Biomarkers of Physical Frailty and Sarcopenia: Coming up to the Place?
Abstract
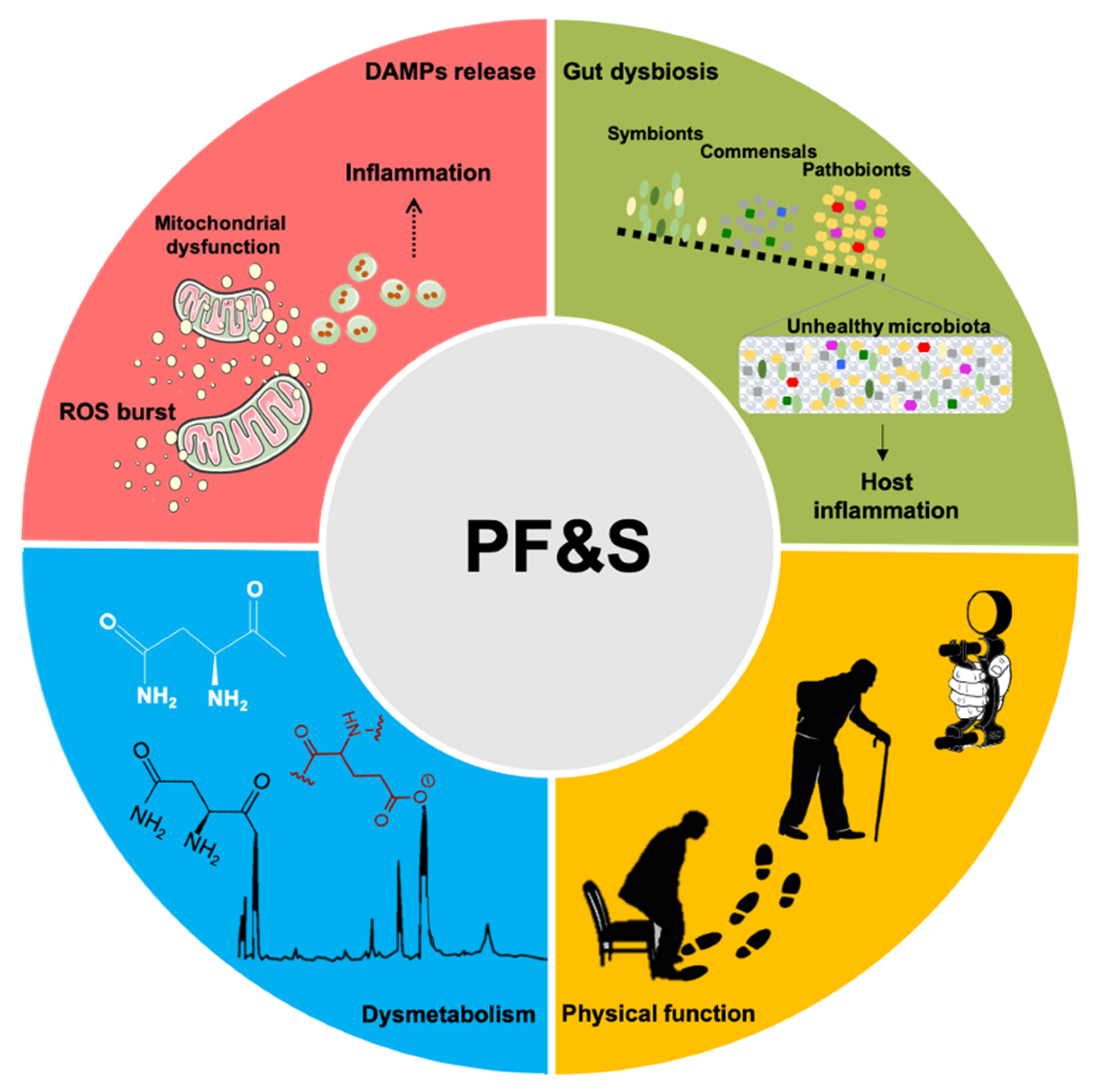
:1. Introduction
2. Imaging and Functional Markers
3. Inflammation-Related Biomarkers
4. Metabolic Markers
5. Gut Microbiota
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5 × STS | Five-time sit-to-stand |
| ADL | Activities of daily living |
| BIOSPHERE | BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons |
| CRP | C-reactive protein |
| CT | Computed tomography |
| DAMPs | Damage-associated molecular patterns |
| DXA | Dual energy x-ray absorptiometry |
| HSP72 | HSP72, heat shock protein 72 |
| IHG | Isometric handgrip strength |
| IL | Interleukin |
| MaSS | Maastricht Sarcopenia study |
| MCP1 | Monocyte chemoattractant protein 1 |
| MDVs | Mitochondrial-derived vesicles |
| MIP1α | Macrophage inflammatory protein 1α |
| MPO | Myeloperoxidase |
| MRI | Magnetic resonance imaging |
| PDGF-BB | Platelet-derived growth factor-BB |
| PF&S | Physical frailty and sarcopenia |
| PLS-DA | Partial Least Squares–Discriminant Analysis |
| SASP | Senescence-associated secretory phenotype |
| SCFAs | Short chain fatty acids |
| SPRINTT | Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies |
| sEVs | Small extracellular vesicle |
| SO-CovSel | Sequential and Orthogonalized Covariance Selection |
| SPPB | Short physical performance battery |
References
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Rizzoli, R.; Bruyère, O.; Reginster, J.; Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health 2014, 72, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvani, R.; Picca, A.; Cesari, M.; Tosato, M.; Marini, F.; Manes-Gravina, E.; Bernabei, R.; Landi, F.; Marzetti, E. Biomarkers for sarcopenia: Reductionism vs. complexity. Curr. Protein Pept. Sci. 2018, 19, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An overview on current definitions, diagnosis and treatment. Curr. Protein Pept. Sci. 2018, 19, 633–638. [Google Scholar] [CrossRef]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in older persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Cesari, M.; Landi, F.; Calvani, R.; Cherubini, A.; Di Bari, M.; Kortebein, P.; Del Signore, S.; Le Lain, R.; Vellas, B.; Pahor, M.; et al. Rationale for a preliminary operational definition of physical frailty and sarcopenia in the SPRINTT trial. Aging Clin. Exp. Res. 2017, 29, 81–88. [Google Scholar] [CrossRef]
- Picca, A.; Beli, R.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Bucci, C.; Guerra, F.; Marzetti, E. Older adults with physical frailty and sarcopenia show increased levels of circulating small extracellular vesicles with a specific mitochondrial signature. Cells 2020, 9, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picca, A.; Ponziani, F.R.; Calvani, R.; Marini, F.; Biancolillo, A.; Coelho-Júnior, H.J.; Gervasoni, J.; Primiano, A.; Putignani, L.; Del Chierico, F.; et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: Results from the Biosphere study. Nutrients 2019, 12, 65. [Google Scholar] [CrossRef] [Green Version]
- Marzetti, E.; Picca, A.; Marini, F.; Biancolillo, A.; Coelho-Junior, H.J.; Gervasoni, J.; Bossola, M.; Cesari, M.; Onder, G.; Landi, F.; et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: The frailty “cytokinome” at its core. Exp. Gerontol. 2019, 122, 129–138. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Júnior, H.J.; Bossola, M.; Urbani, A.; et al. A Distinct pattern of circulating amino acids characterizes older persons with physical frailty and sarcopenia: Results from the Biosphere study. Nutrients 2018, 10, 1691. