Comparison of Phytochemicals, Antioxidant and Anti-Inflammatory Properties of Sun-, Oven- and Freeze-Dried Ginger Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Drying Treatment and Sample Processing
2.3. Extraction Procedures
2.4. Determination of Phenolic Content
2.5. Determination of Flavonoid Content
2.6. Assessment of Antioxidant Activity
2.6.1. Ferric-Reducing Antioxidant Power (FRAP)
2.6.2. Radical Scavenging Activity on ABTS Radical Cation
2.6.3. Radical Scavenging Activity on DPPH Radical
2.7. Assessment of Anti-inflammatory Activity
2.7.1. Cell Culture Experiment
2.7.2. Determination of Secreted NO Amounts
2.7.3. Inhibitory Effect on Lipopolysaccharide (LPS)-Induced Nitric Oxide (NO) Production
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phenolic and Flavonoid Contents of Ginger Extracts
3.2. Antioxidant Activity of Ginger Extracts
3.3. Anti-Inflammatory Activity of Ginger Extracts
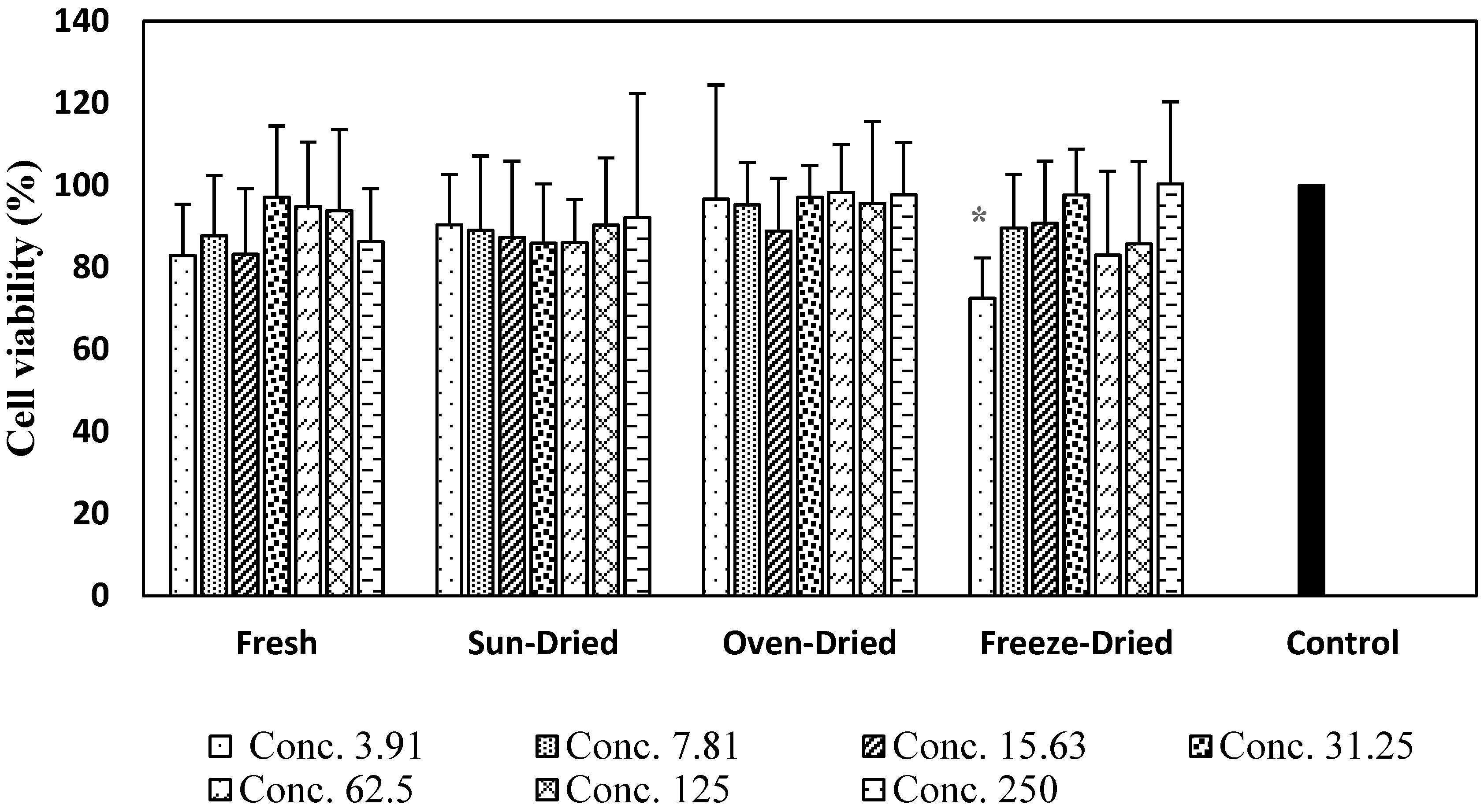
