Effect of Treatment with Peptide Extract from Beef Myofibrillar Protein on Oxidative Stress in the Brains of Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Peptide Extract
2.3. Animals
2.4. Determination of Antioxidant Enzyme Activities
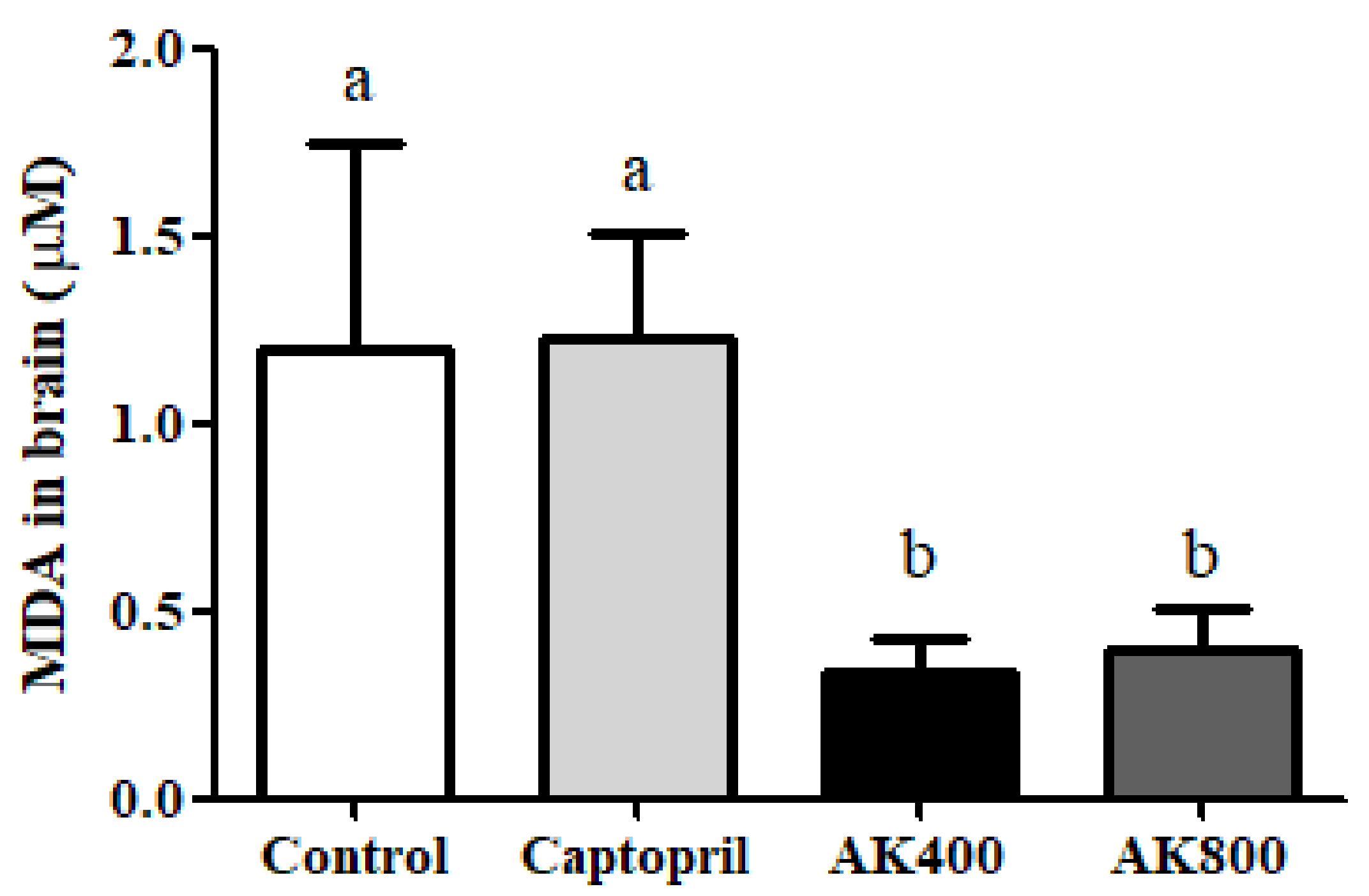
2.4.1. Analysis of Lipid Oxidation Using the Thiobarbituric Acid-Reactive Substance (TBARS) Assay
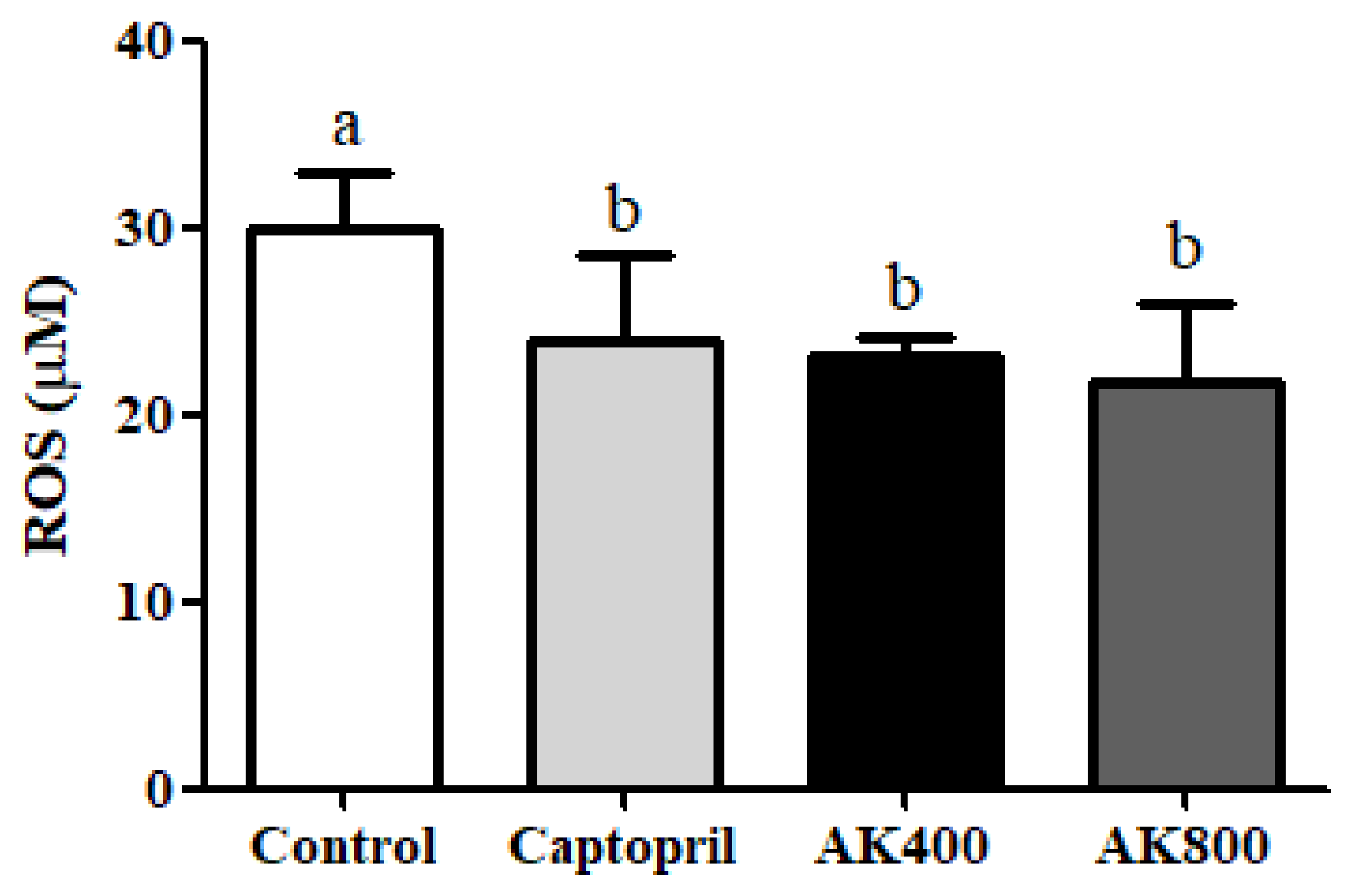
2.4.2. Analysis of ROS Generation
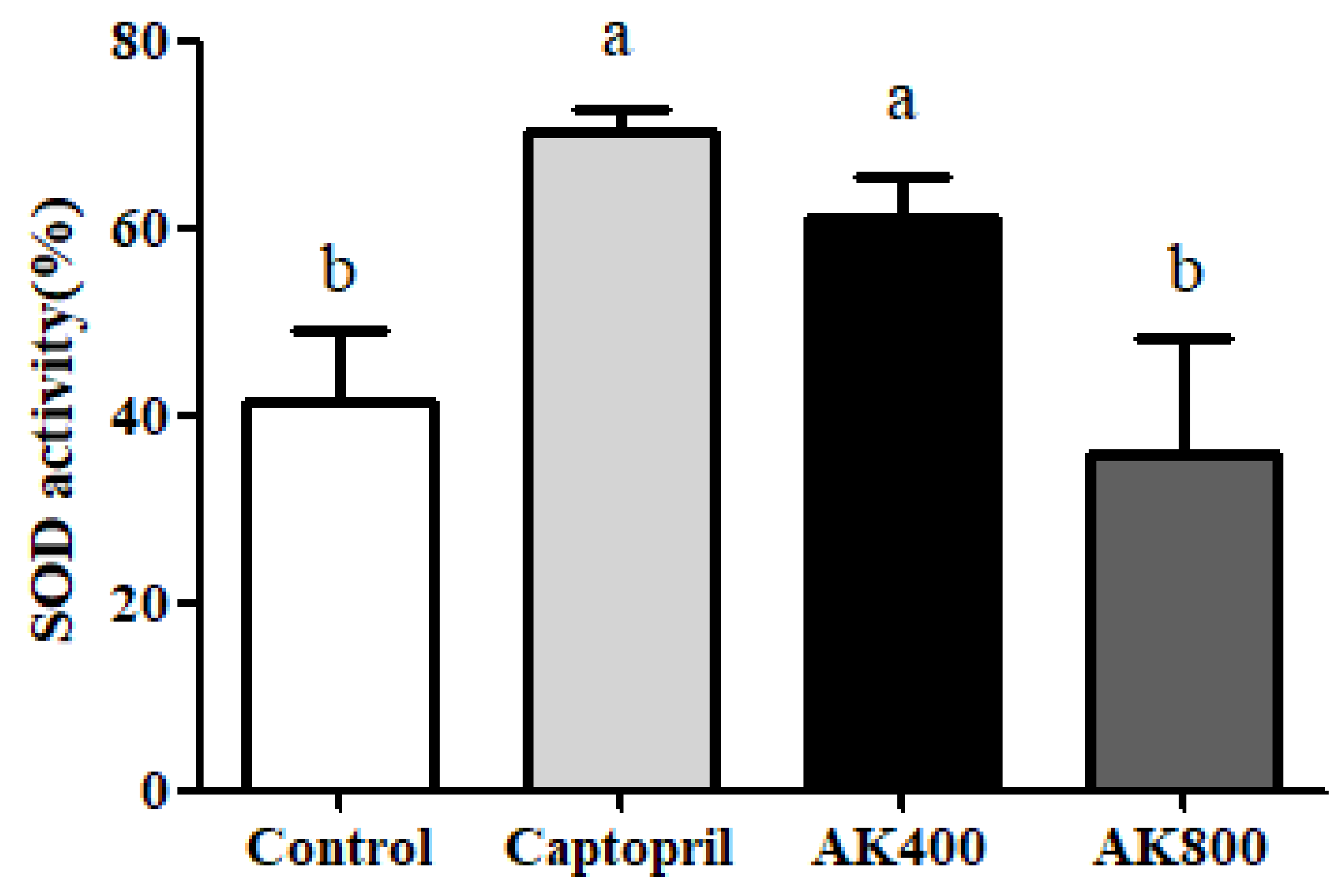
2.4.3. Analysis of SOD Activity
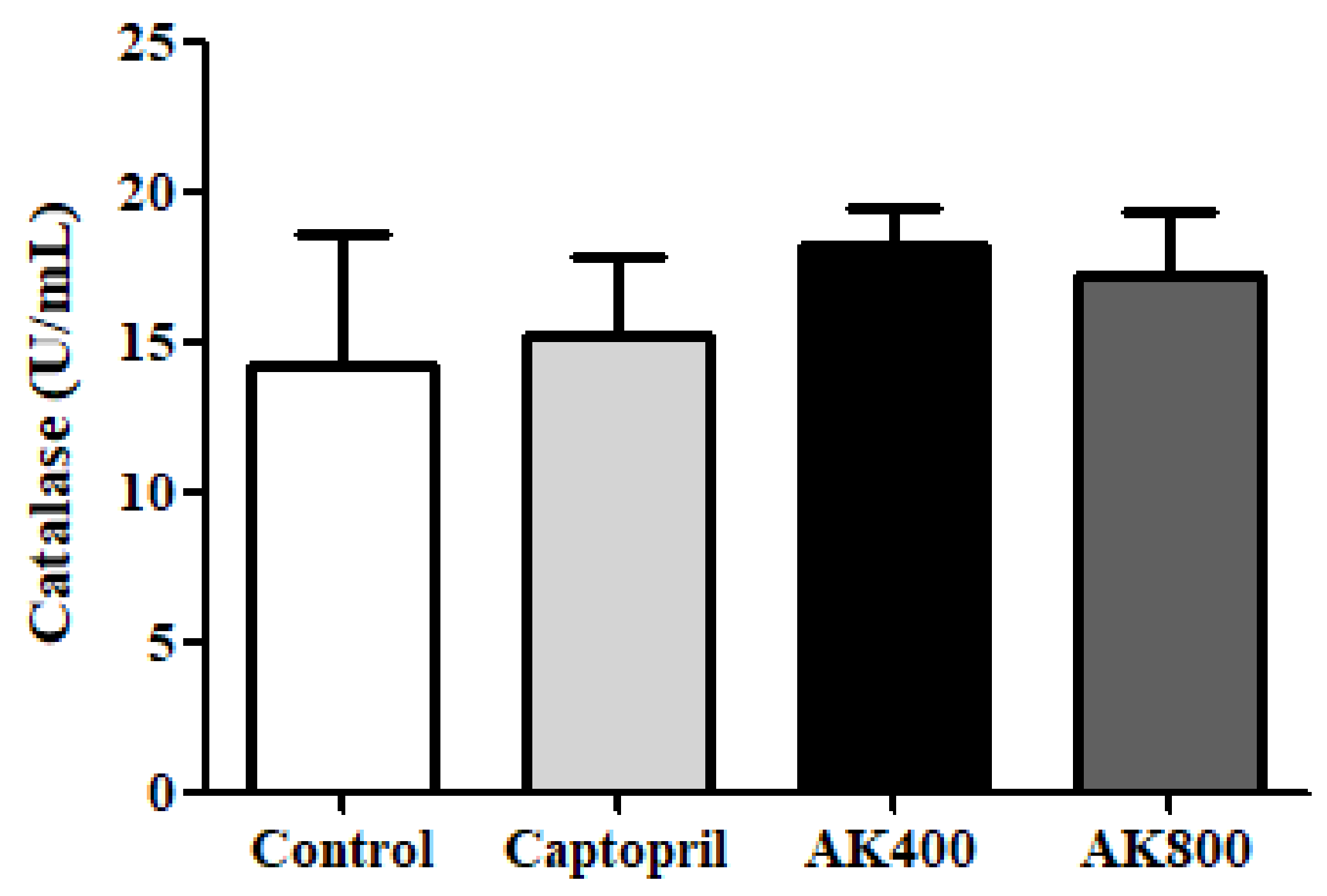
2.4.4. Analysis of Catalase Activity
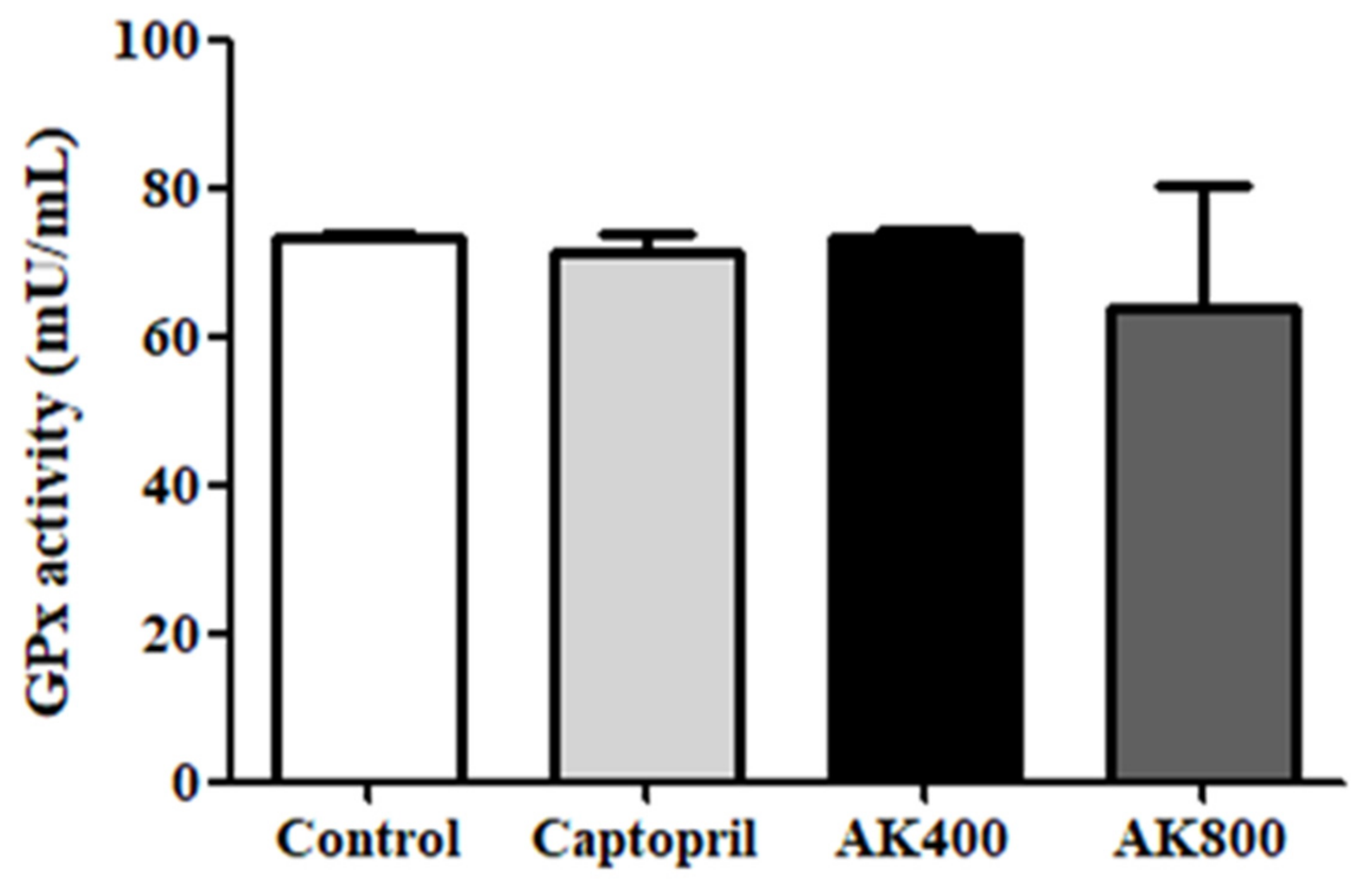
2.4.5. Analysis of GPx Activity
2.5. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grossman, E. Does increased oxidative stress cause hypertension? Diabetes Care 2008, 31, S185–S189. [Google Scholar] [CrossRef]
- Harrison, D.G.; Gongora, M.C. Oxidative stress and hypertension. Med. Clin. N. Am. 2009, 93, 621–635. [Google Scholar] [CrossRef]
- Brown, N.J.; Vaughan, D.E. Angiotensin-converting enzyme inhibitors. Circulation 1998, 97, 1411–1420. [Google Scholar] [CrossRef]
- Mikrut, K.; Kupsz, J.; Koźlik, J.; Krauss, H.; Pruszyńska-Oszmałek, E.; Gibas-Dorna, M. Angiotensin-converting enzyme inhibitors reduce oxidative stress intensity in hyperglicemic conditions in rats independently from bradykinin receptor inhibitors. Croat. Med. J. 2016, 57, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Fiordaliso, F.; Cuccovillo, I.; Bianchi, R.; Bai, A.; Doni, M.; Salio, M.; De Angelis, N.; Ghezzi, P.; Latini, R.; Masson, S. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 2006, 79, 121–129. [Google Scholar] [CrossRef]
