1. Introduction
Type 2 diabetes mellitus (T2D) has reached epidemic proportions worldwide. It has been estimated that, globally, over 8% of adults, corresponding to more than 380 million people, have T2D and this number is set to rise beyond 600 million in less than 25 years [
1]. Furthermore, the prevalence of insulin resistance, the cause of prediabetes and an independent risk factor for cardiovascular disease and metabolic syndrome (MS), is even more widespread [
2,
3,
4]. To date, the number of prediabetic subjects is estimated at 30 million in Europe and at 86 million in the United States (i.e., approximately 35% of the U.S. population). While T2D is irreversible, prediabetes, the most important risk factor to develop T2D, can be reverted to a healthy metabolic condition through lifestyle interventions, which require a lifelong commitment, and suffer low compliance.
While the appropriateness and cost-effectiveness of pharmacological interventions in prediabetic subjects are being debated, nutraceuticals and food supplements are attracting attention as a viable strategy to address the need for safe, lifelong interventions to reduce the risk of developing diabetes and dyslipidemia. Botanicals have been extensively used in traditional medicine throughout the world because of their effectiveness, limited side effects, and relatively low cost [
5]. In particular, some phytochemicals reported to possess hypoglycemic activity have been identified in vegetal extracts such as Bitter melon, Fenugreek, Gymnema, and Morus Alba [
6]. The mechanism of action of these botanicals relies on stimulation of insulin secretion and/or reduction of intestinal glucose absorption [
6].
The plant stress hormone abscisic acid (ABA) has recently been recognized as an animal hormone involved in glycemia homeostasis [
7,
8]. Plasma ABA (ABAp) increases in healthy subjects after an oral glucose load and impairment of this normal ABA response to hyperglycemia occurs in T2D and in gestational diabetes (GDM) [
9]. GDM is the only diabetic condition capable of spontaneous remission to apparent normalcy (after childbirth). Interestingly, the plasma ABA response to a glucose load also is restored after delivery; thus, resolution of the diabetic state after childbirth is accompanied by the restoration of a normal ABA response to oral glucose [
9]. Fasting ABAp was investigated before and after biliopancreatic diversion (BPD) in obese, normal glucose tolerant (NGT) subjects, and in obese T2D patients, in which resolution of diabetes was observed after BPD. Compared to pre-BPD values, basal ABAp significantly increased 1 month after BPD in T2D as well as in NGT subjects, in parallel with a reduction of fasting plasma glucose.
Altogether, these observations lend support to the hypothesis that endogenous (nanomolar) ABA plays a critical role in normal glucose tolerance, and that low-dose exogenous ABA may provide a means to improve glucose tolerance in prediabetes.
Impairment of the normal ABA response to hyperglycemia occurs in T2D [
9], supporting the hypothesis that ABA supplementation might improve glucose tolerance in prediabetes.
Indeed, a single ABA oral dose of 1 µg per Kg body weight (BW), in the form of an ABA-rich vegetal extract or of synthetic ABA, reduces glycemia after glucose load in healthy humans or in rodents, respectively [
7]. The glycemia-lowering effect of low-dose ABA does not depend on stimulation of insulin secretion, as insulinemia is reduced in ABA-treated compared with ABA-untreated controls. The fact that low-dose ABA is not an insulin secretagogue sets ABA apart from most other current therapeutics with glycemia-lowering action, which conversely stimulate insulin release and may accelerate the beta cell demise that eventually occurs in T2D due to lifelong overstimulation.
The aim of this study was twofold: (i) to test the effect of an ABA-rich vegetal extract formulated for human use on glycemia of healthy subjects taking a single carbohydrate-rich meal or fed a Mediterranean diet for 75 days; (ii) to explore the effect of synthetic ABA on glycemia, lipidemia, and body weight in mice fed a high-glucose diet for 4 months.
2. Materials and Methods
2.1. ABA-Containing Food Supplement
The ABA-containing food supplement was developed and notified to the Italian Ministry of Health by Nutravis S.r.l. (Genova, Italy). It contained GSECM-50®, a vegetal source of ABA, providing approximately 55 µg ABA/tablet.