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Junior, H.J.; Uchida, M.C.; Gonçalves, I.O.; Calvani, R.; Rodrigues, B.; Picca, A.; Onder, G.; Landi, F.; Bernabei, R.; Marzetti, E. Age and gender-related changes in physical function in community-dwelling Brazilian adults aged 50 to 102 Years. J. Geriatr. Phys. Ther. 2019. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Heal. Aging 2019, 23, 771–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Marzetti, E.; Hwang, A.C.; Tosato, M.; Peng, L.-N.; Calvani, R.; Picca, A.; Chen, L.-K.; Landi, F. Age-related changes of skeletal muscle mass and strength among Italian and Taiwanese older people: Results from the Milan EXPO 2015 survey and the I-Lan longitudinal aging study. Exp. Gerontol. 2018, 102, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Fusco, D.; Sisto, A.; Ortolani, E.; Savera, G.; Salini, S.; Marzetti, E. Age-related variations of muscle mass, strength, and physical performance in community-dwellers: Results from the Milan EXPO Survey. J. Am. Med. Med Dir. Assoc. 2017, 18, 88.e17–88.e24. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Marzetti, E.; Picca, A.; Calvani, R.; Cesari, M.; Uchida, M. Prevalence of prefrailty and frailty in South America: A systematic review of observational studies. J. Frailty Aging 2020, 36, 1–17. [Google Scholar] [CrossRef]
- Suetta, C.; Maier, A.B. Is muscle failure a better term than sarcopenia? J. Cachex Sarcopenia Muscle 2019, 10, 1146–1147. [Google Scholar] [CrossRef] [Green Version]
- Marzetti, E.; Calvani, R.; Lorenzi, M.; Tanganelli, F.; Picca, A.; Bossola, M.; Menghi, A.; Bernabei, R.; Landi, F. Association between myocyte quality control signaling and sarcopenia in old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp. Gerontol. 2016, 80, 1–5. [Google Scholar] [CrossRef]
- Justice, J.N.; Ferrucci, L.; Newman, A.B.; Aroda, V.R.; Bahnson, J.L.; Divers, J.; Espeland, M.A.; Marcovina, S.; Pollak, M.N.; Kritchevsky, S.B.; et al. A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. GeroScience 2018, 40, 419–436. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Sierra, F. The Emergence of Geroscience as an Interdisciplinary Approach to the enhancement of health span and life span. Cold Spring Harb. Perspect. Med. 2016, 6, a025163. [Google Scholar] [CrossRef] [Green Version]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Júnior, H.J.; Cesari, M.; Bossola, M.; et al. Identification of biomarkers for physical frailty and sarcopenia through a new multi-marker approach: Results from the Biosphere study. GeroScience 2020. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Cesari, M.; Pesce, V.; Lezza, A.M.S.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; et al. The “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (Biosphere) study: Rationale, design and methods. Eur. J. Intern. Med. 2018, 56, 19–25. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [Green Version]
- Fielding, R.A. Effects of exercise training in the elderly: Impact of progressive-resistance training on skeletal muscle and whole-body protein metabolism. Proc. Nutr. Soc. 1995, 54, 665–675. [Google Scholar] [CrossRef]
- Dutta, C.; Hadley, E.C.; Lexell, J. Sarcopenia and physical performance in old age: Overview. Muscle Nerve. Suppl. 1997, 5, S5–S9. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Häkkinen, K.; Häkkinen, A. Muscle cross-sectional area, force production and relaxation characteristics in women at different ages. Graefe’s Arch. Clin. Exp. Ophthalmol. 1991, 62, 410–414. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef]
