Determination of the Total Polyphenols Content and Antioxidant Activity of Echinacea Purpurea Extracts Using Newly Manufactured Glassy Carbon Electrodes Modified with Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Solutions and Sample Preparation
2.3. Apparatus
2.4. Preparation of CNTs in CS
2.5. Preparation of Modified GCE
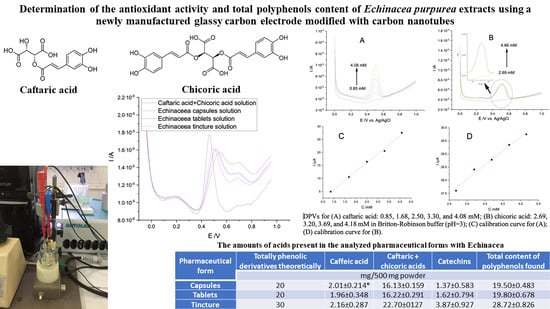
2.6. Determination of the Total Polyphenol Content
2.6.1. Differential Pulse Voltammetry
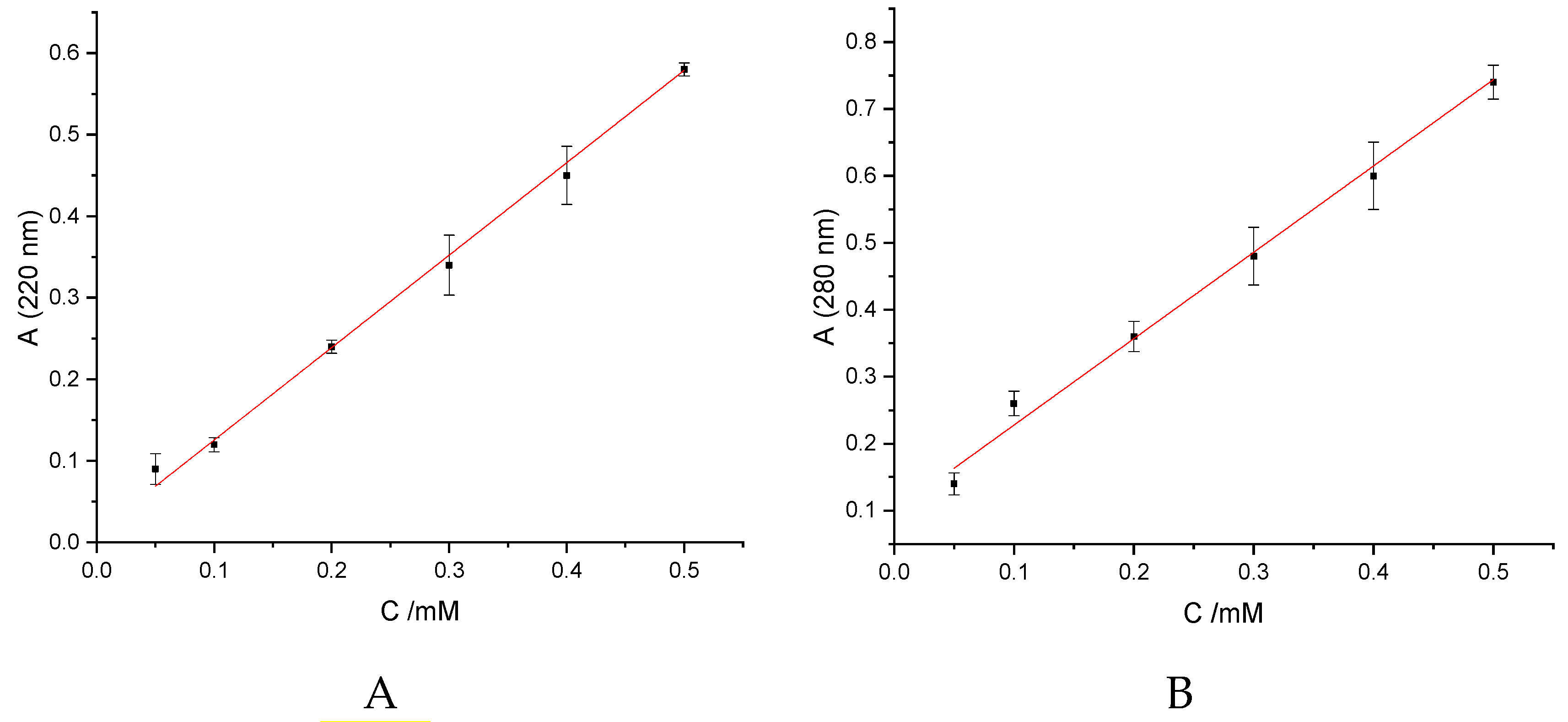
2.6.2. Spectrophotometry
2.7. Antioxidant Activity Determination
2.7.1. Cyclic Voltammetry
2.7.2. DPPH Method
3. Results
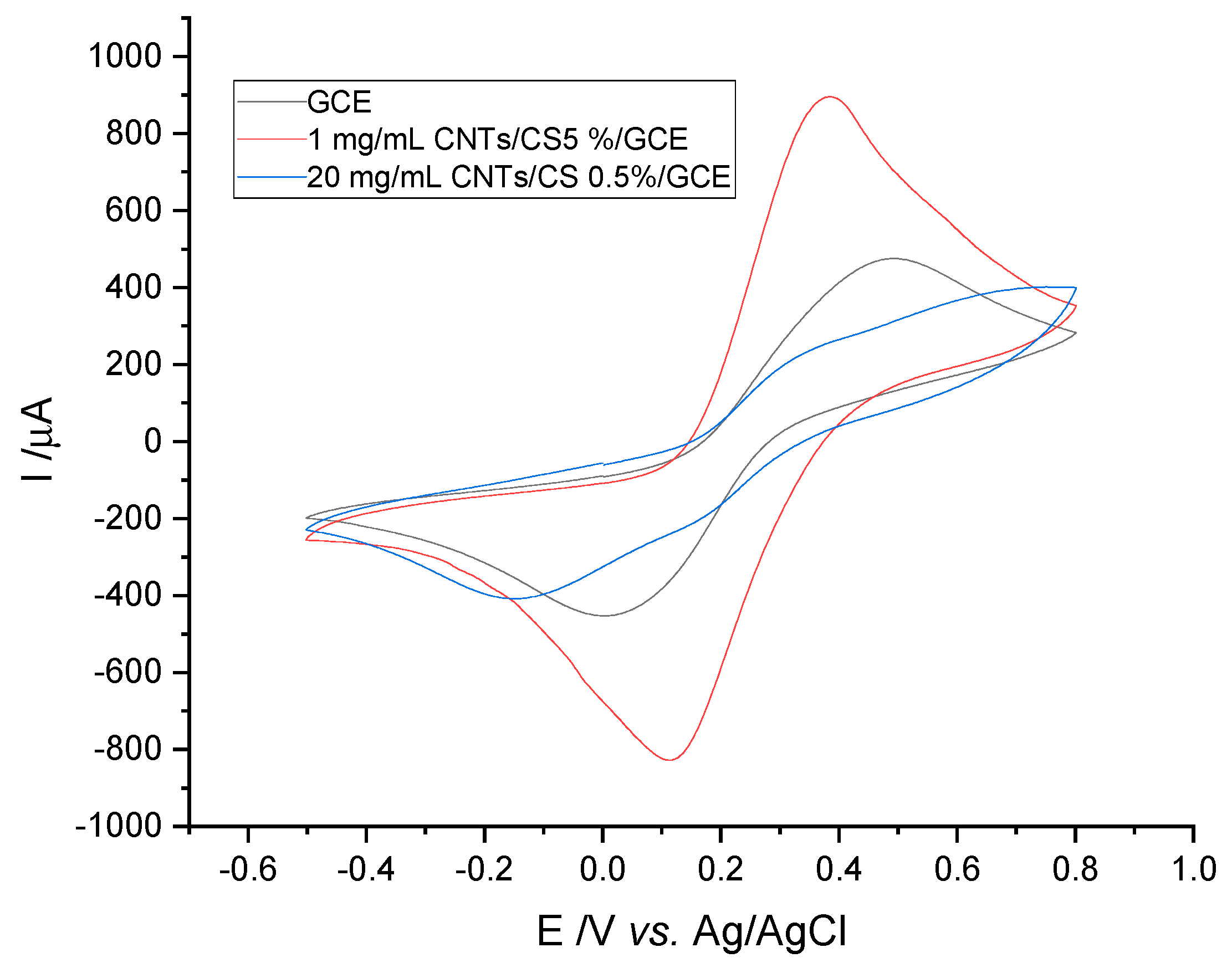
3.1. Electrochemical Characterization of Electrodes by Cyclic Voltammetry
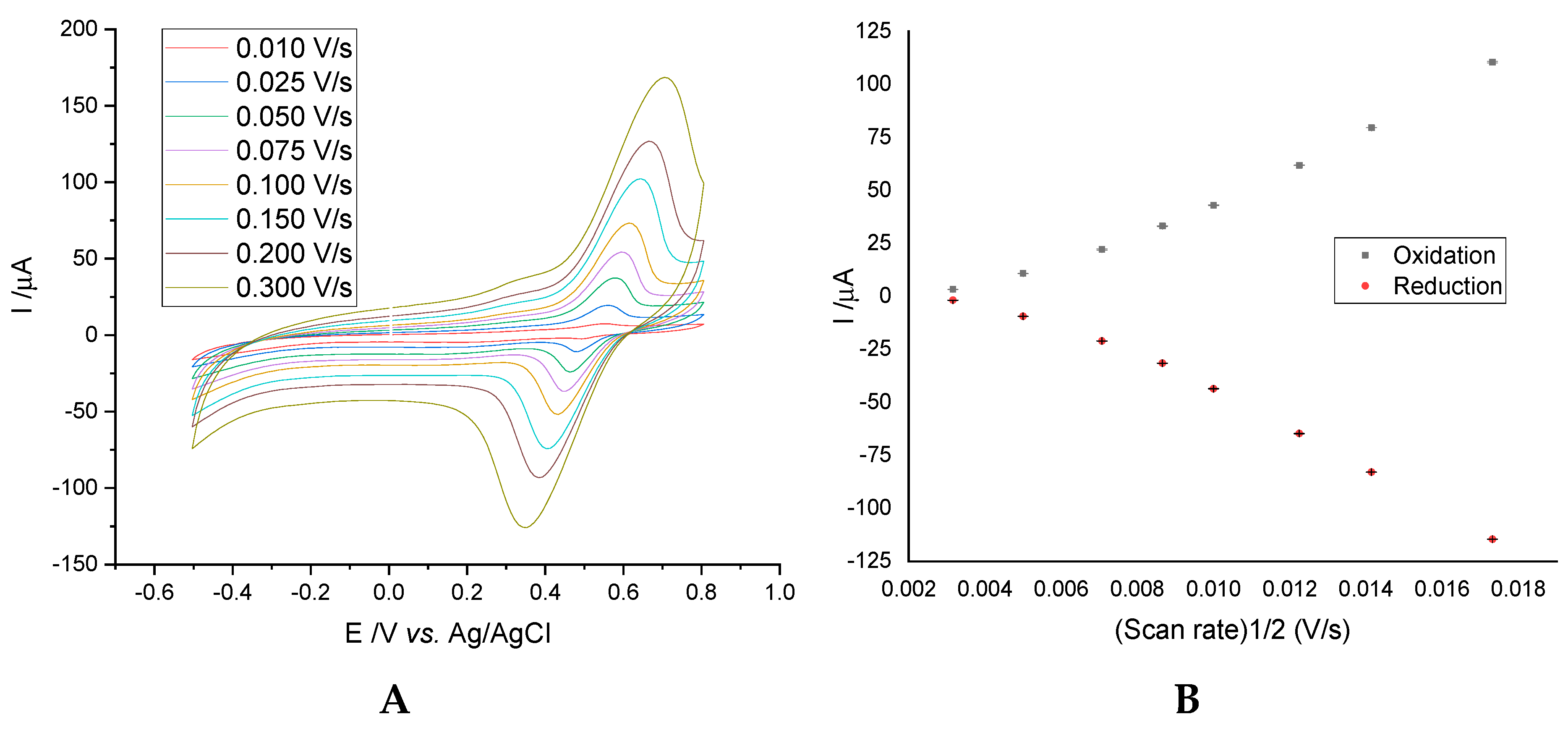
3.1.1. Influence of Scanning Speed on the Intensity of the Anodic/Cathodic Peak
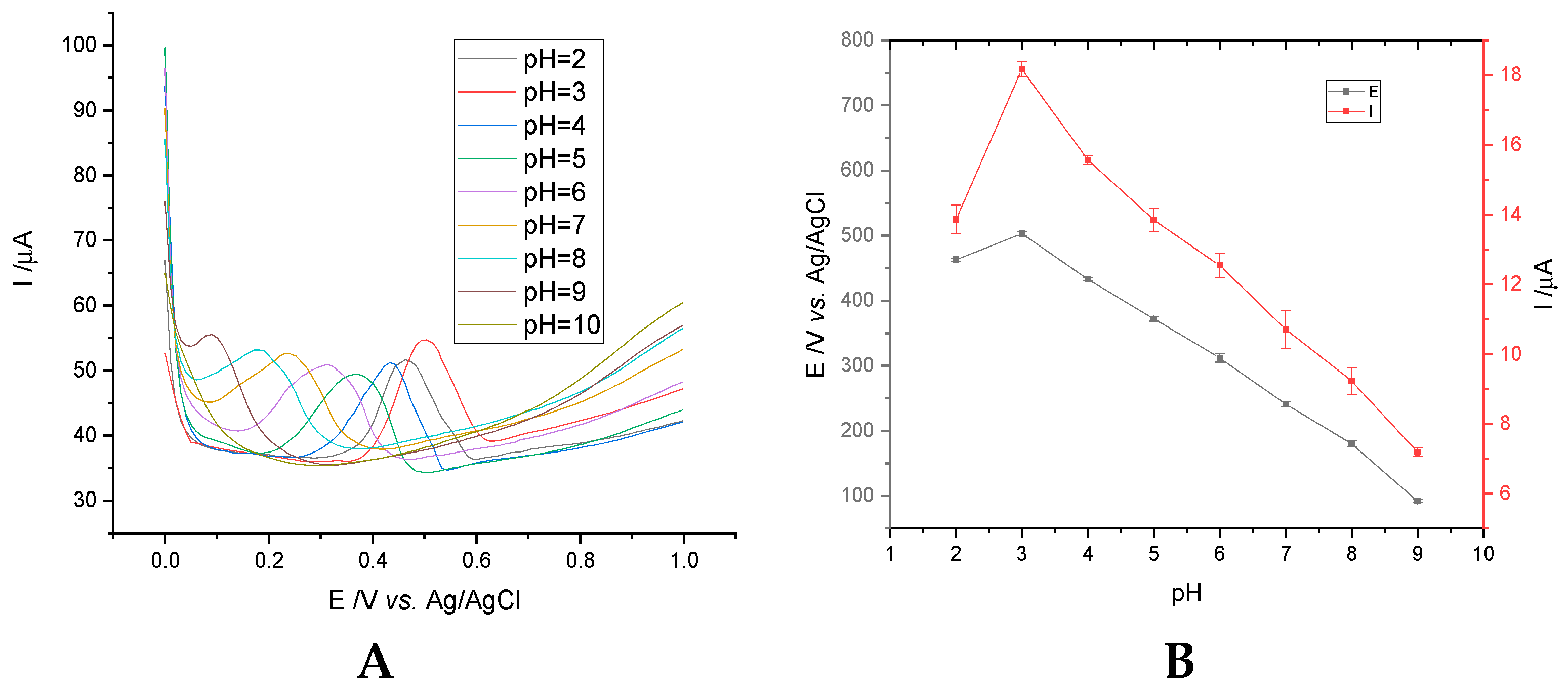
3.1.2. Optimization of the pH Value
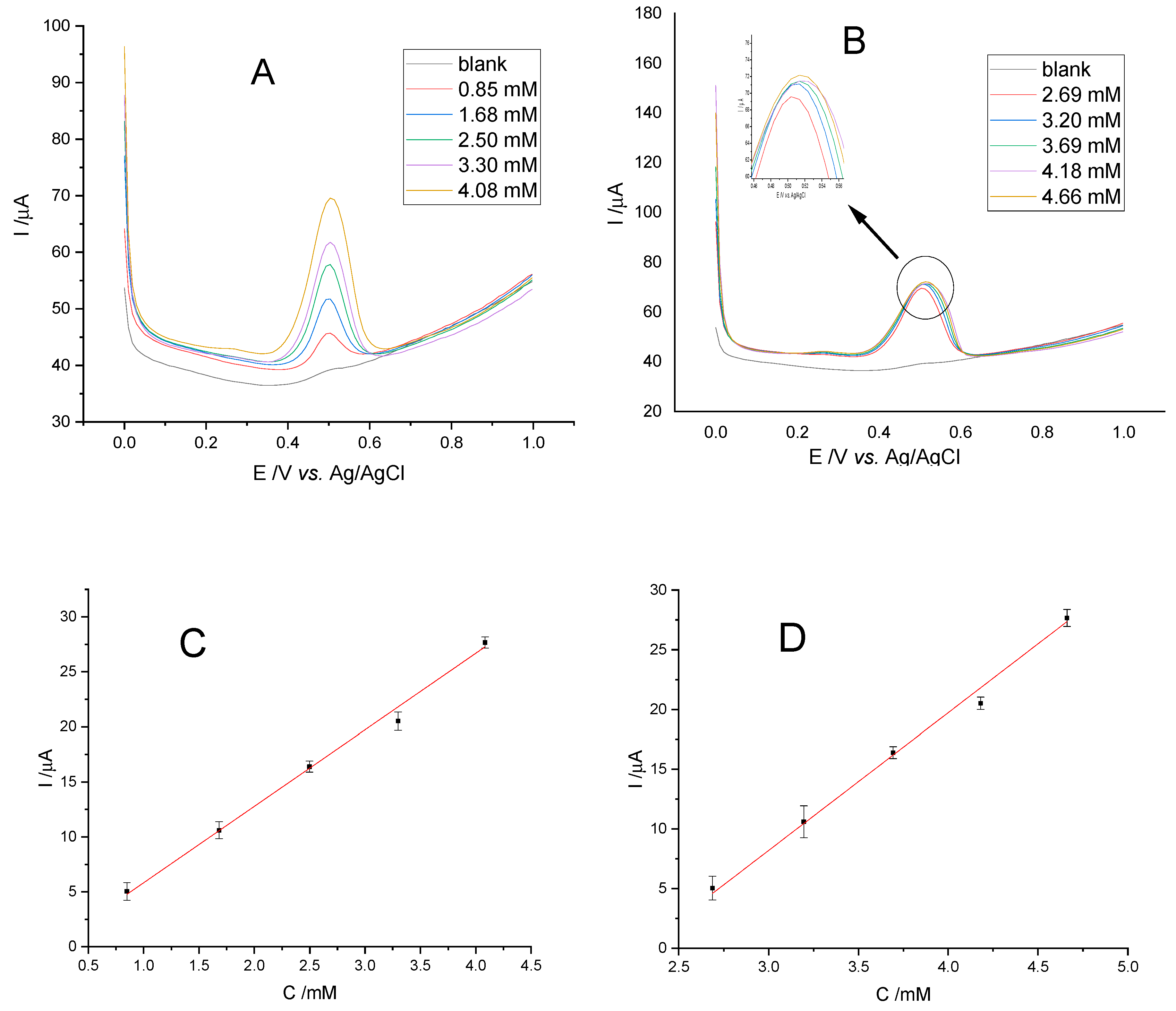
3.1.3. Calibration Iines
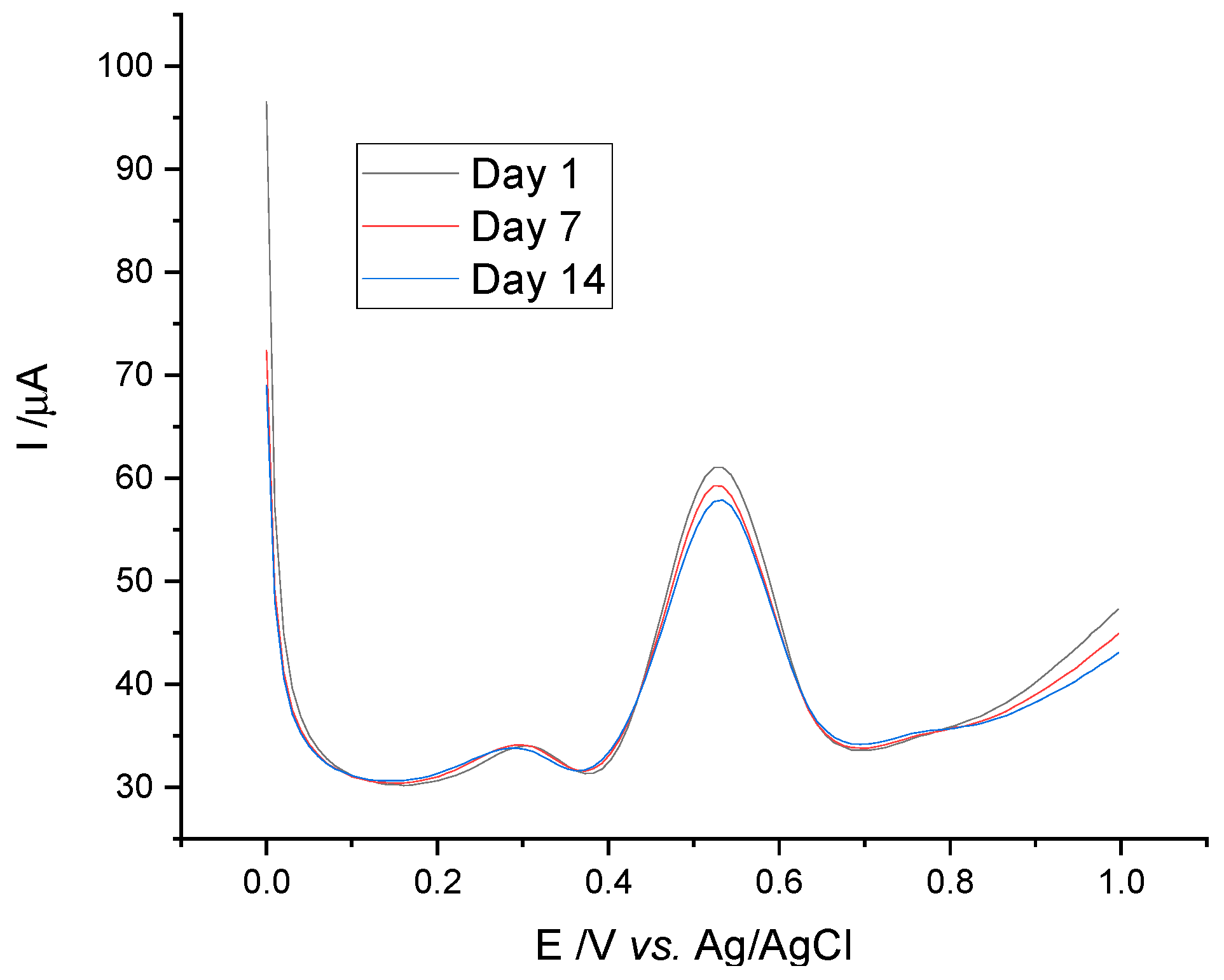
3.1.4. Electrode Stability
3.1.5. Interference Studies
3.2. Voltammetric Study of Echinacea Purpurea in Pharmaceutical Forms
3.3. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hudson, J.B. Applications of the Phytomedicine Echinacea Purpurea (Purple Coneflower) in Infectious Diseases. J. Biomed. Biotechnol. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bungau, S.G.; Popa, V.C. Between Religion and Science Some Aspects Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, 24, 3–18. [Google Scholar]
- Hu, C.; Kitts, D.D. Studies on the Antioxidant Activity of Echinacea Root Extract. J. Agric. Food Chem. 2000, 48, 1466–1472. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Arnason, J.T.; Hudson, J.B. Anti-inflammatory Activities of Echinacea Extracts Do Not Correlate with Traditional Marker Components. Pharm. Biol. 2009, 47, 430–435. [Google Scholar] [CrossRef]