2.2. Human Volunteers
Ten healthy volunteers (6 females and 4 males, aged between 31 and 58 years, mean age 43.6 years) were enrolled. They were selected among 20 prospective candidates meeting the inclusion criteria (see
Table 1) because they showed values of fasting blood glucose (FBG) and/or of total cholesterol close to or slightly above the normal upper limit at the beginning of the study. All subjects gave their informed consent for inclusion before they participated in the study. The clinical trial was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethical Regional Committee (ERC, Genova, Italy; prot.031REG2016, 25 July 2016).
In the first experimental protocol, each subject introduced a standardized carbohydrate-rich breakfast, one with and another without one tablet of the food supplement taken immediately before the meal. The two experiments were scheduled 1 week apart and were performed in the morning after overnight fasting. Glycemia and plasma ABA (ABAp) were measured on blood samples taken before (time zero) and 15, 30, 60, and 120 min after breakfast. Glycemia was measured with a glucometer (Bayer, Milan, Italy) and ABAp was measured by ELISA [
7]. All measures were performed in duplicate.
In the second experimental protocol, the volunteers were instructed not to change their dietary habits during the study and to take one tablet of the ABA-containing food supplement daily before breakfast. At the beginning of the study (day 1) and after 75 days of treatment, a blood sample was taken from each subject after overnight fasting and waist circumference (WC) and body mass index(BMI) were measured. Values of FBG, glycated hemoglobin (HbA1c), and total and HDL cholesterol were determined by the clinical chemistry laboratory of the IRCCS San Martino in Genova.
2.3. Animals
Male CD1 mice (6-week old) purchased from Charles River (Milano, Italy) were housed at the animal facility of the IRCCS San Martino. All protocols of animal use were approved (authorization 349, Italian Ministry of Health, 30 August 2013). As the human prediabetic condition is believed to span a time frame of approximately 15 years, between 30 and 45 years of age, the age of the mice and the duration of treatment (4 months) were chosen in order to match the time frame at which prediabetes develops in diabetes-prone humans.
2.4. Animal Study
Seven-week-old mice (nine/group) fed a standard chow were administered glucose in the drinking water without (controls) or with synthetic (±)-2-cis, 4-trans abscisic acid (ABA) (Sigma Aldrich, Milano, Italy). To achieve the required daily dose of glucose (1 g/Kg BW) and of ABA (1 µg/Kg BW), the daily volume of water drank by the animals was preliminarily established. Based on this volume (5 mL/day) and taking into account an average weight of the mice of 25 g, the water administered to the animals contained 0.005 g/mL of glucose and 0.005 µg/mL of ABA. The animals were weighed weekly and the concentrations of glucose and of ABA in the water were adjusted to the mean BW in each cage. After 4 months of treatment, blood was drawn after overnight fasting to measure HbA1c (Crystal Chem Inc., Elk Grove Village, IL, USA), total cholesterol, and triglycerides (Abcam, Cambridge, UK) and body weight was measured.
2.5. Oral Glucose Tolerance Test (OGTT) in Mice
One week before the end of the 4-month high-glucose diet, mice were fasted for 17 h before the OGTT. Then, 1 g/Kg BW of glucose was administered by gavage in a 150-µL water solution. Blood was drawn from the tail vein before gavage (time zero) and 15, 30, 60, and 120 min after gavage. Glycemia was immediately measured with a glucometer (Bayer, Milan, Italy), and each measure was performed in duplicate.
2.6. Statistical Analysis
A power analysis was performed to calculate the number of participants in the human study and the number of mice in the murine study, starting from results obtained in a previous investigation with an ABA-rich vegetal extract on healthy humans and with synthetic ABA on rodents [
7].
The normal distribution of the values obtained from the human and the murine experiments was assessed with the Vassarstats website for statistical computation [
13]. Continuous variables are presented as mean ±SD. Comparisons were drawn by paired, one-tailed Student’s
t-test, when the same direction of change was observed in all values; if not, a two-tailed
t-test was applied. Statistical significance was always set at
p < 0.05.
4. Discussion
The results of the human study indicate that a single dose of the ABA-containing food supplement ameliorated the glycemia profile of 10healthy subjects after a standardized carbohydrate-rich breakfast and that the daily intake of the food supplement for 75 days reduced FBG, HbA1c, TC, and body weight in the same subjects fed a Mediterranean diet. Approximately the same daily dose of ABA taken by each subject in the form of an ABA-containing vegetal extract (1 µg/Kg) was administered with drinking water as a synthetic molecule to mice fed a high-glucose diet for 4 months. The murine study confirms the significant reduction of glycated hemoglobin, blood lipids, and body weight in the ABA-treated group as compared with untreated controls, replicating with the pure hormone the results obtained in humans with an ABA-containing vegetal extract.