- Clark, R.V.; Walker, A.C.; O’Connor-Semmes, R.L.; Leonard, M.S.; Miller, R.R.; Stimpson, S.A.; Turner, S.M.; Ravussin, E.; Cefalu, W.T.; Hellerstein, M.K.; et al. Total body skeletal muscle mass: Estimation by creatine (methyl-d3) dilution in humans. J. Appl. Physiol. 2014, 116, 1605–1613. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.V.; Walker, A.C.; Miller, R.R.; Semmes, R.L.O.; Ravussin, E.; Cefalu, W.T. Creatine (methyl-d3) dilution in urine for estimation of total body skeletal muscle mass: Accuracy and variability vs. MRI and DXA. J. Appl. Physiol. 2018, 124, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.J.; Hellerstein, M.; Orwoll, E.; Cummings, S.; Cawthon, P.M. D3 Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J. Cachex. Sarcopenia. Muscle 2019, 10, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Cawthon, P.; Orwoll, E.S.; Peters, K.E.; Ensrud, K.E.; Cauley, J.A.; Kado, D.M.; Stefanick, M.L.; Shikany, J.M.; Strotmeyer, E.; Glynn, N.W.; et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2018, 74, 844–852. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Blackwell, T.; Cummings, S.R.; Orwoll, E.S.; Duchowny, K.A.; Kado, D.M.; Stone, K.L.; Ensrud, K.E.; Cauley, J.A.; Evans, W.J.; et al. Muscle mass assessed by D3-Creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community dwelling older men. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2020, glaa111. [Google Scholar] [CrossRef]
- Zanker, J.; Patel, S.; Blackwell, T.; Duchowny, K.; Brennan-Olsen, S.; Cummings, S.R.; Evans, W.J.; Orwoll, E.S.; Scott, D.; Vogrin, S.; et al. Walking speed and muscle mass estimated by the D3-Creatine dilution method are important components of sarcopenia associated with incident mobility disability in older men: A classification and regression tree analysis. J. Am. Med. Dir. Assoc. 2020. [Google Scholar] [CrossRef]
- Orwoll, E.S.; Peters, K.E.; Hellerstein, M.; Cummings, S.R.; Evans, W.J.; Cawthon, P. The Importance of muscle versus fat mass in sarcopenic obesity: A re-evaluation using D3-Creatine muscle mass versus DXA lean mass measurements. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2020, 75, 1362–1368. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet. 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Chen, L.-K.; Lee, W.; Peng, L.-N.; Liu, L.-K.; Arai, H.; Akishita, M. Recent ADVANCES IN SARCOPENIA RESEARCH in Asia: 2016 update from the Asian working group for sarcopenia. J. Am. Med. Dir. Assoc. 2016, 17, 767.e1–767.e7. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, R.; Weber, M.; Schwenk, M.; Ulseth, S.; Helbostad, J.L.; Vereijken, B.; Taraldsen, K. Performance-based clinical tests of balance and muscle strength used in young seniors: A systematic literature review. BMC Geriatr. 2019, 19, 9. [Google Scholar] [CrossRef]
- Bohannon, R.W. Considerations and practical options for measuring muscle strength: A narrative review. BioMed Res. Int. 2019, 2019, 8194537. [Google Scholar] [CrossRef]
- Taekema, D.G.; Gussekloo, J.; Maier, A.B.; Westendorp, R.G.J.; De Craen, A.J.M. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age. Ageing 2010, 39, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Wiśniowska-Szurlej, A.; Ćwirlej-Sozańska, A.; Wołoszyn, N.; Sozańśki, B.; Wilmowska-Pietruszyńska, A. Association between handgrip strength, mobility, leg strength, flexibility, and postural balance in older adults under long-term care facilities. Bio.Med. Res. Int. 2019, 2019, 1042834. [Google Scholar] [CrossRef]
- Yu, H.; Chen, X.; Dong, R.; Zhang, W.; Han, P.; Kang, L.; Ma, Y.; Jia, L.; Fu, L.; Hou, L.; et al. Clinical relevance of different handgrip strength indexes and cardiovascular disease risk factors: A cross-sectional study in suburb-dwelling elderly Chinese. J. Formos. Med. Assoc. 2019, 118, 1062–1072. [Google Scholar] [CrossRef]