- Reza, H.M.; Tabassum, N.; Sagor, M.A.T.; Chowdhury, M.R.H.; Rahman, M.; Jain, P.; Alam, M.A. Angiotensin-converting enzyme inhibitor prevents oxidative stress, inflammation, and fibrosis in carbon tetrachloride-treated rat liver. Toxicol. Mech. Methods 2016, 26, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Touyz, R.M. Oxidative stress and hypertension: Current concepts. Curr. Hypertens. Rep. 2010, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Hwang, J.E.; Eum, S.J.; Paik, H.D. Physiochemical analysis, antioxidant effects, and sensory characteristics of quark cheese supplemented with ginseng Extract. Food Sci. Anim. Resour. 2019, 39, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Loperena, R.; Harrison, D.G. Oxidative stress and hypertensive diseases. Med. Clin. North Am. 2017, 101, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Satonaka, H. Hypertension and oxidative stress. Jpn. Med. Assoc. J. 2001, 44, 540–545. [Google Scholar]
- Nakazono, K.; Watanabe, N.; Matsuno, K.; Sasaki, J.; Sato, T.; Inoue, M. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl. Acad. Sci. USA 1991, 88, 10045–10048. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Touyz, R.M.; Park, J.B.; Schiffrin, E.L. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension 2001, 38, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.J.; Gokce, N.; Holbrook, M.; Hunter, L.M.; Biegelsen, E.S.; Huang, A.; John, F.; Keaney, J.; Vita, J.A. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H528–H534. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.R.; Zanstra, Y. Is the brain the essential in hypertension? NeuroImage 2009, 47, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A. Hypertension and the Brain: A Risk Factor for More than Heart Disease. Cerebrovasc. Dis. 2016, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- He, D.H.; Zhang, L.M.; Lin, L.M.; Ning, R.B.; Wang, H.J.; Xu, C.S.; Lin, J.X. Long-term prehypertension treatment with losartan effectively prevents brain damage and stroke in stroke-prone spontaneously hypertensive rats. Int. J. Mol. Med. 2014, 33, 301–309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Hwang, D.; Lee, E.S.; Hyun, J.W.; Yi, P.H.; Kim, G.S.; Lee, S.E.; Pang, C.; Park, Y.J.; Chung, K.H.; et al. Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu. J. Ethnopharmacol. 2015, 163, 106–112. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Recio, I. Protective effect of milk peptides: Antibacterial and antitumor properties. In Bioactive Components of Milk; Bösze, Z., Ed.; Springer: New York, NY, USA, 2008; pp. 271–294. [Google Scholar]
- Yoon, J.W.; Ahn, S.I.; Jhoo, J.W.; Kim, G.Y. Antioxidant activity of yogurt fermented at low temperature and its anti-inflammatory effect on DSS-induced colitis in mice. Food Sci. Anim. Resour. 2019, 39, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Sakurai, T.; Ochi, D.; Mitsuyama, E.; Yamauchi, K.; Abe, F. Novel angiotensin I-converting enzyme inhibitory peptide derived from bovine casein. Food Chem. 2013, 141, 3781–3789. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Aluko, R.E.; Rai, D.K.; O’Connor, P.; Hayes, M. Identification of bioactive peptides from a papain hydrolysate of bovine serum albumin and assessment of an antihypertensive effect in spontaneously hypertensive rats. Food Res. Int. 2016, 81, 91–99. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Li, S.; Zhou, J.; Xu, J. Physico-chemical properties, antioxidant activities and antihypertensive effects of walnut protein and its hydrolysate. J. Sci. Food Agric. 2016, 96, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Neuroprotective effects of different molecular weight peptide fractions obtained from beef by hydrolysis with commercial enzymes in SH-SY5Y cells. Food Res. Int. 2019, 121, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Purification of novel angiotensin converting enzyme inhibitory peptides from beef myofibrillar proteins and analysis of their effect in spontaneously hypertensive rat model. Biomed. Pharmacother. 