- Burns, J.J.; Zhao, L.; Taylor, E.W.; Spelman, K. The Influence of Traditional Herbal Formulas on Cytokine Activity. Toxicology 2010, 278, 140–159. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Saito, K.; Maruyama, H.; Inoue, M.; Yamashita, T.; Ishida, T.; Gu, Y. Antioxidant and Immuno-Enhancing Effects of Echinacea Purpurea. Biol. Pharm. Bull. 2004, 27, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tang, T.; He, H.; Wu, H.; Hu, Z. Influence of several postharvest processing methods on polyphenol oxidase activity and cichoric acid content of Echinacea purpurea roots. Ind. Crops Prod. 2011, 34, 873–881. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Magro, L.; Melegari, M.; Soragni, F. Analysis of Phenolic Compounds and Radical Scavenging Activity of Echinacea Spp. J. Pharm. Biomed. Anal. 2004, 35, 289–301. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Niazi, A.; Afiuni-Zadeh, S.; Zamani, K. Determination of Cichoric Acid as a Biomarker in Echinacea Purpurea Cultivated in Iran Using High Performance Liquid Chromatography. Chin. Med. 2010, 1, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, L.; Thulin, J.; Mortensen, A.; Skibsted, L.H.; Molgaard, P. Antioxidant Activity of Cichoric Acid and Alkamides from Echinacea purpurea, Alone and in Combination. Food Chem. 2007, 101, 74–81. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical Scavenging Ability of Polyphenolic Compounds Towards DPPH Free Radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Remiger, P.; Wagner, H. Comparative TLC and HPLC analysis of herbal drugs from Echinacea purpurea, E. pallida and E. angustifolia. Dtsch. Apoth. Ztg. 1988, 128, 174–180. [Google Scholar]
- Perry, N.B.; Burgess, E.J.; Glennie, V.L. Echinacea Standardization: Analytical Methods for Phenolic Compounds and Typical Levels in Medicinal Species. J. Agric. Food Chem. 2001, 49, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Chan, M.; Paley, L.; Betz, J.M. Determination of Major Phenolic Compounds in Echinacea Spp. Raw Materials and Finished Products by High-Performance Liquid Chromatography With Ultraviolet Detection: Single-Laboratory Validation Matrix Extension. J. AOAC Int. 2011, 94, 1400–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilmartin, P.A. Electrochemistry applied to the analysis of wine: A mini-review. Electrochem. Commun. 2016, 67, 39–42. [Google Scholar] [CrossRef]