- Martien, S.; Delecluse, C.; Boen, F.; Seghers, J.; Pelssers, J.; Van Hoecke, A.-S.; Van Roie, E. Is knee extension strength a better predictor of functional performance than handgrip strength among older adults in three different settings? Arch. Gerontol. Geriatr. 2015, 60, 252–258. [Google Scholar] [CrossRef]
- Stevens, P.J.; Syddall, H.E.; Patel, H.P.; Martin, H.J.; Cooper, C.; Sayer, A. Is grip strength a good marker of physical performance among community-dwelling older people? J. Nutr. Heal. Aging. 2012, 16, 769–774. [Google Scholar] [CrossRef]
- McLean, R.R.; Shardell, M.D.; Alley, D.E.; Cawthon, P.M.; Fragala, M.S.; Harris, T.B.; Kenny, A.M.; Peters, K.W.; Ferrucci, L.; Guralnik, J.M.; et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 69, 576–583. [Google Scholar] [CrossRef]
- Giampaoli, S.; Ferrucci, L.; Cecchi, F.; Noce, C.L.; Poce, A.; Dima, F.; Santaquilani, A.; Vescio, M.F.; Menotti, A. Hand-grip strength predicts incident disability in non-disabled older men. Age. Ageing 1999, 28, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Rantanen, T.; Avlund, K.; Suominen, H.; Schroll, M.; Frändin, K.; Pertti, E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin. Exp. Res. 2002, 14, 10–15. [Google Scholar]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. JAMA 1999, 281, 558–560. [Google Scholar] [CrossRef] [Green Version]
- Onder, G.; Penninx, B.W.J.H.; Ferrucci, L.; Fried, L.P.; Guralnik, J.M.; Pahor, M. Measures of physical performance and risk for progressive and catastrophic disability: Results from the women’s health and aging study. J. Gerontol. Ser. A Boil. Sci. Med.Med Sci. 2005, 60, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W. Reference values for the Five-Repetition Sit-to-Stand Test: A Descriptive meta-analysis of data from elders. Percept. Mot. Ski. 2006, 103, 215–222. [Google Scholar] [CrossRef]
- Pinheiro, P.A.; Carneiro, J.; Coqueiro, R.; Pereira, R.; Fernandes, M. “Chair stand test” as simple tool for sarcopenia screening in elderly women. J. Nutr. Heal. Aging 2016, 20, 56–59. [Google Scholar] [CrossRef]
- Suzuki, T.; Bean, J.F.; Fielding, R.A. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J. Am. Geriatr. Soc. 2001, 49, 1161–1167. [Google Scholar] [CrossRef]
- Bean, J.F.; Leveille, S.; Kiely, D.K.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. A comparison of leg power and leg strength within the InCHIANTI study: Which influences mobility more? J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, M728–M733. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.X.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachex. Sarcopenia. Muscle 2019, 11, 3–25. [Google Scholar] [CrossRef] [Green Version]
- Taaffe, D.R.; Harris, T.B.; Ferrucci, L.; Rowe, J.; Seeman, T.E. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2000, 55, M709–M715. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.J.H.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Williams, G.R.; Guralnik, J.M.; Ferrucci, L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2004, 59, M242–M248. [Google Scholar] [CrossRef] [Green Version]
- Ferrucci, L.; Penninx, B.W.J.H.; Volpato, S.; Harris, T.B.; Bandeen-Roche, K.; Balfour, J.; Leveille, S.G.; Fried, L.P.; Guralnik, J.M. Change in muscle strength explains accelerated decline of physical function in older women with high Interleukin-6 serum levels. J. Am. Geriatr. Soc. 2002, 50, 1947–1954. [Google Scholar] [CrossRef] [Green Version]
