Effect of Moringa oleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Step 1—Chemical assessment to nutritionally characterize the MO leaf powder produced at Saharawi refugee camps.
- Step 2—Enzyme assay to evaluate the ability of MO leaf powder extract to inhibit α-amylase activity.
- Step 3—Acceptability test to evaluate the sensory characteristics and the overall acceptability of a traditional meal added with 20 g of MO leaf powder.
- Step 4—Glycemic response test to evaluate how a traditional meal with an added 20 g of MO leaf powder affects postprandial glucose response in diabetic and non-diabetic subjects.
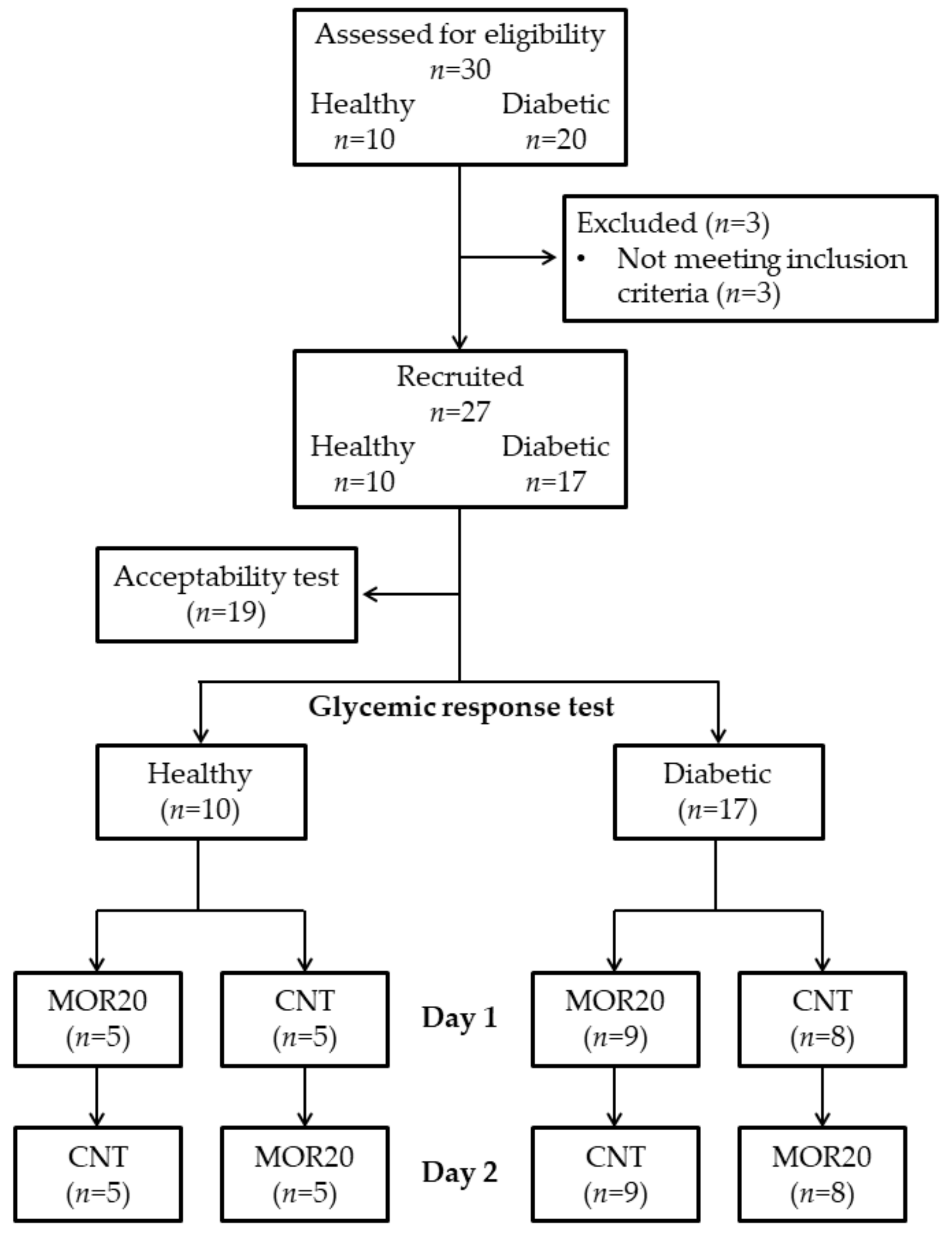
2.2. Sample
2.3. Subjects
2.4. Meal Preparation
2.5. Experimental Protocol
2.5.1. Chemical Assessment
2.5.2. Enzyme Assay
2.5.3. Acceptability Test
2.5.4. Glycemic Response Test
2.6. Statistical Analysis
3. Results
3.1. Chemical Assessment
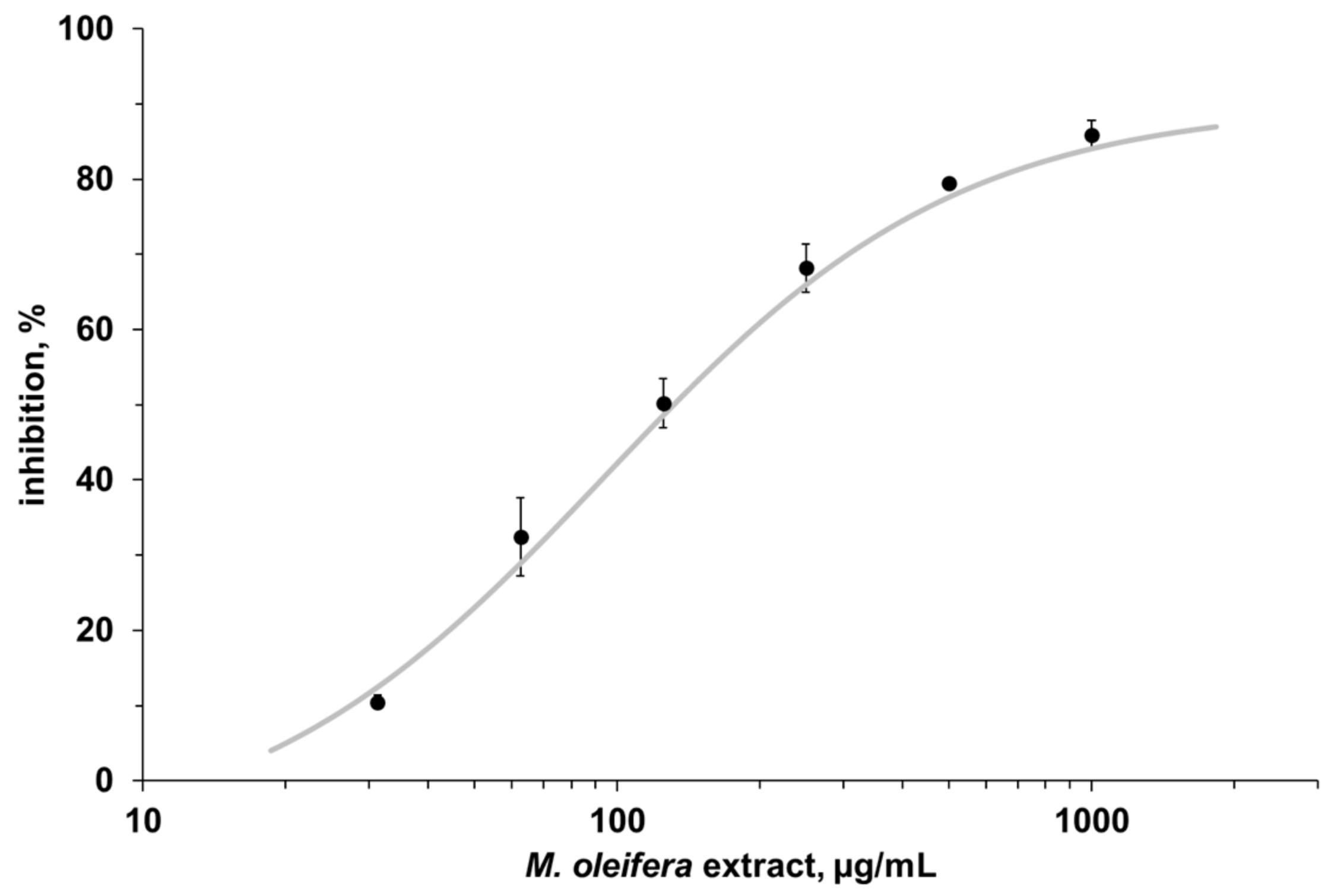
3.2. Enzyme Assay
3.3. Acceptability and Glycemic Response Tests
3.3.1. Acceptability Test
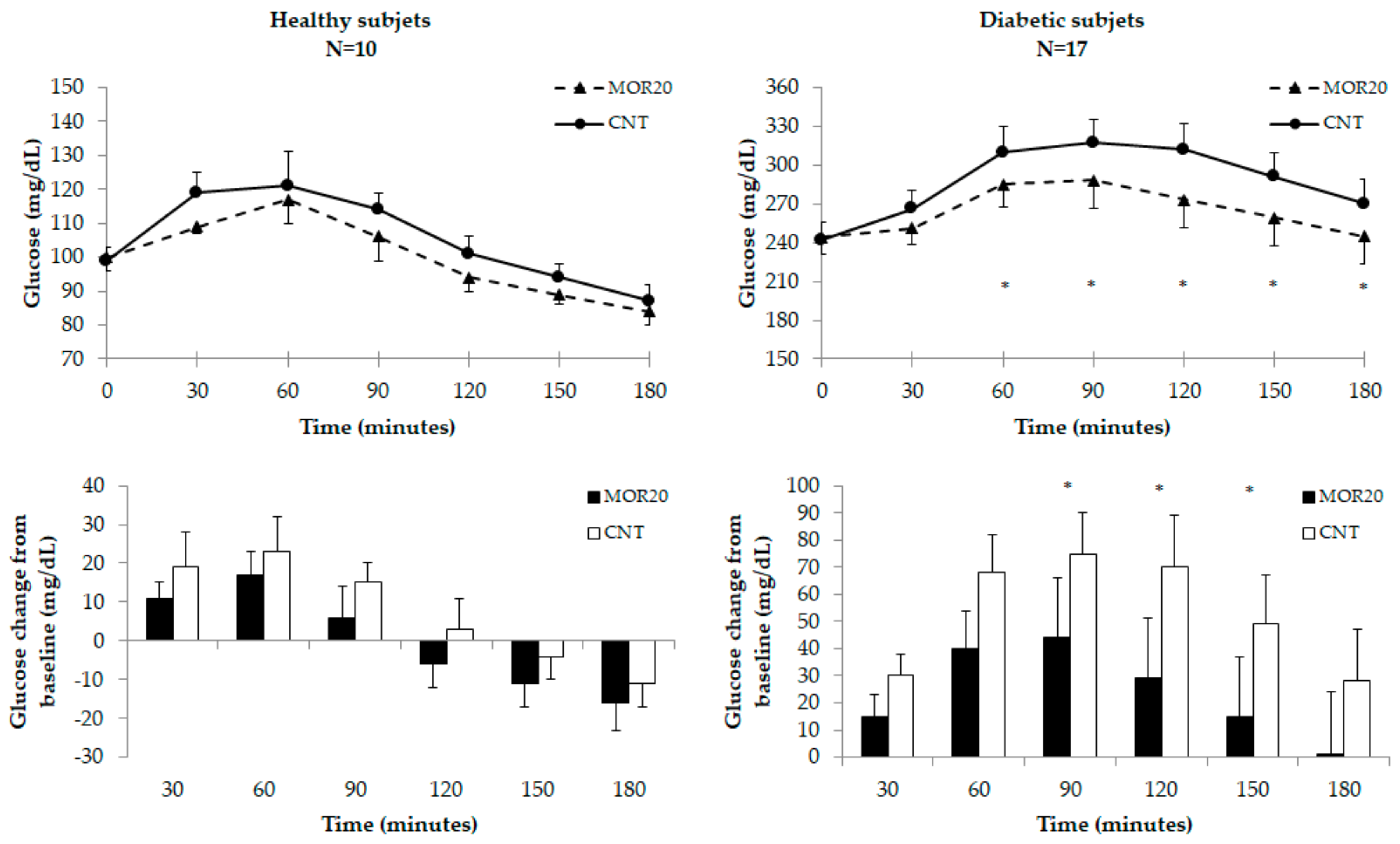
3.3.2. Glycemic Response Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera seeds and oil: Characteristics and uses for human health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [PubMed]