- Karaosmanoglu, H.; Suthanthangjai, W.; Travas-Sejdic, J.; Kilmartin, P.A. Electrochemical analysis of beverage phenolics using an electrode modified with poly(3,4-ethylenedioxythiophene). Electrochim. Acta 2016, 201, 366–373. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Newair, E.F. Adsorptive stripping voltammetric determination of gallic acid using an electrochemical sensor based on polyepinephrine/glassy carbon electrode and its determination in black tea sample. J. Electroanal. Chem. 2013, 704, 32–37. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. The use of cyclic voltammetry for wine analysis: Determination of polyphenols and free sulfur dioxide. Anal. Chim. Acta 2010, 668, 155–165. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; He, J.; Xu, Y.; Gao, R.; Zhang, J.; Chong, T.; Wang, L.; Tang, X. Surface Imprinted Polymers Based on Amino-Hyperbranched Magnetic Nanoparticles for Selective Extraction and Detection of Chlorogenic Acid in Honeysuckle Tea. Talanta 2018, 181, 271–277. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Miguel, E.M.; Silva, J.D.S.; Silva, C.B.D.; Goulart, M.O.F.; Kubota, L.T.; Gonzaga, F.B.; Santos, W.J.R.; Lima, P.R. Application of a Nanostructured Platform and Imprinted Sol-Gel Film for Determination of Chlorogenic Acid in Food Samples. Talanta 2016, 156–157, 119–125. [Google Scholar] [CrossRef]
- Newair, E.F.; Kilmartin, P.A.; Garcia, F. Square wave voltammetric analysis of polyphenol content and antioxidant capacity of red wines using glassy carbon and disposable carbon nanotubes modified screen-printed electrodes. Eur. Food Res. Technol. 2018, 244, 1225–1237. [Google Scholar] [CrossRef]
- Daniel, S.; Rao, T.P.; Rao, K.S.; Rani, S.U.; Lee, H.Y.; Kawai, T.; Naidu, G.R.K. A review of DNA functionalized/grafted carbon nanotubes and their characterization. Sens. Actuators B Chem. 2007, 122, 672–682. [Google Scholar] [CrossRef]
- Ovádeková, R.; Labuda, J. Electrochemical DNA biosensors for the investigation of dsDNA host-guest interactions and damage. Curr. Opin. Electrochem. 2006, 11, 21–56. [Google Scholar]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Wang, Y.R.; Hu, P.; Liang, Q.L.; Luo, G.A.; Wang, Y.M. Application of Carbon Nanotube Modified Electrode in Bioelectroanalysis. Chin. J. Anal. Chem. 2008, 36, 1011–1016. [Google Scholar] [CrossRef]