- Cesari, M.; Pahor, M.; Bartali, B.; Cherubini, A.; Penninx, B.W.J.H.; Williams, G.R.; Atkinson, H.; Martin, A.; Guralnik, J.M.; Ferrucci, L. Antioxidants and physical performance in elderly persons: The invecchiare in Chianti (InCHIANTI) study. Am. J. Clin. Nutr. 2004, 79, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Calvani, R.; Leeuwenburgh, C.; Coelho-Júnior, H.J.; Bernabei, R.; Landi, F.; Marzetti, E. Targeting mitochondrial quality control for treating sarcopenia: Lessons from physical exercise. Expert Opin. Ther. Targets 2018, 23, 153–160. [Google Scholar] [CrossRef]
- Calvani, R.; Brasili, E.; Praticò, G.; Capuani, G.; Tomassini, A.; Marini, F.; Sciubba, F.; Finamore, A.; Roselli, M.; Marzetti, E.; et al. Fecal and urinary NMR-based metabolomics unveil an aging signature in mice. Exp. Gerontol. 2014, 49, 5–11. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Varadhan, R.; Yao, W.; Matteini, A.; Beamer, B.A.; Xue, Q.-L.; Yang, H.; Manwani, B.; Reiner, A.; Jenny, N.; Parekh, N.; et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 69, 165–173. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Butcher, S.; Chahel, H.; Lord, J.M. Ageing and the neutrophil: No appetite for killing? Immunology 2000, 100, 411–416. [Google Scholar] [CrossRef]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Busti, F.; Campostrini, N.; Martinelli, N.; Girelli, D. Iron deficiency in the elderly population, revisited in the hepcidin era. Front. Pharmacol. 2014, 5, 83. [Google Scholar] [CrossRef]
- Picca, A.; Mankowski, R.T.; Kamenov, G.D.; Anton, S.D.; Manini, T.M.; Buford, T.W.; Saini, S.K.; Calvani, R.; Landi, F.; Bernabei, R.; et al. advanced age is associated with iron dyshomeostasis and mitochondrial DNA damage in human skeletal muscle. Cells 2019, 8, 1525. [Google Scholar] [CrossRef] [Green Version]
- Yahiaoui, L.; Gvozdic, D.; Danialou, G.; Mack, M.; Petrof, B.J. CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J. Physiol. 2008, 586, 3991–4004. [Google Scholar] [CrossRef]
- Martinez, C.O.; McHale, M.J.; Wells, J.T.; Ochoa, O.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R832–R842. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1high macrophage infiltration. J. Immunol. 2014, 193, 5149–5160. [Google Scholar] [CrossRef] [Green Version]
- Scully, D.; Sfyri, P.; Verpoorten, S.; Papadopoulos, P.; Muñoz-Turrillas, M.C.; Mitchell, R.; Aburima, A.; Patel, K.; Gutiérrez, L.; Naseem, K.M.; et al. Platelet releasate promotes skeletal myogenesis by increasing muscle stem cell commitment to differentiation and accelerates muscle regeneration following acute injury. Acta Physiol. 2018, 225, e13207. [Google Scholar] [CrossRef]
- Liu, B.; Poon, M.; Taubman, M.B. PDGF-BB enhances monocyte chemoattractant protein-1 mRNA stability in smooth muscle cells by downregulating ribonuclease activity. J. Mol. Cell. Cardiol. 2006, 41, 160–169. [Google Scholar] [CrossRef]
- Coppe, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Boil. 2008, 6, 2853–2868. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Scheibye-Knudsen, M.; Fang, E.F.; Croteau, D.L.; Wilson, D.M.; Bohr, V.A. Protecting the mitochondrial powerhouse. Trends. Cell. Boil. 2014, 25, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. Maturitas 2017, 96, 10–15. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcarcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Landi, F.; Bernabei, R.; Marzetti, E. Fueling inflamm-aging through mitochondrial dysfunction: Mechanisms and molecular targets. Int. J. Mol. Sci. 2017, 18, 933. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, Danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Krysko, D.; Agostinis, P.; Krysko, O.; Garg, A.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011, 32, 157–164. [Google Scholar] [CrossRef]
- Collins, L.V.; Hajizadeh, S.; Holme, E.; Jonsson, I.-M.; Tarkowski, A. Endogenously oxidized mitochondrial DNA induces In Vivo and In Vitro inflammatory responses. J. Leukoc. Boil. 2004, 75, 995–1000. [Google Scholar] [CrossRef]