3.3.1. Cell Viability
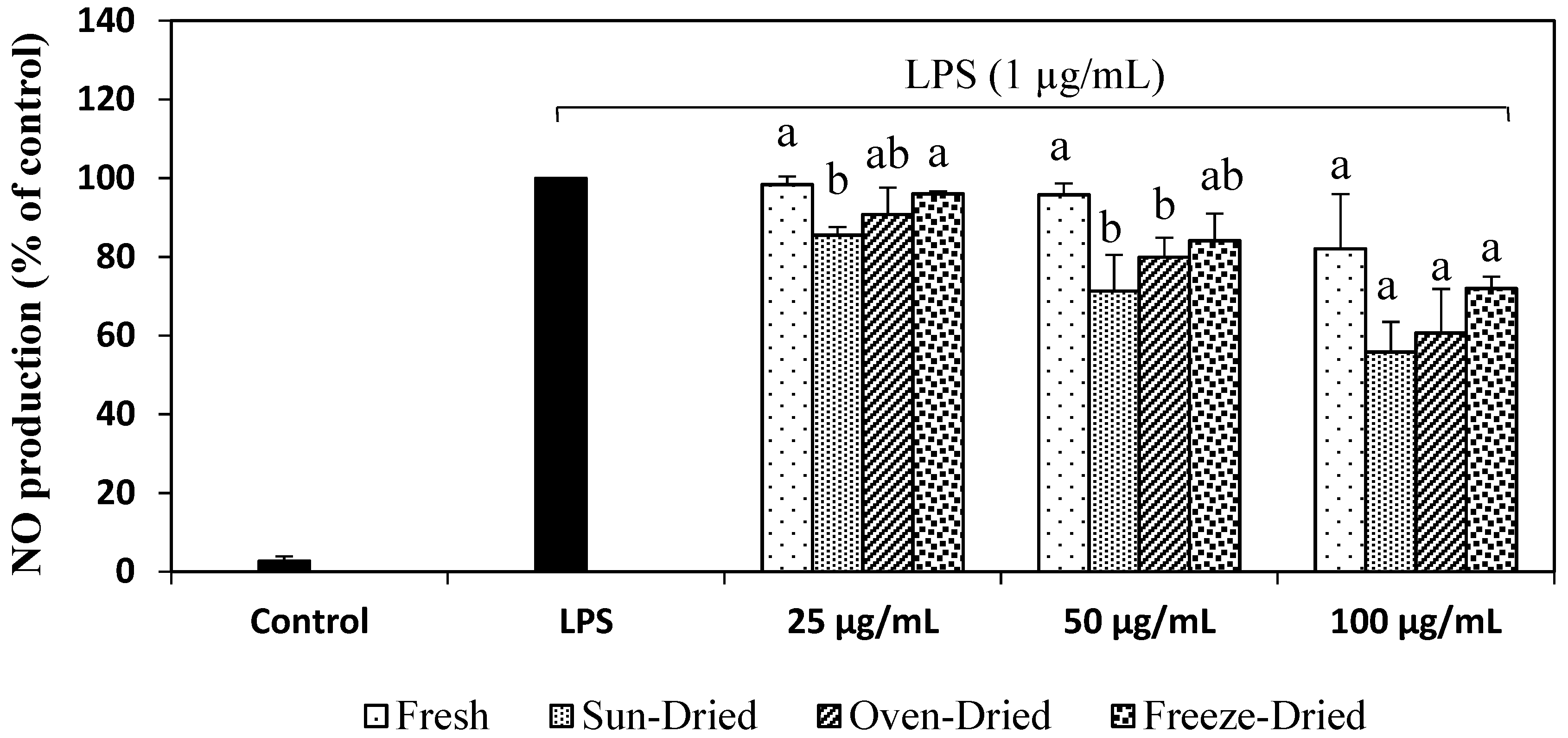
3.3.2. NO Production in LPS-Stimulated Cells
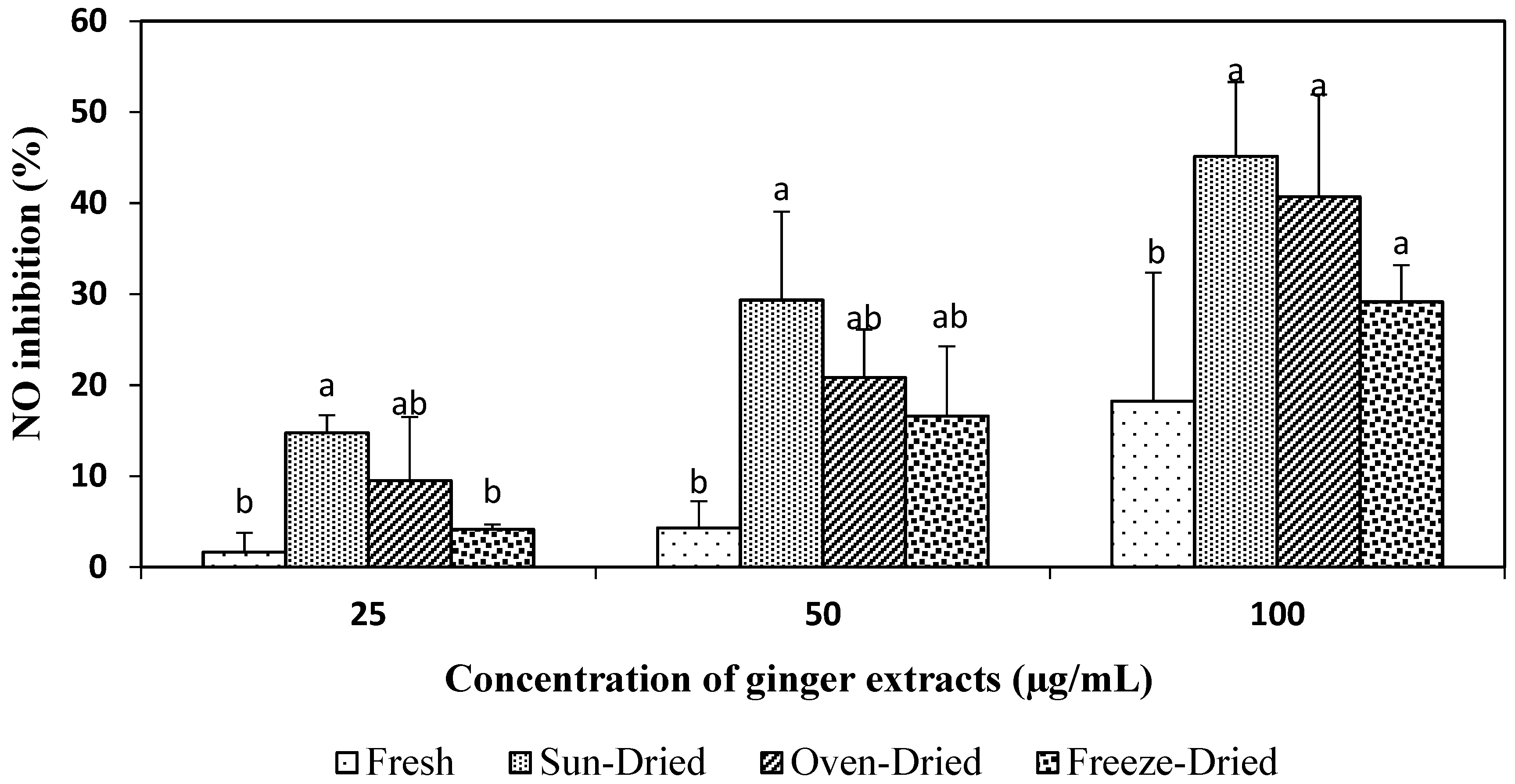
3.3.3. NO-Inhibitory Activity of Ginger Extracts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-C.; Su, M.-S. Optimized heat treatment enhances the anti-inflammatory capacity of ginger. Int. J. Food Prop. 2016, 19, 1884–1898. [Google Scholar] [CrossRef]
- Shetty, K.; McCue, P. Phenolic antioxidant biosynthesis in plants for functional food application: Integration of systems biology and biotechnological approaches. Food Biotechnol. 2003, 17, 67–97. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.; Chuang, C.; Chen, H.; Wan, C.; Chen, T.; Lin, L. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT Food Sci. Technol. 2013, 55, 329–334. [Google Scholar] [CrossRef]