- Otrisal, P.; Obsel, V.; Forus, S.; Bungau, C.; Aleya, L.; Bungau, S. Protecting emergency workers and armed forces from volatile toxic compounds: Applicability of reversible conductive polymer-based sensors in barrier materials. Sci. Total Environ. 2019, 694. [Google Scholar] [CrossRef]
- Qu, L.; Yang, S. Application of Carbon Nanotubes Modified Electrode in Pharmaceutical Analysis. In Carbon Nanotubes—Growth and Applications; Naraghi, M., Ed.; IntechOpen: Rijieka, Croatia, 2011. [Google Scholar]
- Zhao, L.; Gao, L. Novel In Situ Synthesis of MWNTs–Hydroxyapatite Composites. Carbon 2004, 42, 423–426. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Chenite, A.; Chaput, C.; Guirguis, S.; Leroux, J. Characterization of Thermosensitive Chitosan Gels for the Sustained Delivery of Drugs. Int. J. Pharm. 2000, 203, 89–98. [Google Scholar] [CrossRef]
- Cirillo, G.; Vittorio, O.; Kunhardt, D.; Valli, E.; Voli, F.; Farfalla, A.; Curcio, M.; Spizzirri, U.G.; Hampel, S. Combining Carbon Nanotubes and Chitosan for the Vectorization of Methotrexate to Lung Cancer Cells. Materials 2019, 12, 2889. [Google Scholar] [CrossRef] [Green Version]
- Sengiz, C.; Congur, G.; Eksin, E.; Erdem, A. Multiwalled Carbon Nanotubes-Chitosan Modified Single-Use Biosensors for Electrochemical Monitoring of Drug-DNA Interactions. Electroanalysis 2015, 27, 1855–1863. [Google Scholar] [CrossRef]
- Fritea, L.; Bănică, F.; Costea, T.O.; Moldovan, L.; Iovan, C.; Cavalu, S. A gold nanoparticles-Graphene based electrochemical sensor for sensitive determination of nitrazepam. J. Electroanal. Chem. 2018, 830–831, 63–71. [Google Scholar] [CrossRef]
- Cavalu, S.; Simon, V.; Banica, F. In vitro study of collagen coating by electrodeposition on acrylic bone cement with antimicrobial potential. Dig. J. Nanomater. Biostruct. 2010, 6, 89–97. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH.Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Zanello, P. Inorganic Electrochemistry: Theory, Practice and Application; The Royal Society of Chemistry, Thomas Graham House: Cambridge, UK, 2003. [Google Scholar]
