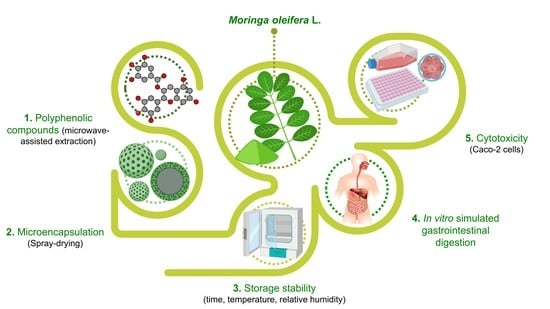
Moringa oleifera—Storage Stability, In Vitro-Simulated Digestion and Cytotoxicity Assessment of Microencapsulated Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Extraction of Polyphenolic Compounds
2.3. Microencapsulation Process
2.4. Stability During Storage
2.5. Release of Compounds during In Vitro Digestion
2.6. Activities Assessment
2.6.1. Preparation of Samples
2.6.2. Determination of Total Polyphenol Content (TPC)
2.6.3. DPPH radical Scavenging Activity
2.7. Cell Toxicity
2.7.1. Cell Culture
2.7.2. Cell Viability Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Influence of Storage Conditions on the Polyphenolic Content
3.2. Influence of Storage Conditions on Antioxidant Activity
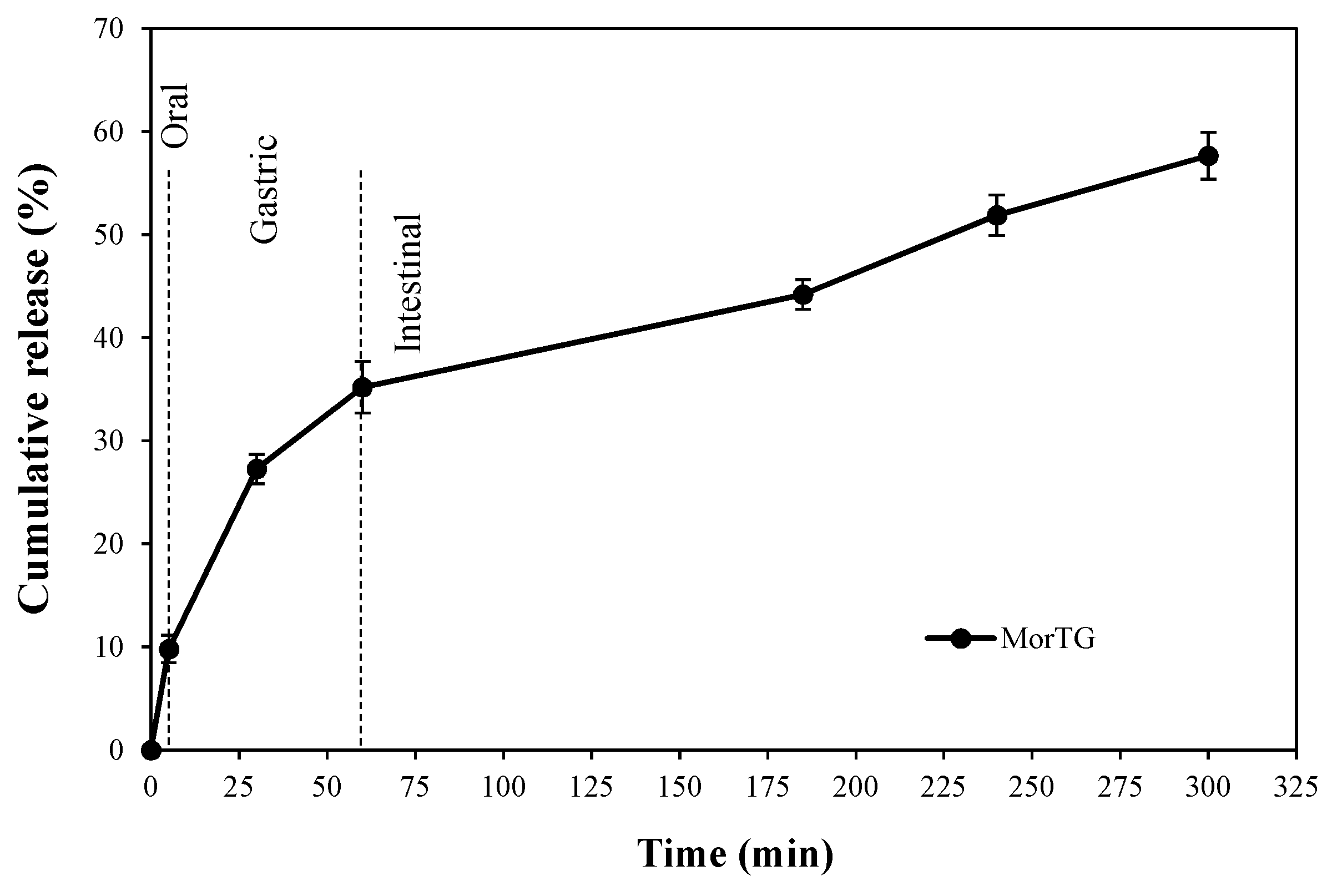
3.3. Release of Polyphenolic Compounds during In Vitro Digestion
3.4. Antioxidant Activity During in Vitro Digestion
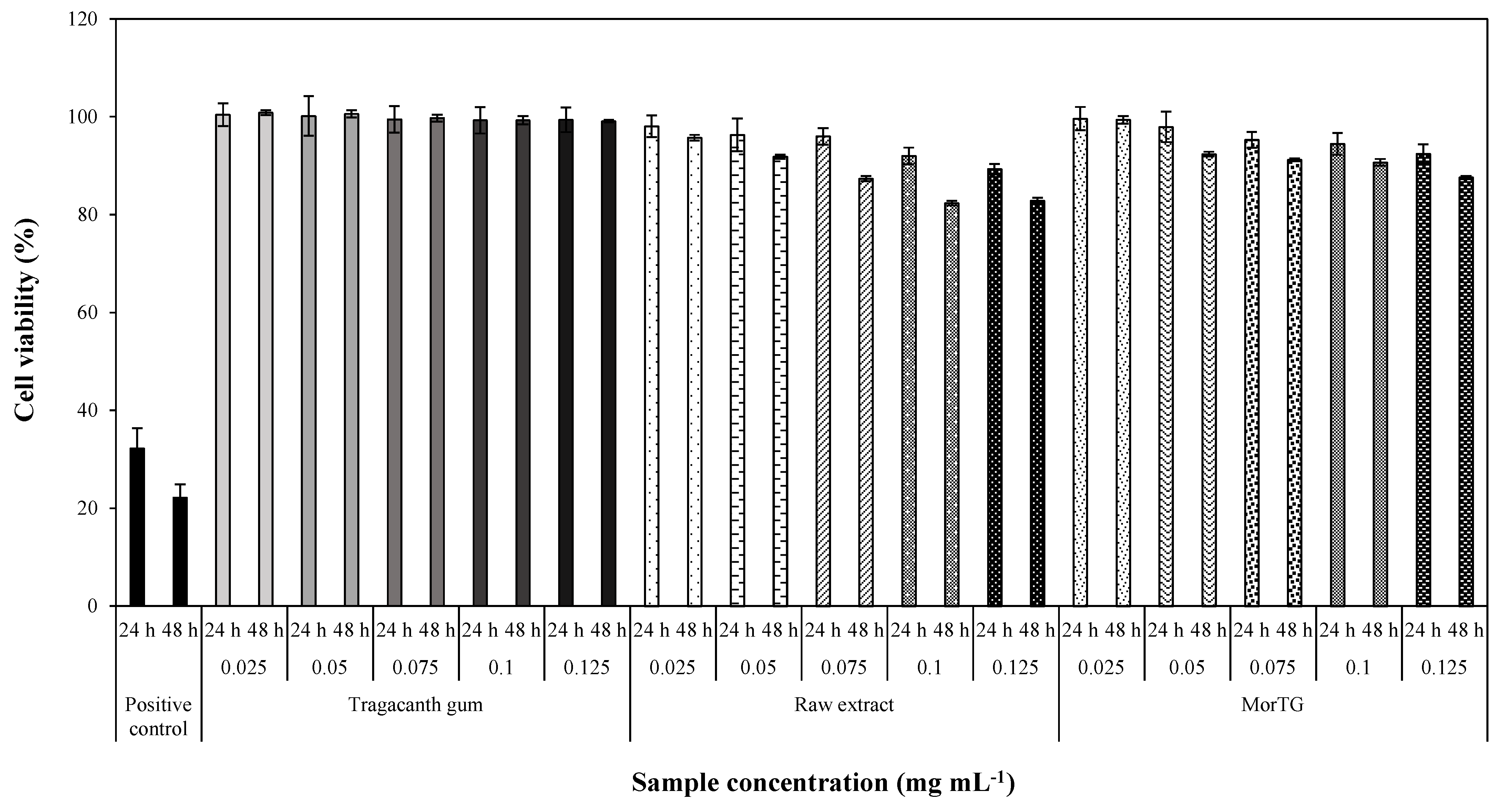
3.5. Effect in Caco-2 Cells Viability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onwulata, C.I. Encapsulation of new active ingredients. Annu. Rev. Food Sci. Technol. 2011, 3, 183–202. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Torres-Castillo, J.A.; Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Ngangyo-Heya, M.; Martínez-Ávila, G.C.G. Moringa plants: Bioactive compounds and promising applications in food products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Bholah, K.; Ramful-Baboolall, D.; Neergheen-Bhujun, V.S. Antioxidant activity of polyphenolic rich Moringa oleifera Lam. Extracts in food Systems. J. Food Biochem. 2015, 39, 733–741. [Google Scholar] [CrossRef]
- Nadeem, M.; Abdullah, M.; Hussain, I.; Inayat, S.; Javid, A.; Zahoor, Y. Antioxidant potential of Moringa oleifera leaf extract for the stabilisation of butter at refrigeration temperature. Czech J. Food Sci. 2013, 31, 332–339. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wadhwa, S.S.; Waterhouse, G.I.N. Spray-drying microencapsulation of polyphenol bioactives: A comparative study using different natural fibre polymers as encapsulants. Food Bioprocess Technol. 2013, 6, 2376–2388. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crop. Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols-A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds: A review. J. Pharm. 2011, 4, 793–829. [Google Scholar] [CrossRef]
- Laine, P.; Kylli, P.; Heinonen, M.; Jouppila, K. Storage stability of microencapsulated cloudberry (Rubus chamaemorus) phenolics. J. Agric. Food Chem. 2008, 56, 11251–11261. [Google Scholar] [CrossRef]
- Castro-López, C.; Ventura-Sobrevilla, J.M.; González-Hernández, M.D.; Rojas, R.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Martínez-Ávila, G.C.G. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem. 2017, 237, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Núñez-Ramírez, D.M.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation by spray-drying of laurel infusions (Litsea glaucescens) with maltodextrin. Ind. Crop. Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried acai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; Alvim, I.D.; de Aguirre, J.M. Storage stability of spray-dried blackberry powder produced with maltodextrin or gum arabic. Dry. Technol. 2013, 31, 470–478. [Google Scholar] [CrossRef]
- Ahmad, M.; Qureshi, S.; Maqsood, S.; Gani, A.; Masoodi, F.A. Micro-encapsulation of folic acid using horse chestnut starch and β-cyclodextrin: Microcapsule characterization, release behavior & antioxidant potential during GI tract conditions. Food Hydrocoll. 2017, 66, 154–160. [Google Scholar]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Emter, R.; Natsch, A. A fast Resazurin-based live viability assay is equivalent to the MTT-test in the keratino sens assay. Toxicol. In Vitro 2015, 29, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Díaz, D.I.; Beristain, C.I.; Azuara, E.; Luna, G.; Jimenez, M. Effect of wall material on the antioxidant activity and physicochemical properties of Rubus fruticosus juice microcapsules. J. Microencapsul. 