2019, 116, 109046. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Angiotensin converting enzyme inhibitory and antioxidant activities of enzymatic hydrolysates of korean native cattle (Hanwoo) myofibrillar protein. BioMed Res. Int. 2017, 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Michihara, A.; Shimatani, M.; Anraku, M.; Tomida, H.; Akasaki, K. High levels of oxidative stress exist in the brain than serum or kidneys in stroke-prone spontaneously hypertensive rats at ten weeks of age. Biol. Pharm. Bull. 2010, 33, 518–521. [Google Scholar] [CrossRef]
- Lee, M.C.; Shoji, H.; Miyazaki, H.; Yoshino, F.; Hori, N.; Toyoda, M.; Ikeda, Y.; Anzai, K.; Ikota, N.; Ozawa, T. Assessment of oxidative stress in the spontaneously hypertensive rat brain using electron spin resonance (ESR) imaging and in Vivo L-band ESR. Hypertens. Res. 2004, 27, 485–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.J.; Sujiwo, J.; Kim, H.J.; Jang, A. Effects of dipping chicken breast meat inoculated with Listeria monocytogenes in lyophilized scallion, garlic, and kiwi extracts on its physicochemical quality. Food Sci. Anim. Resour. 2019, 39, 418–429. [Google Scholar] [CrossRef]
- Kishi, T.; Hirooka, Y. Oxidative stress in the brain causes hypertension via sympathoexcitation. Front. Physiol. 2012, 3, 335. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Ni, Z.; Oveisi, F.; Trnavsky-Hobbs, D.L. Effect of antioxidant therapy on blood pressure and NO synthase expression in hypertensive rats. Hypertension 2000, 36, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Inagami, T. The renin-angiotensin system. Essays Biochem. 1994, 28, 147–164. [Google Scholar] [PubMed]