- Hernanz-Vila, D.; Jara-Palacios, M.J.; Escudero-Gilete, M.L.; Heredia, F.J. Applications of Voltammetric Analysis to Wine Products. In Applications of the Voltammetry; Stoitcheva, M., Zlatev, R., Eds.; IntechOpen: Rijieka, Croatia, 2017. [Google Scholar]
- Sloley, B.D.; Urichuk, L.J.; Tywin, C.; Coutts, R.T.; Pang, P.K.; Shan, J.J. Comparison of Chemical Components and Antioxidants Capacity of Different Echinacea Species. J. Pharm. Pharmacol. 2001, 53, 849–857. [Google Scholar] [CrossRef]
- Oniszczuk, T.; Oniszczuk, A.; Gondek, E.; Guz, L.; Puk, K.; Kocira, A.; Kusz, A.; Kasprzak, K.; Wójtowicz, A. Active Polyphenolic Compounds, Nutrient Contents and Antioxidant Capacity of Extruded Fish Feed Containing Purple Coneflower (Echinacea purpurea (L.) Moench.). Saudi J. Biol. Sci. 2019, 26, 24–30. [Google Scholar] [CrossRef]
- Cosio, M.S.; Buratti, S.; Mannino, S.; Benedetti, S. Use of an electrochemical method to evaluate the antioxidant activity of herb extracts from the Labiatae family. Food Chem. 2006, 97, 725–731. [Google Scholar] [CrossRef]
- Dar, R.A.; Brahman, P.K.; Khurana, N.; Wagay, J.A.; Lone, Z.A.; Ganaie, M.A.; Pitre, K.S. Evaluation of antioxidant activity of crocin, podophyllotoxin and kaempferol by chemical, biochemical and electrochemical assays. Arab. J. Chem. 2017, 10, S1119–S1128. [Google Scholar] [CrossRef] [Green Version]
- Badea, M.; Chiperea, S.; Balan, M.; Floroian, L.; Restani, P.; Marty, J.L.; Iovan, C.; Tit, D.M.; Bungau, S.; Taus, N. New approaches for electrochemical detection of ascorbic acid. Farmacia 2018, 66, 83–87. [Google Scholar]
- Fritea, L.; Tertiş, M.; Cristea, C.; Cosnier, S.; Săndulescu, R. Simultaneous Determination of Ascorbic and Uric Acids in Urine Using an Innovative Electrochemical Sensor Based on β-Cyclodextrin. Anal. Lett. 2015, 48, 89–99. [Google Scholar] [CrossRef]
- Badea, M.; di Modugno, F.; Floroian, L.; Tit, D.M.; Restani, P.; Bungau, S.; Iovan, C.; Badea, G.E.; Aleya, L. Electrochemical Strategies for Gallic Acid Detection: Potential for Application in Clinical, Food or Environmental Analyses. Sci. Total Environ. 2019, 672, 129–140. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Bakr, A.; Newair, E.F.; Garcia, F. Simultaneous Voltammetric Determination of Gallic and Protocatechuic Acids in Mango Juice Using a Reduced Graphene Oxide-Based Electrochemical Sensor. Beverages 2019, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical Analysis of the Relationship Between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. de Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Hottaa, H.; Uedaa, M.; Naganoa, S.; Tsujinob, Y.; Koyamac, J.; Osakaia, T. Mechanistic Study of the Oxidation of Caffeic Acid by Digital Simulation of Cyclic Voltammograms. Anal. Biochem. 2002, 303, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Newair, E.F.; Abdel-Hamid, R.; Kilmartin, P.A. Mechanism of Chicoric Acid Electrochemical Oxidation and Identification of Oxidation Products by Liquid Chromatography and Mass Spectrometry. Electroanalysis 2016, 28, 1–12. [Google Scholar] [CrossRef]
- Zayova, E.; Stancheva, I.; Geneva, M.; Petrova, M.; Vasilevska-Ivanova, R. Morphological evaluation and antioxidant activity of in vitro- and in vivo-derived Echinacea purpurea plants. Open Life Sci. 2012, 7, 698–707. [Google Scholar] [CrossRef]


| Reagent | Purity | CAS | Supplier | Purpose of Usage |
|---|---|---|---|---|
| 1,1-diphenyl-2-picrylylhydrazyl | ≥98% (HPLC) | 1898-66-4 | Sigma-Aldrich, USA | DPPH method |
| Acetone | p.a. ≥ 99% | 67-64-1 | Chempur, Poland | Solvent |
| Boric acid | p.a. ≥ 99% | 10043-35-3 | Micromchim, Romania | Buffer B-R preparation |
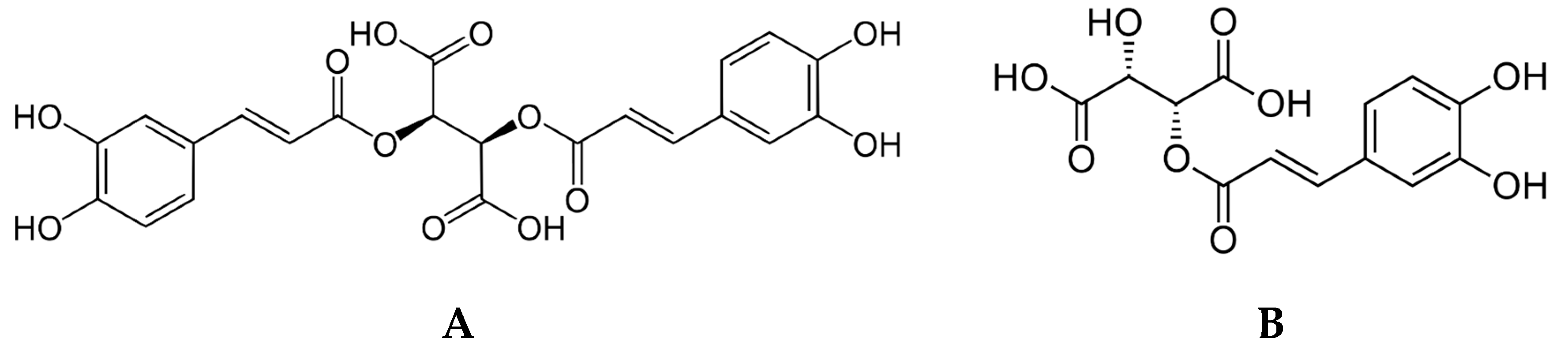
| Caftaric acid (2-Caffeoyl-L-tartaric acid) | ≥97.0% | 67879-58-7 | Sigma-Aldrich, USA | Dosage |
| Cichoric/Chicoric acid (2,3-Dicaffeoyl-L-tartaric acid) | ≥95%, (HPLC) | 6537-80-0 | Sigma-Aldrich, USA | Dosage |
| Copper (II) sulphate pentahydrate | ≥98% | 7758-99-8 | Sigma-Aldrich, USA | Interferent |
| D-(+)-glucose monohydrate | 7.0–9.5% water | 14431-43-7 | Sigma-Aldrich, USA | Interferent |
| Ethanol | 95% | 64-17-5 | Chempur, Poland | Solvent |
| Folin-Ciocalteu reagent | - | 12111-13-6 | Sigma-Aldrich, USA | Total content of polyphenolic compounds determination |
| Gallic acid | ≥98% (HPLC) | 5995-86-8 | Sigma-Aldrich, USA | DPPH antioxidant activity determination |