The present study originates from a previous finding that low-dose oral ABA improves glucose tolerance and reduces insulinemia in humans and rats [
7,
14]. The mechanism underlying the insulin-sparing effect of ABA was hypothesized to rely on stimulation by ABA of muscle glucose uptake, causing a reduction of blood glucose levels and consequently of insulin secretion. Indeed, in vitro studies have demonstrated a direct, insulin-independent effect of ABA on GLUT4 expression and membrane translocation in murine myoblasts [
8]. Insulin-independent stimulation of glucose transport in the skeletal muscle may account for the glycemia-lowering effect of ABA observed both under acute (
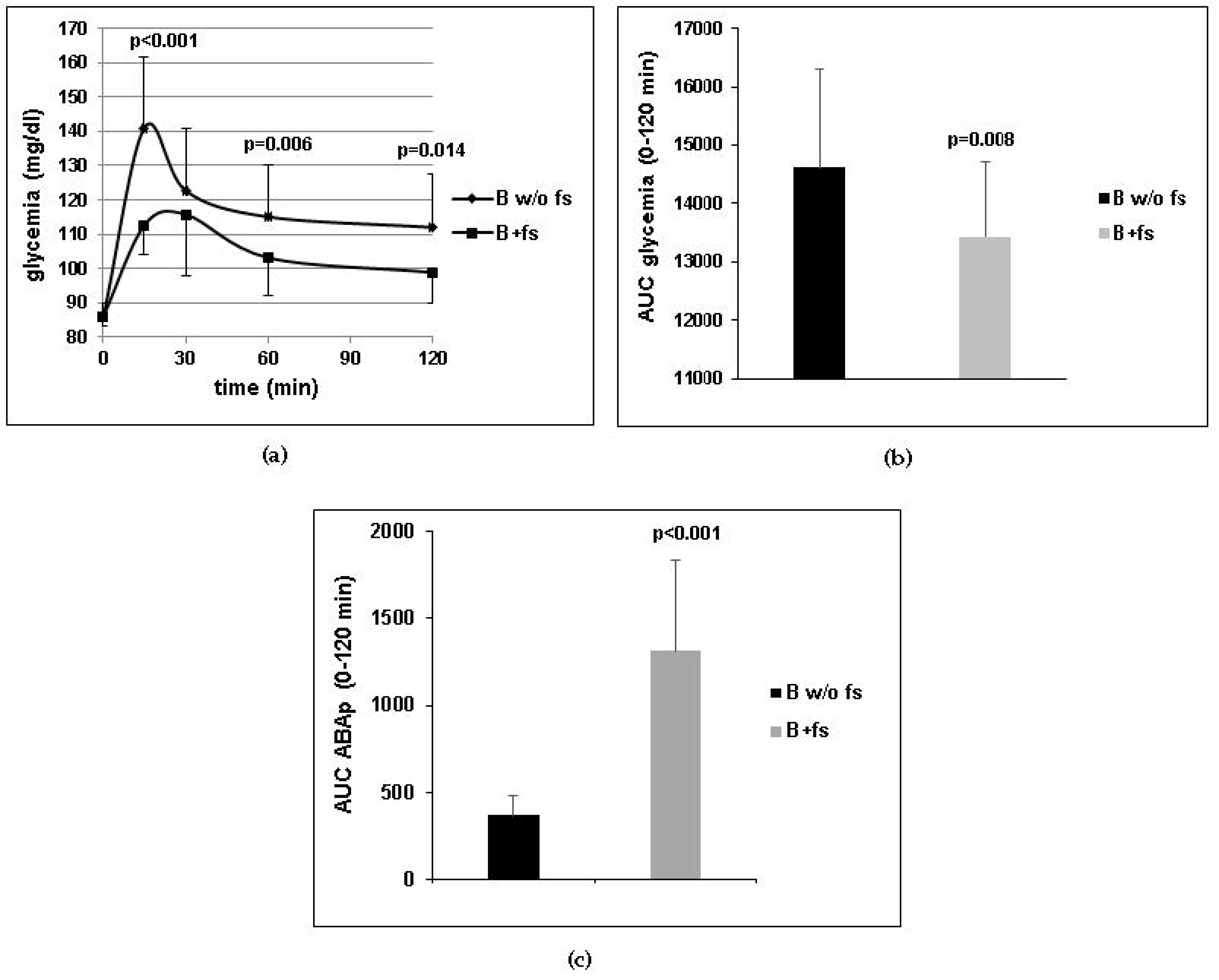
Figure 1a) and chronic (
Table 4) treatment. The sparing effect of ABA on insulin release may also be partly responsible for the reduction of BMI and waist circumference observed in the clinical study (
Table 3) and the reduced body weight gain observed in the ABA-treated mice fed a high-glucose diet (
Figure 3d). Another possible mechanism underlying the effect of ABA on body mass is the recently reported stimulation by chronic, low-dose ABA of the expression of brown adipose tissue (BAT) marker genes in the white adipose tissue (WAT). In vitro, treatment of human and murine adipocytes with ABA induces lower triglyceride accumulation and glucose-derived fatty acid synthesis compared with insulin, increases transcription of adiponectin, and upregulates the expression of several BAT marker genes, including energy-dissipating uncoupling protein-1 (UCP1). In vivo, a single dose of ABA at 1 µg/Kg increases BAT glucose uptake twofold in rats and ABA treatment at the same dose for 1 month significantly increases expression of BAT genes in the WAT of treated mice [
15].