- Wojakowski, W.; Gminski, J.; Siemianowicz, K.; Goss, M.; Machalski, M. The influence of angiotensin converting enzyme inhibitors on lipid peroxidation in sera and aorta of rabbits in diet-induced hypercholesterolemia. Int. J. Mol. Med. 2000, 6, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Lopes De Faria, J.B.; Biswas, S.K.; Lopes De Faria, J.B. Which comes first: Renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic. Res. 2007, 41, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Nakamura, H.; Kwon, Y.W.; Teratani, A.; Masutani, H.; Shioji, K.; Kishimoto, C.; Ohira, A.; Horie, R.; Yodoi, J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid. Redox Signal. 2004, 6, 89–97. [Google Scholar] [CrossRef]
- Kimoto-Kinoshita, S.; Nishida, S.; Tomura, T.T. Age-related change of antioxidant capacities in the cerebral cortex and hippocampus of stroke-prone spontaneously hypertensive rats. Neurosci. Lett. 1999, 273, 41–44. [Google Scholar] [CrossRef]
- Kishi, T.; Hirooka, Y.; Kimura, Y.; Ito, K.; Shimokawa, H.; Takeshita, A. I Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 2004, 109, 2357–2362. [Google Scholar] [CrossRef]
- Pasini, A.F.; Stranieri, C.; Nava, M.C.; Garbin, U.; Boccioletti, V.; Lo Cascio, V.; Cominacini, L.; Luchetta, M.L.; Pellegrini, M.; Fabrizzi, P. Effect of sulfhydryl and non-sulfhydryl angiotensin-converting enzyme inhibitors on endothelial function in essential hypertensive patients. Am. J. Hypertens. 2007, 20, 443–450. [Google Scholar] [CrossRef][Green Version]
- Aruoma, O.I.; Akanmu, D.; Cecchini, R.; Halliwell, B. Evaluation of the ability of the angiotensin-converting enzyme inhibitor captopril to scavenge reactive oxygen species. Chem. Biol. Interact. 1991, 77, 303–314. [Google Scholar] [CrossRef]
- Lapenna, D.; De Gioia, S.; Ciofani, G.; Daniele, F.; Cuccurullo, F. Captopril has no significant scavenging antioxidant activity in human plasma in vitro or in vivo. Br. J. Clin. Pharmacol. 1996, 42, 451–456. [Google Scholar] [CrossRef]