| Glacial acetic acid | p.a. ≥ 99% | 64-19-7 | Chempur, Poland | Buffer B-R preparation |
| Chitosan | Low molecular weight | 9012-76-4 | Merck, Germany | Modified electrode preparation |
| L-ascorbic acid | ≥99% | 50-81-7 | Sigma-Aldrich, USA | DPPH antioxidant activity determination |
| Magnesium chloride | Anhydrous, ≥98% | 7786-30-3 | Sigma-Aldrich, USA | Interferent |
| Methanol | ≥99% | 67-56-1 | Chempur, Poland | Solvent |
| MWCNT | ≤100% | 308068-56-6 | Sigma-Aldrich, USA | Modified electrode preparation |
| Nickel (II) sulphate hexahydrate | ≥98% | 10101-97-0 | Chempur, Poland | Interferent |
| Phenol | 99–100.5% | 108-95-2 | Sigma-Aldrich, USA | Interferent |
| Phosphoric acid | ACS reagent, ≥85 wt.% in H2O | 7664-38-2 | Merck, Germany | Buffer B-R preparation |
| Potassium ferricyanide, Red prussiate | ≥99% | 13746-66-2 | Merck, Germany | Redox couple |
| Potassium ferrocyanide, Yellow prussiate | ≥98.5% | 14459-95-1 | Merck, Germany | Redox couple |
| Potassium nitrate | ≥99% | 7757-79-1 | Merck, Germany | Interferent |
| Resorcinol | ≥99% | 108-46-3 | Chempur, Poland | Interferent |
| Sodium carbonate | BioXtra, ≥99.0% | 497-1908 | Chempur, Poland | Total content of polyphenolic compounds determination |
| Sodium hydroxide | BioXtra, ≥98.0% (acidimetric), pellets (anhydrous) | 1310-73-2 | Chempur, Poland | Interferent |
| Sodium chloride | ≥99% | 7647-14-5 | Sigma-Aldrich, USA | Interferent |
| Tartaric acid | ≥99.5% | 87-69-4 | Sigma-Aldrich, USA | Interferent |
| Uric acid | ≥99% | 69-93-2 | Chempur, Poland | Interferent |
| Manufacturer | Content | Category | |
|---|---|---|---|
| Capsules 500 mg | Cosmopharm, Bucharest, Romania | Concentrated Echinacea extract, 20 mg polyphenols | Natural immunostimulant |
| Tablets 500 mg | Alevia, Fălticeni, Romania | Echinacea Purpurea standardized extract, 4% polyphenols | Food supplement |
| Tincture 50 mL | Dacia Plant, Bod, Romania | Extract 1: 3.75 in hydroalcoholic solution (ethyl alcohol/water—35/65 by mass) from aerial parts of Echinacea purpurea | Food supplement |
| Acid | Linearity Range | Limit of Detection (mM) | Limit of Quantification |
|---|---|---|---|
| Caftaric | 0.850–4.084 | 0.283 | 0.850 |