Interestingly, the subjects with borderline values of FBG, TC, and/or BMI at the start of the study benefited more from the daily supplementation with ABA. As summarized in
Table 5B, the reduction of FBG, TC, and HbA1c was significantly higher in the subjects with the highest levels at day 1 compared with those who had lower starting values. Indeed, borderline subjects improved significantly more compared with normal subjects for each parameter investigated (
Figure 2a). This result suggests that prediabetic subjects should benefit from oral low-dose ABA supplementation, similar to the borderline subjects of this study. In this regard, it is noteworthy that the cardiovascular risk index was reduced by a significantly higher percentage in the subjects with borderline values of TC compared with those with normal starting TC values (
Table 5B,
Figure 2a).
Calculation of the Framingham point score for each subject at days 1 and 75 showed a reduction of the score in all subjects, except for #10, yielding a highly significant mean reduction (
Figure 2b). Accordingly, the mean 10-year risk percentage value was also significantly decreased (
Figure 2c).
Of note is that the significant improvement of the metabolic parameters occurred without lifestyle interventions, such as dietary restrictions or increased physical activity. One of the inclusion criteria was the dietary habit of a Mediterranean diet, defined on the basis of published criteria [
10,
11,
12] and investigated during the interview of the candidates (
Table 1). All 10volunteers were instructed not to change their dietary regimen or otherwise significantly modify their lifestyle during the study. Thus, each individual subject at the start of the study was his/her own control.
It is possible that in subjects fed a high-fat and/or high-carbohydrate diet, the effect of the ABA-containing nutraceutical could be different compared with what was observed in subjects under a Mediterranean diet. A high-fat/high-carbohydrate diet should induce faster and more pronounced changes in glucose and lipid tolerance (in diabetes-prone individuals) compared with a Mediterranean diet. Since the subjects with borderline values were those who benefited more from the nutraceutical intervention, it is possible that the beneficial effect of ABA supplementation may be more pronounced under a dietary regimen that accelerates the onset of prediabetes.
