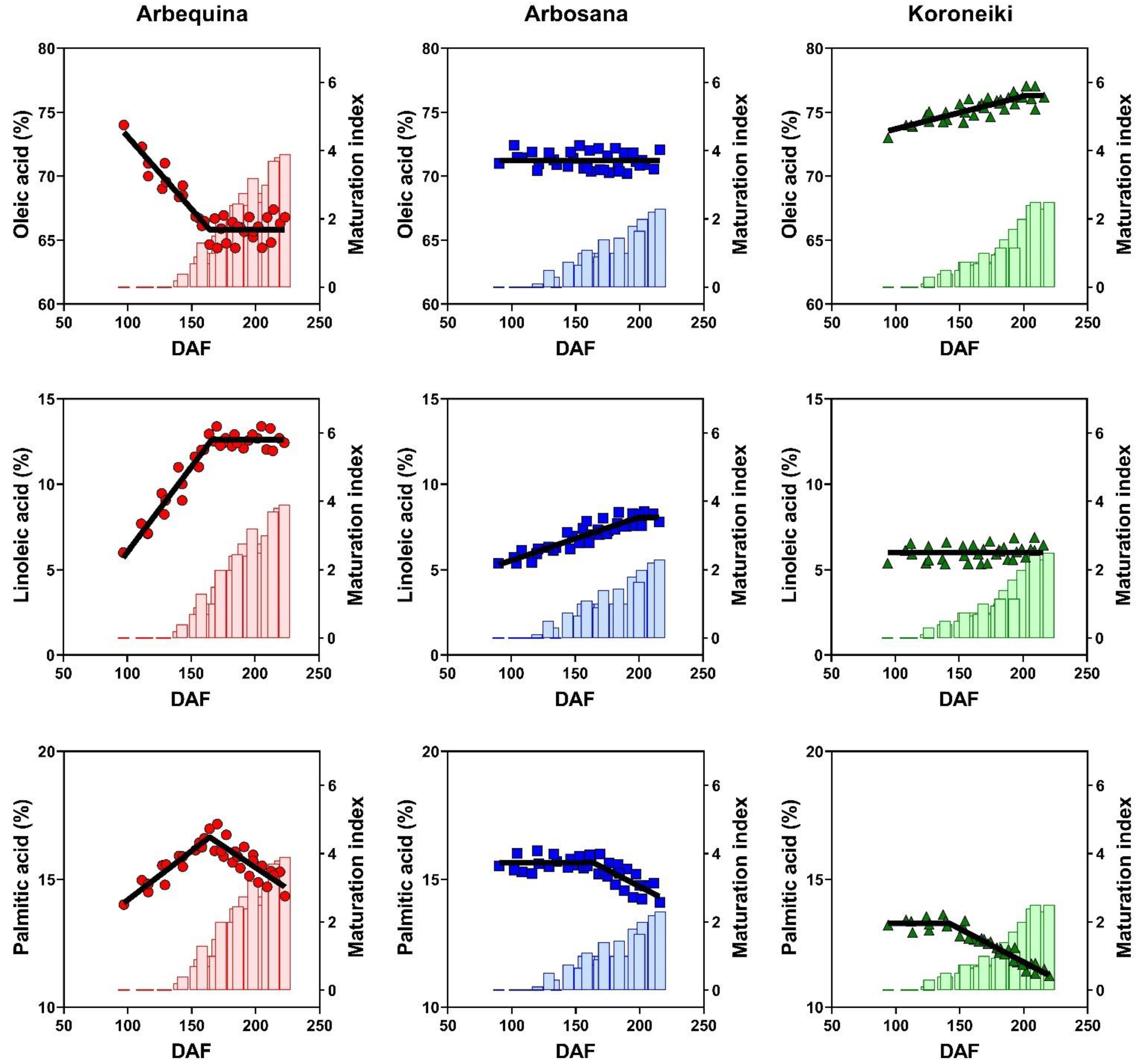
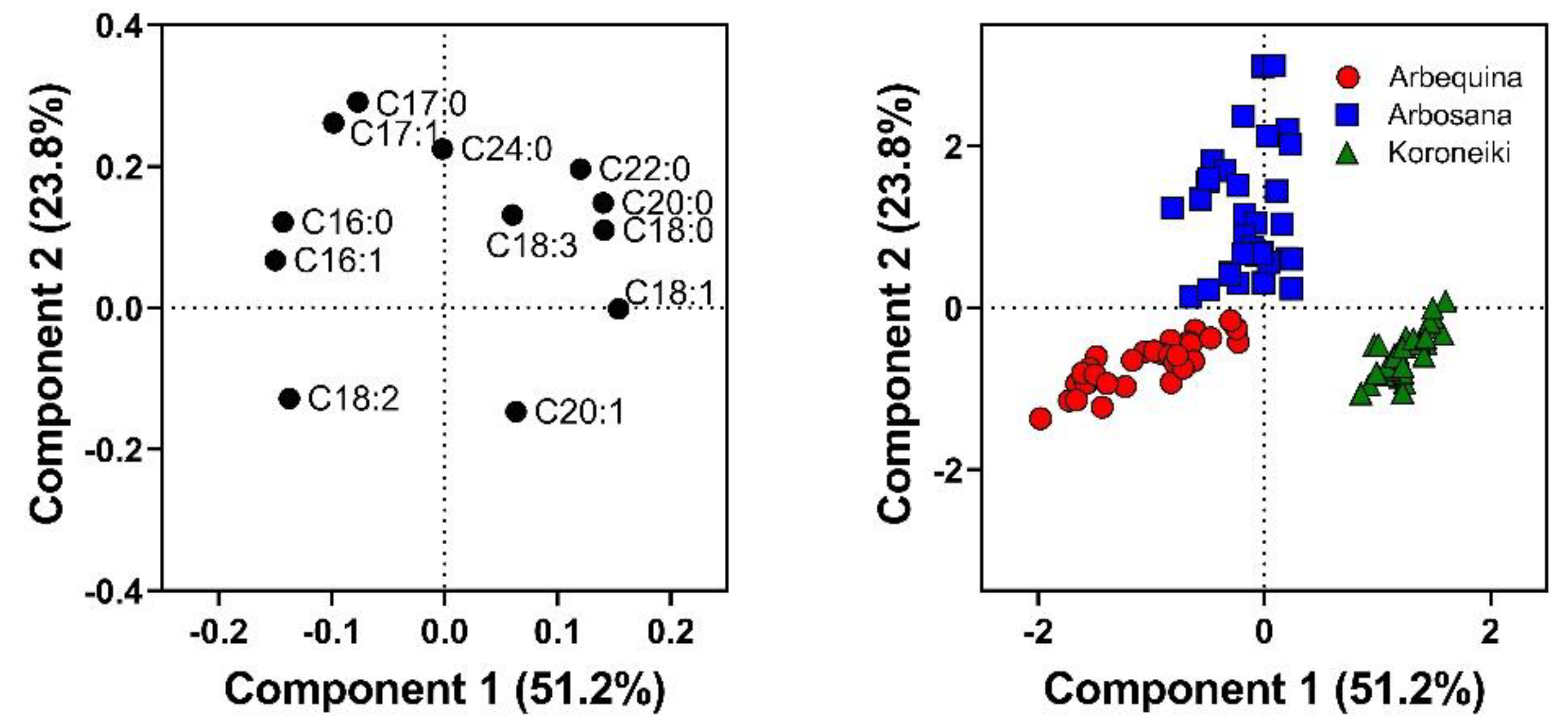
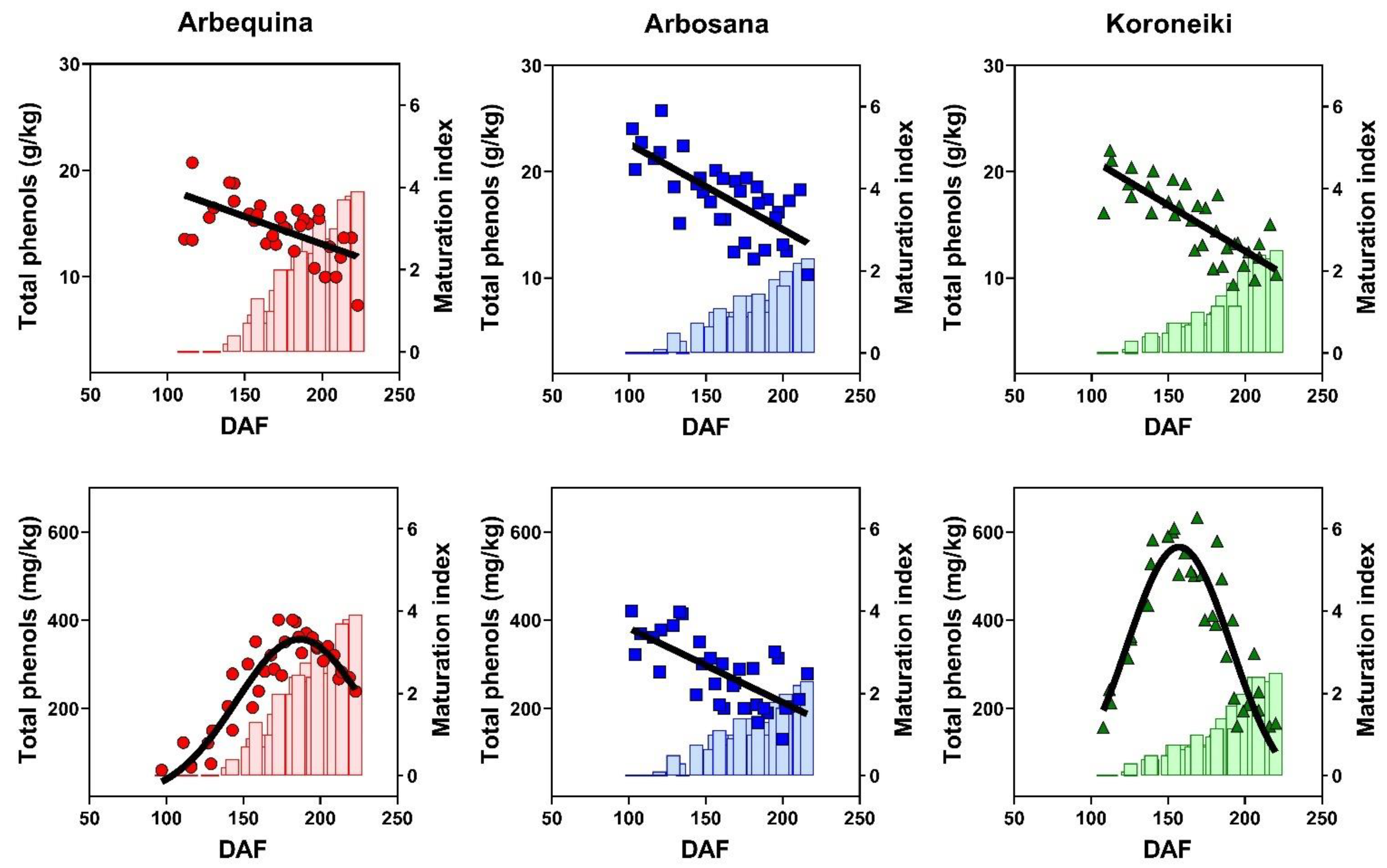
| Cultivar | Max Oil Conc. (%) | Rate (%/DAF) | Onset (DAF) | Duration (DAF) |
|---|---|---|---|---|
| Arbequina | 41.8 ± 1.6 b | 0.41 ± 0.04 a | 60 ± 10 a | 172 ± 4 b |
| Arbosana | 45.4 ± 1.6 a | 0.32 ± 0.03 b | 36 ± 10 b | 179 ± 5 b |
| Koroneiki | 45.7 ± 1.3 a | 0.31 ± 0.02 b | 43 ± 10 b | 192 ± 4 a |
| Fatty Acid | Arbequina | Arbosana | Koroneiki |
|---|---|---|---|
| Palmitic acid (C16:0) | 15.76 ± 0.69 a | 15.32 ± 0.61 b | 12.31 ± 1.32 c |
| Palmitoleic acid (C16:1) | 1.44 ± 0.32 a | 1.38 ± 0.17 b | 0.66 ± 0.09 c |
| Margaric acid (C17:0) | 0.12 ± 0.03 c | 0.18 ± 0.05 b | 0.06 ± 0.08 a |
| Heptadecenoic acid (C17:1) | 0.26 ± 0.04 b | 0.35 ± 0.06 a | 0.07 ± 0.01 c |