| Chicoric | 2.691–4.661 | 0.897 | 2.691 |
| Voltammetry | Spectrophotometry | |||||
|---|---|---|---|---|---|---|
| Pharm. Form | Total Phenolic Derivatives Theoretically | Caffeic Acid | Caftaric + Cichoric Acids | Catechins | Total Content of Polyphenols Found | Total Content of Polyphenols Found mg GAE/500 mg Powder |
| mg/500 mg Powder | ||||||
| Capsules | 20 | 2.006 ± 0.214 * | 16.129 ± 0.159 | 1.366 ± 0.583 | 19.501 ± 0.483 | 2.638 ± 0.258 |
| Tablets | 20 | 1.958 ± 0.348 | 16.222 ± 0.291 | 1.622 ± 0.794 | 19.802 ± 0.678 | 2.682 ± 0.592 |
| Tincture | 30 | 2.161 ± 0.287 | 22.701 ± 0127 | 3.866 ± 0.927 | 28.728 ± 0.826 | 3.890 ± 0.156 |
| Pharmaceutical Form | Antioxidant Activity, mg acid/g Extract | |||
|---|---|---|---|---|
| Voltammetry | Spectrophotometry | |||
| AA | GA | AA | GA | |
| Capsules | 6.125 ± 0.428 a | 0.952 ± 0.819 | 8.917 ± 0.482 | 3.009 ± 0.294 |
| Tablets | 6.354 ± 0.181 | 1.133 ± 0.483 | 9.457 ± 0.824 | 3.191 ± 0.326 |
| Tincture | 6.722 ± 0.537 | 1.245 ± 0.724 | 9.826 ± 0.624 | 3.457 ± 0.753 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banica, F.; Bungau, S.; Tit, D.M.; Behl, T.; Otrisal, P.; Nechifor, A.C.; Gitea, D.; Pavel, F.-M.; Nemeth, S. Determination of the Total Polyphenols Content and Antioxidant Activity of Echinacea Purpurea Extracts Using Newly Manufactured Glassy Carbon Electrodes Modified with Carbon Nanotubes. Processes 2020, 8, 833. https://doi.org/10.3390/pr8070833
Banica F, Bungau S, Tit DM, Behl T, Otrisal P, Nechifor AC, Gitea D, Pavel F-M, Nemeth S. Determination of the Total Polyphenols Content and Antioxidant Activity of Echinacea Purpurea Extracts Using Newly Manufactured Glassy Carbon Electrodes Modified with Carbon Nanotubes. Processes. 2020; 8(7):833. https://doi.org/10.3390/pr8070833
Chicago/Turabian StyleBanica, Florin, Simona Bungau, Delia Mirela Tit, Tapan Behl, Pavel Otrisal, Aurelia Cristina Nechifor, Daniela Gitea, Flavia-Maria Pavel, and Sebastian Nemeth. 2020. "Determination of the Total Polyphenols Content and Antioxidant Activity of Echinacea Purpurea Extracts Using Newly Manufactured Glassy Carbon Electrodes Modified with Carbon Nanotubes" Processes 8, no. 7: 833. https://doi.org/10.3390/pr8070833