- Cai, X.; Chiu, Y.-H.; Chen, Z.J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell 2014, 54, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Bossola, M.; Manes-Gravina, E.; Landi, F.; Bernabei, R.; Marzetti, E. Circulating Mitochondrial DNA at the crossroads of mitochondrial dysfunction and inflammation during aging and muscle wasting disorders. Rejuvenation Res. 2018, 21, 350–359. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Inter-organelle membrane contact sites and mitochondrial quality control during aging: A geroscience view. Cells 2020, 9, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, A.E.; Turnbull, D.M.; Eisner, V.; Hajnóczky, G.; Picard, M. Mitochondrial nanotunnels. Trends Cell Boil. 2017, 27, 787–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, M.L.; Robertson, G.L.; Gama, V. Break on through: Golgi-derived vesicles aid in mitochondrial fission. Cell Metab. 2020, 31, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.; Abadir, P.M.; Marx, R.; Westbrook, R.; Cooke, C.; Yang, H.; Walston, J. Impaired mitochondrial degradation by autophagy in the skeletal muscle of the aged female interleukin 10 null mouse. Exp. Gerontol. 2016, 73, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Júnior, H.J.; Bossola, M.; Landi, F.; Bernabei, R.; Bucci, C.; Marzetti, E. Generation and release of mitochondrial-derived vesicles in health, aging and disease. J. Clin. Med. 2020, 9, 1440. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Bucci, C.; Monaco, M.R.L.; Bentivoglio, A.R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial dysfunction and aging: Insights from the analysis of extracellular vesicles. Int. J. Mol. Sci. 2019, 20, 805. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Calvani, R.; Lorenzi, M.; Menghi, A.; Galli, M.; Vitiello, R.; Randisi, F.; Bernabei, R.; Landi, F.; Marzetti, E. Mitochondrial dynamics signaling is shifted toward fusion in muscles of very old hip-fractured patients: Results from the Sarcopenia in HIp FracTure (SHIFT) exploratory study. Exp. Gerontol. 2017, 96, 63–67. [Google Scholar] [CrossRef]
- Basisty, N.B.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS. Boil. 2020, 18, e3000599. [Google Scholar] [CrossRef] [Green Version]
- Takasugi, M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 2018, 17, e12734. [Google Scholar] [CrossRef]
- Belov, L.; Hallal, S.; Best, G.; Matic, K.J.; Mulligan, S.P.; Christopherson, R.I. Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J. Extracell. Vesicles. 2016, 5, 25355. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant. Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Wilson, I.D. Understanding ’global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C. Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Yap, I.K.S.; Chan, Q.; Ebbels, T.; De Iorio, M.; Brown, I.J.; Veselkov, K.; et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008, 453, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tasigchana, R.F.; León-Muñoz, L.M.; Lopez-Garcia, E.; Gutierrez-Fisac, J.L.; Laclaustra, M.; Rodríguez-Artalejo, F.; Guallar-Castillon, P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: A prospective cohort study. Age Ageing 2017, 46, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Calvani, R.; Rodriguez-Mañas, L.; Picca, A.; Marini, F.; Biancolillo, A.; Laosa, O.; Pedraza, L.; Gervasoni, J.; Primiano, A.; Conta, G.; et al. Identification of a circulating amino acid signature in frail older persons with type 2 diabetes mellitus: Results from the Metabofrail study. Nutrients 2020, 12, 199. [Google Scholar] [CrossRef] [Green Version]
- Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Pérez-Schindler, J.; Philp, A.; Smith, K.; Atherton, P.J. Skeletal muscle homeostasis and plasticity in youth and ageing: Impact of nutrition and exercise. Acta. Physiol. 2015, 216, 15–41. [Google Scholar] [CrossRef] [Green Version]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Boil. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef]
- Yoon, M.-S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; D’Angelo, E.; Sisto, A.; Marzetti, E. Protein intake and muscle health in old age: From biological plausibility to clinical evidence. Nutrients 2016, 8, 295. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F.S. Protein-amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lustgarten, M.S.; Price, L.L.; Chale, A.; Phillips, E.M.; Fielding, R.A. Branched chain amino acids are associated with muscle mass in functionally limited older adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 69, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moaddel, R.; Fabbri, E.; Khadeer, M.A.; Carlson, O.D.; González-Freire, M.; Zhang, P.; Semba, R.D.; Ferrucci, L. Plasma biomarkers of poor muscle quality in older men and women from the Baltimore longitudinal study of aging. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 71, 1266–1272. [Google Scholar] [CrossRef] [Green Version]
- Ottestad, I.; Ulven, S.M.; Øyri, L.K.L.; Sandvei, K.S.; Gjevestad, G.O.; Bye, A.; Sheikh, N.A.; Biong, A.S.; Andersen, L.F.; Holven, K.B. Reduced plasma concentration of branched-chain amino acids in sarcopenic older subjects: A cross-sectional study. Br. J. Nutr. 2018, 120, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, K.; Nakamura, M.; Adachi, Y.; Imaizumi, A.; Hakamada, T.; Abe, Y.; Kaneko, E.; Takahashi, S.; Shimokado, K. Increased plasma proline concentrations are associated with sarcopenia in the elderly. PLoS ONE 2017, 12, e0185206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, Y.; Ono, N.; Imaizumi, A.; Muramatsu, T.; Andou, T.; Shimodaira, Y.; Nagao, K.; Kageyama, Y.; Mori, M.; Noguchi, Y.; et al. Plasma amino acid profile in severely frail elderly patients in Japan. Int. J. Gerontol. 2018, 12, 290–293. [Google Scholar] [CrossRef]
- He, Q. Metabonomics and its role in amino acid nutrition research. Front. Biosci. 2011, 16, 2451–2460. [Google Scholar] [CrossRef] [Green Version]
- Ter Borg, S.; Luiking, Y.C.; Van Helvoort, A.; Boirie, Y.; Schols, J.M.G.A.; De Groot, L.C.P.G.M. Low levels of branched chain amino acids, eicosapentaenoic acid and micronutrients are associated with low muscle mass, strength and function in community-dwelling older adults. J. Nutr. Heal. Aging 2018, 23, 27–34. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Microbiome–health interactions in older people. Cell. Mol. Life Sci. 2017, 75, 119–128. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Raes, J.; Bork, P. The Human gut microbiome: From association to modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif. Tissue. Int. 2017, 102, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: Is there a gut–muscle axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut dysbiosis and muscle aging: Searching for novel targets against sarcopenia. Mediat. Inflamm. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [Green Version]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.-P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, G.T.; Allison, C.; Gibson, S.A.W.; Cummings, J.H. Contribution of the microflora to proteolysis in the human large intestine. J. Appl. Bacteriol. 1988, 64, 37–46. [Google Scholar] [CrossRef]