2015, 32, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.A.; Jafari, S.M.; Assadpour, E.; Ghorbani, M. Storage stability of encapsulated barberry’s anthocyanin and its application in jelly formulation. J. Food Eng. 2016, 181, 59–66. [Google Scholar] [CrossRef]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Ambroziak, W.; Adamiec, J.; Czyżowska, A. Preservation of antioxidant activity and polyphenols in chokeberry juice and wine with the use of microencapsulation. J. Food Process. Preserv. 2016, 41, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Chen, Z.; Liu, W.; Chen, H. Characterization and storage properties of a new microencapsulation of tea polyphenols. Ind. Crop. Prod. 2016, 89, 152–156. [Google Scholar] [CrossRef]
- Zheng, L.; Ding, Z.; Zhang, M.; Sun, J. Microencapsulation of bayberry polyphenols by ethyl cellulose: Preparation and characterization. J. Food Eng. 2011, 104, 89–95. [Google Scholar] [CrossRef]
- Fracassetti, D.; Del Bo, C.; Simonetti, P.; Gardana, C.; Klimis-Zacas, D.; Ciappellano, S. Effect of time and storage temperature on anthocyanin decay and antioxidant activity in wild blueberry (Vaccinium angustifolium) powder. J. Agric. Food Chem. 2013, 61, 2999–3005. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.E. Effect of in vitro gastrointestinal digestion on encapsulated and no encapsulated phenolic compounds of Carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Pathumban, S.; Yoovidhya, T. Effect of entrapped α-tocopherol on mucoadhesivity and evaluation of the release, degradation, and swelling characteristics of zein-chitosan composite electrospun fibers. J. Food Eng. 2014, 120, 110–117. [Google Scholar] [CrossRef]
- Asghari-Varzaneh, E.; Shahedi, M.; Shekarchizadeh, H. Iron microencapsulation in gum tragacanth using solvent evaporation method. Int. J. Biol. Macromol. 2017, 103, 640–647. [Google Scholar] [CrossRef]