- Lu, C.-C.; Yen, G.-C. Antioxidative and anti-inflammatory activity of functional foods. Curr. Opin. Food Sci. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Tsai, P.-J.; Ho, S.-C. Antioxidant and anti-inflammatory activities of several commonly used compounds. Food Chem. Toxicol. 2005, 70, 93–97. [Google Scholar]
- USDA (United States Department of Agriculture) Nutrient Database. National Nutrient Database for Standard Reference Release 28. 2016. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 20 August 2016).
- Ding, S.H.; An, K.J.; Zhao, C.P.; Li, Y.; Guo, Y.H.; Wang, Z.F. Effect of drying methods on volatiles of Chinese ginger (Zingiber officinale Roscoe). Food Bioprod. Process 2012, 90, 515–524. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Arya, S.S. Effect of drying and storage on bioactive components of jambhul and wood apple. J. Food Sci. Technol. 2014, 52, 2833–2841. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C. Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chem. 2003, 83, 85–92. [Google Scholar] [CrossRef]
- Nolle, N.; Argyropoulos, D.; Ambacher, S.; Muller, J.; Biesalski, H.K. Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. LWT Food Sci. Technol. 2016, 85, 400–404. [Google Scholar] [CrossRef]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; O’Brien, N.; Krumbein, A. UV-B-induced secondary plant metabolites—Potential benefits for plant and human health. Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Eze, J.; Agbo, K. Comparative studies of sun and solar drying of peeled and unpeeled ginger. Am. J. Sci. Ind. Res. 2011, 2, 136–143. [Google Scholar] [CrossRef]
- Jayashree, E.; Visvanathan, R.; John Zachariah, T. Quality of dry ginger (Zingiber officinale) by different drying methods. J. Food Sci. Technol. 2012, 51, 3190–3198. [Google Scholar]
- FAO (Food and Agriculture Organization) Price Statistics. 2015. Available online: https://knoema.com/search?query=price%20ginger-malaysia (accessed on 10 February 2017).