- Macfarlane, G.; Cummings, J.; Macfarlane, S.; Gibson, G. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J. Appl. Bacteriol. 1989, 67, 521–527. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Carlisle, E.M.; Alverdy, J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically Ill. Surg. Clin. North. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Metges, C.C. Contribution of microbial amino acids to amino acid homeostasis of the host. J. Nutr. 2000, 130, 1857S–1864S. [Google Scholar] [CrossRef]
- Bergen, W.G.; Wu, G. Intestinal nitrogen recycling and utilization in health and disease. J. Nutr. 2009, 139, 821–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besten, G.D.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-activated protein kinase and stimulation of energy metabolism by acetic acid in L6 myotube cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sovran, B.; Hugenholtz, F.; Elderman, M.; Van Beek, A.A.; Graversen, K.; Huijskes, M.; Boekschoten, M.V.; Savelkoul, H.F.J.; De Vos, P.; Dekker, J.; et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Stecher, B.; Hardt, W.-D. The role of microbiota in infectious disease. Trends. Microbiol. 2008, 16, 107–114. [Google Scholar] [CrossRef]
- MacPherson, A.J.; Geuking, M.B.; McCoy, K.D. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology 2005, 115, 153–162. [Google Scholar] [CrossRef]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The gut microbiota in immune-mediated inflammatory diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkilä, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Schiffrin, E.J.; Morley, J.E.; Donnet-Hughes, A.; Guigoz, Y. The inflammatory status of the elderly: The intestinal contribution. Mutat. Res. Mol. Mech. Mutagen 2010, 690, 50–56. [Google Scholar] [CrossRef]
- Shapiro, H.; Thaiss, C.A.; Levy, M.; Elinav, E. The cross talk between microbiota and the immune system: Metabolites take center stage. Curr. Opin. Immunol. 2014, 30, 54–62. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability In Vitro and In Vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD. Am. J. Pathol. 2012, 182, 375–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tongeren, S.P.; Slaets, J.P.J.; Harmsen, H.J.M.; Welling, G.W.; Viterbo, A.; Harel, M.; Horwitz, B.A.; Chet, I.; Mukherjee, P.K. Fecal microbiota composition and frailty. Appl. Environ. Microbiol. 2005, 71, 6241–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Londhe, P.; Guttridge, D.C. Inflammation induced loss of skeletal muscle. Bone 2015, 80, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
| Biological Domain | Biomarkers |
|---|---|
| Inflammation | CRP, HSP72, IL1β, IL6, IL8, MCP1, MIP1α, MPO, PDGF-BB |
| Amino acid metabolism | Asparagine, aspartic acid, citrulline, ethanolamine, glutamic acid, sarcosine, taurine, threonine |
| Gut microbiota | Barnesiellaceae, Christensenellaceae, Oscillospira, Ruminococcus |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picca, A.; Calvani, R.; Cesari, M.; Landi, F.; Bernabei, R.; Coelho-Júnior, H.J.; Marzetti, E. Biomarkers of Physical Frailty and Sarcopenia: Coming up to the Place? Int. J. Mol. Sci. 2020, 21, 5635. https://doi.org/10.3390/ijms21165635
Picca A, Calvani R, Cesari M, Landi F, Bernabei R, Coelho-Júnior HJ, Marzetti E. Biomarkers of Physical Frailty and Sarcopenia: Coming up to the Place? International Journal of Molecular Sciences. 2020; 21(16):5635. https://doi.org/10.3390/ijms21165635
Chicago/Turabian StylePicca, Anna, Riccardo Calvani, Matteo Cesari, Francesco Landi, Roberto Bernabei, Hélio José Coelho-Júnior, and Emanuele Marzetti. 2020. "Biomarkers of Physical Frailty and Sarcopenia: Coming up to the Place?" International Journal of Molecular Sciences 21, no. 16: 5635. https://doi.org/10.3390/ijms21165635