- Hostler, A.C. Hydrocolloids: Practical guides for the food industry. In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Stability Testing of New Drug Substances and Products Q 1A (R2), Geneva, Switzerland; Eagan Press Handbook Series; Eagan Press: St. Paul, MI, USA, 2004. [Google Scholar]
- Sansone, F.; Picerno, P.; Mencherini, T.; Russo, P.; Gasparri, F.; Giannini, V.; Lauro, M.R.; Puglisi, G.; Aquino, R.P. Enhanced technological and permeation properties of a microencapsulated soy isoflavones extract. J. Food Eng. 2013, 115, 298–305. [Google Scholar] [CrossRef]
- Allison, S.D. Effect of structural relaxation on the preparation and drug release behavior of poly (lactic-co-glycolic) acid microparticle drug delivery systems. J. Pharm. Sci. 2008, 97, 2022–2035. [Google Scholar] [CrossRef]
- Nur, M.; Vasiljevic, T. Insulin inclusion into a tragacanth hydrogel: An oral delivery system for insulin. Materials 2018, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Pathania, D.; Anjum, S.; Gupta, B. Smart designing of tragacanth gum by graft functionalization for advanced materials. Macromol. Mater. Eng. 2020, 1900762. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Phillips, D.R.; Kong, F. In vitro release properties of encapsulated blueberry (Vaccinium ashei) extracts. Food Chem. 2015, 168, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ashraf, B.; Gani, A.; Gani, A. Microencapsulation of saffron anthocyanins using β-glucan and β-cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in vitro digestion. Int. J. Biol. Macromol. 2018, 109, 435–442. [Google Scholar] [PubMed]
- You, L.J.; Zhao, M.M.; Regenstein, J.M.; Ren, J.Y. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010, 120, 810–816. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- ISO (International Organization for Standardization). ISO 10993–5:2009 Standard “Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity”. Available online: https://www.iso.org/standard/36406.html (accessed on 15 June 2020).