- Haenen, G.R.M.M.; Vermeulen, N.P.E.; Timmerman, H.; Bast, A. Effect of thiols on lipid peroxidation in rat liver microsomes. Chem. Biol. Interact. 1989, 71, 201–212. [Google Scholar] [CrossRef]
- Montezano, A.C.; Touyz, R.M. Reactive oxygen species, vascular Noxs, and hypertension: Focus on translational and clinical research. Antioxid. Redox Signal. 2014, 20, 164–182. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef] [PubMed]
- Hanna, I.R.; Taniyama, Y.; Szöcs, K.; Rocic, P.; Griendling, K.K. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid. Redox Signal. 2002, 4, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.C.; Davisson, R.L. Redox signaling in central neural regulation of cardiovascular function. Prog. Biophys. Mol. Biol. 2004, 84, 125–149. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Chan, J.Y. Brain stem NOS and ROS in neural mechanisms of hypertension. Antioxid. Redox Signal. 2013, 20, 146–163. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358. [Google Scholar]
- Zhong, M.K.; Gao, J.; Zhang, F.; Xu, B.; Fan, Z.D.; Wang, W.; Zhu, G.Q. Reactive oxygen species in rostral ventrolateral medulla modulate cardiac sympathetic afferent reflex in rats. Acta Physiol. 2009, 197, 297–304. [Google Scholar] [CrossRef]
- Tanriverdi, L.H.; Parlakpinar, H.; Ozhan, O.; Ermis, N.; Polat, A.; Vardi, N.; Tanbek, K.; Yildiz, A.; Acet, A. Inhibition of NADPH oxidase by apocynin promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress in rats. Free Radic. Res. 2017, 51, 772–786. [Google Scholar] [CrossRef]
- Nasri, H.; Behradmanesh, S.; Maghsoudi, A.R.; Ahmadi, A.; Nasri, P.; Rafieian-Kopaei, M. Efficacy of supplementary vitamin D on improvement of glycemic parameters in patients with type 2 diabetes mellitus; a randomized double blind clinical trial. J. Ren. Inj. Prev. 2014, 3, 31. [Google