| Stearic acid (C18:0) | 1.83 ± 0.15 c | 2.28 ± 0.23 b | 2.58 ± 0.17 a |
| Oleic acid (C18:1) | 67.47 ± 2.02 c | 71.16 ± 1.59 b | 75.31 ± 1.02 a |
| Linoleic acid (C18:2) | 11.00 ± 1.55 a | 6.96 ± 1.26 b | 6.10 ± 0.75 c |
| Linolenic acid (C18:3) | 0.71 ± 0.19 b | 0.80 ± 0.15 a | 0.8 ± 0.13 a |
| Arachidic acid (C20:0) | 0.41 ± 0.02 c | 0.47 ± 0.03 b | 0.49 ± 0.03 a |
| Gondoic acid (C20:1) | 0.31 ± 0.03 c | 0.29 ± 0.04 b | 0.32 ± 0.02 a |
| Behenic acid (C22:0) | 0.13 ± 0.01 b | 0.18 ± 0.09 a | 0.17 ± 0.01 a |
| Lignoceric acid (C24:0) | 0.07 ± 0.01 b | 0.12 ± 0.10 a | 0.08 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polari, J.J.; Crawford, L.M.; Wang, S.C. Cultivar Determines Fatty Acids and Phenolics Dynamics for Olive Fruit and Oil in Super-High-Density Orchards. Agronomy 2021, 11, 313. https://doi.org/10.3390/agronomy11020313
Polari JJ, Crawford LM, Wang SC. Cultivar Determines Fatty Acids and Phenolics Dynamics for Olive Fruit and Oil in Super-High-Density Orchards. Agronomy. 2021; 11(2):313. https://doi.org/10.3390/agronomy11020313
Chicago/Turabian StylePolari, Juan J., Lauren M. Crawford, and Selina C. Wang. 2021. "Cultivar Determines Fatty Acids and Phenolics Dynamics for Olive Fruit and Oil in Super-High-Density Orchards" Agronomy 11, no. 2: 313. https://doi.org/10.3390/agronomy11020313
APA StylePolari, J. J., Crawford, L. M., & Wang, S. C. (2021). Cultivar Determines Fatty Acids and Phenolics Dynamics for Olive Fruit and Oil in Super-High-Density Orchards. Agronomy, 11(2), 313. https://doi.org/10.3390/agronomy11020313