- Popoola, J.O.; Obembe, O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional characterization and phenolic profiling of Moringa oleifera leaves grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef] [PubMed]
- Barichella, M.; Pezzoli, G.; Faierman, S.A.; Raspini, B.; Rimoldi, M.; Cassani, E.; Bertoli, S.; Battezzati, A.; Leone, A.; Iorio, L.; et al. Nutritional characterisation of Zambian Moringa oleifera: Acceptability and safety of short-term daily supplementation in a group of malnourished girls. Int. J. Food. Sci. Nutr. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tshingani, K.; Donnen, P.; Mukumbi, H.; Duez, P.; Dramaix-Wilmet, M. Impact of Moringa oleifera lam. Leaf powder supplementation versus nutritional counseling on the body mass index and immune response of hiv patients on antiretroviral therapy: A single-blind randomized control trial. BMC Complement. Altern. Med. 2017, 17, 420. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W. Moringa oleifera: A Review of the Medicinal Potential; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2017; pp. 209–224. [Google Scholar]
- Ntila, S.; Ndhlala, A.R.; Kolanisi, U.; Abdelgadir, H.; Siwela, M. Acceptability of a moringa-added complementary soft porridge to caregivers in Hammanskraal, Gauteng province and Lebowakgomo, Limpopo province, South Africa. S. Afr. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Ndong, M.; Uehara, M.; Katsumata, S.; Suzuki, K. Effects of oral administration of Moringa oleifera Lam on glucose tolerance in goto-kakizaki and wistar rats. J. Clin. Biochem. Nutr. 2007, 40, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Mathur, M.; Bajaj, V.K.; Katariya, P.; Yadav, S.; Kamal, R.; Gupta, R.S. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J. Diabetes 2012, 4, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Olayaki, L.A.; Irekpita, J.E.; Yakubu, M.T.; Ojo, O.O. Methanolic extract of Moringa oleifera leaves improves glucose tolerance, glycogen synthesis and lipid metabolism in alloxan-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Choi, E.J.; Han, W.C.; Oh, M.; Kim, J.; Hwang, J.Y.; Park, P.J.; Moon, S.H.; Kim, Y.S.; Kim, E.K. Moringa oleifera from cambodia ameliorates oxidative stress, hyperglycemia, and kidney dysfunction in type 2 diabetic mice. J. Med. Food 2017, 20, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Parveen, R.; Chester, K.; Parveen, S.; Ahmad, S. Hypoglycemic potential of aqueous extract of Moringa oleifera leaf and in vivo GC-MS metabolomics. Front. Pharmacol. 