- Courtney, R.; Sirdaarta, J.; White, A.; Cock, I.E. Inhibition of Caco-2 and HeLa proliferation by Terminalia carpentariae C. T. white and Terminalia grandiflora benth. extracts: Identification of triterpenoid components. Pharmacogn. J. 2017, 9, 441–451. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Santana-Méridas, O.; Polissiou, M.; Vioque, J.; Astraka, K.; Alaiz, M.; Herraiz-Peñalver, D.; Tarantilis, P.A.; Girón-Calle, J. Polyphenol composition and in vitro antiproliferative effect of corm, tepal and leaf from Crocus sativus L. on human colon adenocarcinoma cells (Caco-2). J. Funct. Foods 2016, 24, 18–25. [Google Scholar] [CrossRef]
- Szewczyk, K.; Lewandowska, U.; Owczarek, K.; Sosnowska, D.; Gorlach, S.; Koziołkiewicz, M.; Hrabec, Z.; Hrabec, E. Influence of polyphenol extract from evening primrose (Oenothera paradoxa) seeds on proliferation of Caco-2 cells and on expression, synthesis and activity of matrix metalloproteinases and their inhibitors. Pol. J. Food Nutr. Sci. 2014, 64, 181–191. [Google Scholar] [CrossRef]
- Baeza, G.; Amigo-Benavent, M.; Sarriá, B.; Goya, L.; Mateos, R.; Bravo, L. Green coffee hydroxycinnamic acids but not caffeine protects human HepG2 cells against oxidative stress. Food Res. Int. 2014, 62, 1038–1046. [Google Scholar] [CrossRef]
- Wang, S.; Mateos, R.; Goya, L.; Amigo-Benavent, M.; Sarriá, B.; Bravo, L. A phenolic extract from grape by-products and its main hydroxybenzoic acids protect Caco-2 cells against pro-oxidant induced toxicity. Food Chem. Toxicol. 2016, 88, 65–74. [Google Scholar] [CrossRef] [PubMed]
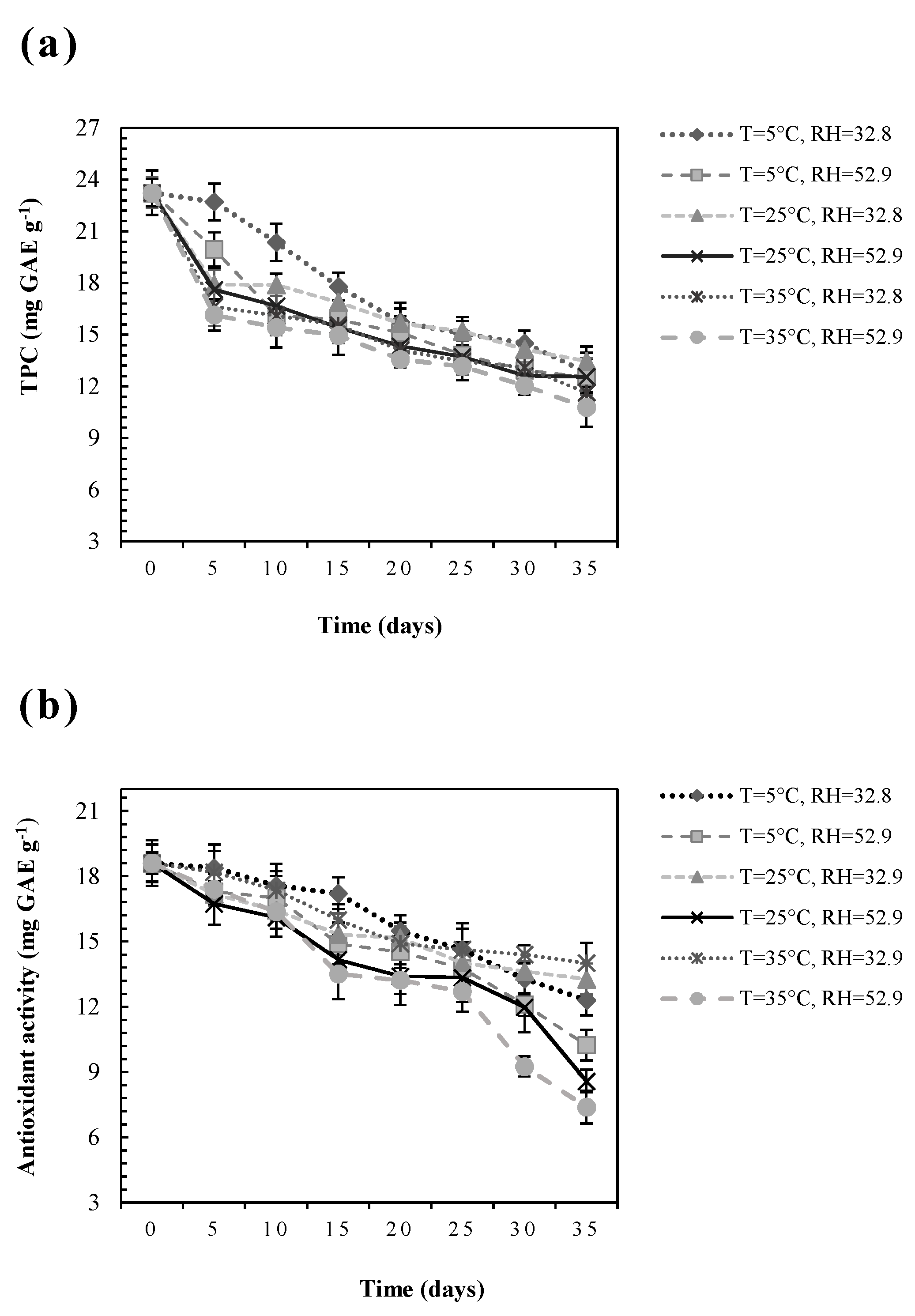
| Storage Conditions | k (days−1) | t1/2 (days) | R2 | Q10 | |
|---|---|---|---|---|---|
| Temperature (°C) | Water Activity (aw) | ||||
| 5 | 0.328 | 0.0170 | 40.75 | 0.989 | |