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 2010, 123, 85–91. [Google Scholar] [CrossRef]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A.H. Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Re, R.; Pellergrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Siddhuraju, P.; Manian, S. Antioxidant and free radical scavenging capacity of the underutilized legume, Vigna vexillata (L.) A. Rich. J. Food Compos. Anal. 2011, 24, 160–165. [Google Scholar] [CrossRef]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamon, E.; Villares, A.; Martinez, J.A.; Garcia-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations-A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Gordon, M.F. The mechanism of antioxidant action in vitro. In Food Antioxidants; Hudson, B.J.F., Ed.; Elsevier Applied Science: London, UK, 1990; pp. 1–18. [Google Scholar]
- Barros, L.; Ferreira, M.J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Ling, A.L.M.; Yasir, S.; Matanjun, P.; Abu Bakar, M.F. Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii. J. Appl. Phycol. 2014, 27, 1717–1723. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar] [PubMed]
- Tanaka, J. Anti-inflammatory properties of red ginger (Zingiber officinale var. Rubra) extract and suppression of nitric oxide production by its constituents. J. Med. Food 2010, 13, 156–162. [Google Scholar]
- Li, F.; Wang, Y.; Parkin, K.L.; Nitteranon, V.; Liang, J.; Yang, W.; Li, Y.; Zhang, G.; Hu, Q. Isolation of quinone reductase (QR) inducing agents from ginger rhizome and their in vitro anti-inflammatory activity. Food Res. Int. 2011, 44, 1597–1603. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Hunaefi, D.; Akumo, D.N.; Smetanska, I. Effect of fermentation on antioxidant properties of red cabbages. Food Biotechnol. 2013, 27, 66–85. [Google Scholar] [CrossRef]
| Drying | Extract Yield (%) | Phenolic Content (mg GAE/g Dry Extract) | Flavonoid Content (mg RE/g Dry Extract) |
|---|---|---|---|
| Fresh | 3.40 ± 0.80 b | 10.53 ± 0.21 d | 96.5 ± 4.08 c |
| Sun-dried | 9.87 ± 1.31 a | 18.94 ± 0.29 b | 584.8 ± 48.64 a |
| Oven-dried | 8.21 ± 2.79 a | 16.08 ± 0.52 c | 508.2 ± 38.80 ab |
| Freeze-dried | 9.50 ± 1.66 a | 20.07 ± 0.52 a | 473.2 ± 24.94 b |
| Drying | FRAP (mmol Fe (II)/g Dry Extract) | ABTS•+ (mmol TEAC/g Dry Extract) | DPPH IC50 µg/mL |
|---|---|---|---|
| Fresh | 1021 ± 29.7 d | 829 ± 44.0 c | 65.82 ± 6.83 a |
| Sun-dried | 4033 ± 29.9 a | 1712 ± 1.7 a | 14.69 ± 2.34 c |
| Oven-dried | 3584 ± 61.1 b | 1428 ± 51.8 b | 27.97 ± 1.92 b |
| Freeze-dried | 3219 ± 72.5 c | 1336 ± 72.8 b | 28.59 ± 0.83 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, I.; Chin, N.L.; Fakurazi, S.; Palanisamy, A. Comparison of Phytochemicals, Antioxidant and Anti-Inflammatory Properties of Sun-, Oven- and Freeze-Dried Ginger Extracts. Foods 2019, 8, 456. https://doi.org/10.3390/foods8100456
Mustafa I, Chin NL, Fakurazi S, Palanisamy A. Comparison of Phytochemicals, Antioxidant and Anti-Inflammatory Properties of Sun-, Oven- and Freeze-Dried Ginger Extracts. Foods. 2019; 8(10):456. https://doi.org/10.3390/foods8100456
Chicago/Turabian StyleMustafa, Iswaibah, Nyuk Ling Chin, Sharida Fakurazi, and Arulselvan Palanisamy. 2019. "Comparison of Phytochemicals, Antioxidant and Anti-Inflammatory Properties of Sun-, Oven- and Freeze-Dried Ginger Extracts" Foods 8, no. 10: 456. https://doi.org/10.3390/foods8100456