2017, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Abd El Latif, A.; El Bialy Bel, S.; Mahboub, H.D.; Abd Eldaim, M.A. Moringa oleifera leaf extract ameliorates alloxan-induced diabetes in rats by regeneration of beta cells and reduction of pyruvate carboxylase expression. Biochem. Cell Biol. 2014, 92, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Yassa, H.D.; Tohamy, A.F. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced diabetes mellitus in adult rats. Acta Histochem. 2014, 116, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Spadafranca, A.; Rinelli, S.; Riva, A.; Morazzoni, P.; Magni, P.; Bertoli, S.; Battezzati, A. Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br. J. Nutr. 2013, 109, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Taweerutchana, R.; LumLerdkij, N.; Vannasaeng, S.; Akarasereenont, P.; Sriwijitkamol, A. Effect of Moringa oleifera leaf capsules on glycemic control in therapy-naive type 2 diabetes patients: A randomized placebo controlled study. Evid. Based Complement. Altern. Med. 2017, 2017, 6581390. [Google Scholar] [CrossRef] [PubMed]
- UNHCR. Humanitarian Needs of Sahrawi Refugees in Algeria. Available online: http://reporting.unhcr.org/sites/default/files/Humanitarian%20Needs%20of%20Sahrawi%20Refugees%20in%20Algeria%202016-2017%20-%20June%202016.pdf (accessed on 13 July 2018).