| 0.529 | 0.0175 | 39.42 | 0.971 | ||
| 25 | 0.328 | 0.0155 | 44.47 | 0.978 | 1.26 |
| 0.529 | 0.0175 | 39.42 | 0.986 | ||
| 35 | 0.328 | 0.0197 | 35.04 | 0.961 | 1.24 |
| 0.529 | 0.0218 | 31.65 | 0.983 | ||
| Digestion Phase | Duration of Digestion | DPPH 1 |
|---|---|---|
| MorTG | ||
| Oral | 5 min | 5.23 (±0.36) |
| Gastric | 30 min | 8.54 (±0.51) |
| 60 min | 11.07 (±0.46) | |
| Intestinal | 2 h | 13.19 (±0.73) |
| 3 h | 15.20 (±0.51) | |
| 4 h | 16.76 (±0.15) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-López, C.; Gonçalves, C.; Ventura-Sobrevilla, J.M.; Pastrana, L.M.; Aguilar-González, C.N.; Martínez-Ávila, G.C.G. Moringa oleifera—Storage Stability, In Vitro-Simulated Digestion and Cytotoxicity Assessment of Microencapsulated Extract. Processes 2020, 8, 770. https://doi.org/10.3390/pr8070770
Castro-López C, Gonçalves C, Ventura-Sobrevilla JM, Pastrana LM, Aguilar-González CN, Martínez-Ávila GCG. Moringa oleifera—Storage Stability, In Vitro-Simulated Digestion and Cytotoxicity Assessment of Microencapsulated Extract. Processes. 2020; 8(7):770. https://doi.org/10.3390/pr8070770
Chicago/Turabian StyleCastro-López, Cecilia, Catarina Gonçalves, Janeth M. Ventura-Sobrevilla, Lorenzo M. Pastrana, Cristóbal N. Aguilar-González, and Guillermo C. G. Martínez-Ávila. 2020. "Moringa oleifera—Storage Stability, In Vitro-Simulated Digestion and Cytotoxicity Assessment of Microencapsulated Extract" Processes 8, no. 7: 770. https://doi.org/10.3390/pr8070770
APA StyleCastro-López, C., Gonçalves, C., Ventura-Sobrevilla, J. M., Pastrana, L. M., Aguilar-González, C. N., & Martínez-Ávila, G. C. G. (2020). Moringa oleifera—Storage Stability, In Vitro-Simulated Digestion and Cytotoxicity Assessment of Microencapsulated Extract. Processes, 8(7), 770. https://doi.org/10.3390/pr8070770