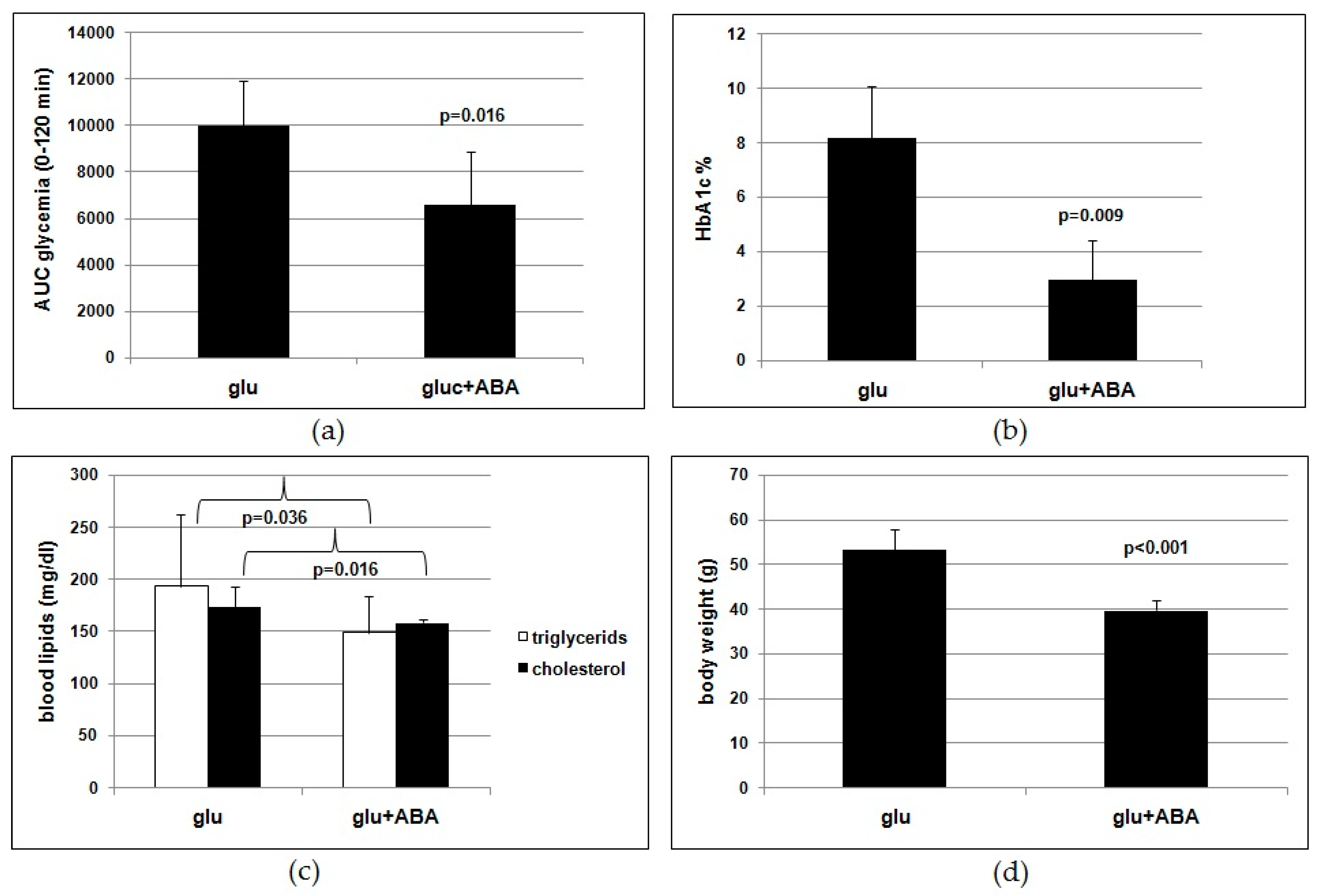
Results obtained on the murine model lend support to the conclusion that ABA is indeed the effective molecule in the food supplement. Rodents fed a high-glucose diet with synthetic ABA for 4 months show an improved glucose tolerance (
Figure 3a,b) and reduced lipidemia and body weight gain (
Figure 3c,d) compared with ABA-untreated controls. Thus, the outcome of the study of chronic ABA treatment in mice confirms that low-dose ABA improves the same metabolic parameters in rodents as in humans and is certainly unaffected by the presence of other vegetal-derived molecules as in the ABA-containing food supplement or by possible modifications of feeding behavior during the study.
The dose of ABA administered in the clinical and murine studies (1 µg/Kg BW) is not attainable from a vegetal-rich diet, although ABA is present in most leafy vegetables, seeds, legumes, and fruits [
7,
14]. Thus, intake of a food supplement containing the appropriate amount of ABA is required to achieve this dose. Absorption of ingested ABA has been already documented [
7] and has been confirmed here (
Figure 1c), probably occurring by simple diffusion of the protonated molecule at pH values below the pKa of ABA (4.6), such as those present in the stomach. The long half-life of ABA in the bloodstream, inferred from the elevated ABAp levels observed in humans several hours after intake of an ABA-rich extract [
7], is likely due to binding of ABA to plasma proteins. Indeed, ABA binding to fatty-acid-free human albumin has been observed in vitro [
7] and is reminiscent of the behavior of steroid and thyroid hormones, which share a relatively long half-life. In addition to preventing rapid urinary excretion, plasma protein binding of lipophylic hormones provides a natural slow-release reservoir, useful in lifelong treatments, where a single daily dose replenishes the bound reservoir.