- Grijalva-Eternod, C.S.; Wells, J.C.; Cortina-Borja, M.; Salse-Ubach, N.; Tondeur, M.C.; Dolan, C.; Meziani, C.; Wilkinson, C.; Spiegel, P.; Seal, A.J. The double burden of obesity and malnutrition in a protracted emergency setting: A cross-sectional study of Western Sahara refugees. PLoS Med. 2012, 9, e1001320. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analisys, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W.; Furda, I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: Interlaboratory study. J. Assoc. Off. Anal. Chem. 1988, 71, 1017–1023. [Google Scholar] [PubMed]
- Rocklin, R.D.; Pohl, C.A. Determination of carbohydrates by anion exchange chromatography with pulsed amperometric detection. J. Liq. Chromatogr. 1983, 6, 1577–1590. [Google Scholar] [CrossRef]
- Mawlong, I.; Sujith Kumar, M.S.; Gurung, B.; Singh, K.H.; Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.; Tícona, E.; Carrasco, C.; Flores, Y.; Almanza, G.R. Cuantificaci n de saponinas en residuos de quinua real chenopodium quinoa willd. Rev. Boliv. Quím. 2012, 29, 131–138. [Google Scholar]
- Bernfeld, P. Amylases, alpha and beta. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 149–158. [Google Scholar]
- Forster, N.; Ulrichs, C.; Schreiner, M.; Arndt, N.; Schmidt, R.; Mewis, I. Ecotype variability in growth and secondary metabolite profile in Moringa oleifera: Impact of sulfur and water availability. J. Agric. Food Chem. 2015, 63, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Reddy, A.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Padma, P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Chodur, G.M.; Olson, M.E.; Wade, K.L.; Stephenson, K.K.; Nouman, W.; Fahey, J.W. Wild and domesticated Moringa oleifera differ in taste, glucosinolate composition, and antioxidant potential, but not myrosinase activity or protein content. Sci. Rep. 2018, 8, 7995. [Google Scholar] [CrossRef] [PubMed]
- Boateng, L.; Nyarko, R.; Asante, M.; Steiner-Asiedu, M. Acceptability of complementary foods that incorporate Moringa oleifera leaf powder among infants and their caregivers. Food Nutr. Bull. 2018, 39, 137–148. [Google Scholar] [CrossRef] [PubMed]
- William, F.; Lakshminarayanan, S.; Chegu, H. Effect of some indian vegetables on the glucose and insulin response in diabetic subjects. Int. J. Food Sci. Nutr. 1993, 44, 191–195. [Google Scholar] [CrossRef]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of alpha-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory kinetics and mechanism of kaempferol on alpha-glucosidase. Food Chem. 2016, 190, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.F.; Cazarolli, L.H.; Lavado, C.; Mengatto, V.; Figueiredo, M.S.; Guedes, A.; Pizzolatti, M.G.; Silva, F.R. Effects of flavonoids on alpha-glucosidase activity: Potential targets for glucose homeostasis. Nutrition 2011, 27, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid inhibition of sodium-dependent vitamin c transporter 1 (svct1) and glucose transporter isoform 2 (glut2), intestinal transporters for vitamin c and glucose. J. Biol. Chem. 2002, 277, 15252–15260. [Google Scholar] [CrossRef] [PubMed]