Scholar]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B.; Dodia, C.; Feinstein, S.I. Identification of small peptides that inhibit NADPH oxidase (Nox2) activation. Antioxidants 2018, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Terpiłowska, S.; Siwicka-Gieroba, D.; Siwicki, A.K. Cytotoxicity of iron (III), molybdenum (III), and their mixtures in BALB/3T3 and HepG2 cells. J. Vet. Res. 2018, 62, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate - induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Cecarini, V.; Gee, J.; Fioretti, E.; Amici, M.; Angeletti, M.; Eleuteri, A.M.; Keller, J.N. Protein oxidation and cellular homeostasis: Emphasis on metabolism. BBA Mol. Cell Res. 2007, 1773, 93–104. [Google Scholar] [CrossRef]
- Dasuri, K.; Zhang, L.; Keller, J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic. Biol. Med. 2013, 62, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Lucassen, P.J.; Krsnik, Ž.; Krušlin, B.; Kostović, I.; Winblad, B.; Bogdanović, N. nNOS expression in reactive astrocytes correlates with increased cell death related DNA damage in the hippocampus and entorhinal cortex in Alzheimer’s disease. Exp. Neurol. 2000, 165, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Amirkhizi, F.; Siassi, F.; Djalali, M.; Foroushani, A.R. Assessment of antioxidant enzyme activities in erythrocytes of pre-hypertensive and hypertensive women. J. Res. Med. Sci. 2010, 15, 270–278. [Google Scholar] [PubMed]
- Shou, I.; Wang, L.N.; Suzuki, S.; Fukui, M.; Tomino, Y. Effects of anti hypertensive drugs on antioxidant enzyme activities and renal function in stroke-prone spontaneously hypertensive rats. Am. J. Med. Sci. 1997, 314, 377–384. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; González-Córdova, A.F.; Astiazarán-García, H.; Esparza-Romero, J.; Vallejo-Córdoba, B. Mechanistic pathways underlying the antihypertensive effect of fermented milk with Lactococcus lactis NRRL B-50571 in spontaneously hypertensive rats. Nutrients 2018, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Greig, J.A.; Shirley, R.; Graham, D.; Denby, L.; Dominiczak, A.F.; Work, L.M.; Baker, A.H. Vascular-targeting antioxidant therapy in a model of hypertension and stroke. J. Cardiovasc. Pharmacol. 2010, 56, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Beswick, H.; Clapperton, M.; Dargie, H.; Smith, W.; McMurray, J. Antioxidant effects of angiotensin-converting enzyme (ACE) inhibitors: Free radical and oxidant scavenging are sulfhydryl dependent, but lipid peroxidation is inhibited by both sulfhydryl-and nonsulfhydryl-containing ACE inhibitors. J. Cardiovasc. Pharmacol. 1992, 19, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Decker, E.A.; Feustman, C. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 1994, 34, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Hur, S.J. Effect of Treatment with Peptide Extract from Beef Myofibrillar Protein on Oxidative Stress in the Brains of Spontaneously Hypertensive Rats. Foods 2019, 8, 455. https://doi.org/10.3390/foods8100455
Lee SY, Hur SJ. Effect of Treatment with Peptide Extract from Beef Myofibrillar Protein on Oxidative Stress in the Brains of Spontaneously Hypertensive Rats. Foods. 2019; 8(10):455. https://doi.org/10.3390/foods8100455
Chicago/Turabian StyleLee, Seung Yun, and Sun Jin Hur. 2019. "Effect of Treatment with Peptide Extract from Beef Myofibrillar Protein on Oxidative Stress in the Brains of Spontaneously Hypertensive Rats" Foods 8, no. 10: 455. https://doi.org/10.3390/foods8100455
APA StyleLee, S. Y., & Hur, S. J. (2019). Effect of Treatment with Peptide Extract from Beef Myofibrillar Protein on Oxidative Stress in the Brains of Spontaneously Hypertensive Rats. Foods, 8(10), 455. https://doi.org/10.3390/foods8100455