- Welsch, C.A.; Lachance, P.A.; Wasserman, B.P. Dietary phenolic compounds: Inhibition of Na+-dependent d-glucose uptake in rat intestinal brush border membrane vesicles. J. Nutr. 1989, 119, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [PubMed]
- Domènech, G.; Escortell, S.; Gilabert, R.; Lucena, M.; Martínez, M.C.; Mañes, J.; Soriano, J.M. Assessment of energy and nutrient intakes among saharawi children hosted in spain. Int. J. Child Health Hum. Dev. 2013, 6, 193. [Google Scholar]
- Boyd, R.; Leigh, B.; Stuart, P. Capillary versus venous bedside blood glucose estimations. Emerg. Med. J. 2005, 22, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Colagiuri, S.; Sandbaek, A.; Carstensen, B.; Christensen, J.; Glumer, C.; Lauritzen, T.; Borch-Johnsen, K. Comparability of venous and capillary glucose measurements in blood. Diabet Med. 2003, 20, 953–956. [Google Scholar] [CrossRef] [PubMed]
| Nutrients | ||
|---|---|---|
| Proteins | g/100 g | 30.6 ± 0.8 |
| Lipids | g/100 g | 5.6 ± 0.3 |
| Total fiber | g/100 g | 32.8 ± 0.2 |
| Soluble fiber | g/100 g | 5.7 ± 0.1 |
| Insoluble fiber | g/100 g | 27.1 ± 0.2 |
| Starch (estimated by difference) | g/100 g | 11.3 |
| Sugars | g/100 g | 4.6 ± 0.1 |
| Ash | g/100 g | 15.1 ± 0.3 |
| Sodium | mg/100 g | 502 ± 5 |
| Potassium | mg/100 g | 1492 ± 13 |
| Calcium | mg/100 g | 2997 ± 27 |
| Iron | mg/100 g | 30.2 ± 0.3 |
| Total polyphenols | mg GAE/g | 23.91 ± 0.2 |
| Total glucosinolates | mg SE/g | 21.22 ± 3.7 |
| Total saponins | mg OAE/g | 16.92 ± 0.6 |
| Sensory Characteristics | MOR20 | CNT | p-Value |
|---|---|---|---|
| Color | 5.0 ± 0.5 | 6.6 ± 0.5 | 0.003 |
| Taste | 5.4 ± 0.4 | 6.4 ± 0.4 | 0.024 |
| Texture | 5.8 ± 0.6 | 6.8 ± 0.6 | 0.064 |
| Acceptability | 5.2 ± 0.5 | 6.4 ± 0.5 | 0.055 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, A.; Bertoli, S.; Di Lello, S.; Bassoli, A.; Ravasenghi, S.; Borgonovo, G.; Forlani, F.; Battezzati, A. Effect of Moringa oleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps. Nutrients 2018, 10, 1494. https://doi.org/10.3390/nu10101494
Leone A, Bertoli S, Di Lello S, Bassoli A, Ravasenghi S, Borgonovo G, Forlani F, Battezzati A. Effect of Moringa oleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps. Nutrients. 2018; 10(10):1494. https://doi.org/10.3390/nu10101494
Chicago/Turabian StyleLeone, Alessandro, Simona Bertoli, Sara Di Lello, Angela Bassoli, Stefano Ravasenghi, Gigliola Borgonovo, Fabio Forlani, and Alberto Battezzati. 2018. "Effect of Moringa oleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps" Nutrients 10, no. 10: 1494. https://doi.org/10.3390/nu10101494
APA StyleLeone, A., Bertoli, S., Di Lello, S., Bassoli, A., Ravasenghi, S., Borgonovo, G., Forlani, F., & Battezzati, A. (2018). Effect of Moringa oleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps. Nutrients, 10(10), 1494. https://doi.org/10.3390/nu10101494








