African Nightshade (Solanum scabrum Mill.): Impact of Cultivation and Plant Processing on Its Health Promoting Potential as Determined in a Human Liver Cell Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Solanum scabrum
Processing of S. scabrum Leaf Material
2.3. Extract Preparation by Sonication
2.4. Determination of S. scabrum Phytochemical Content
2.5. Cell Cultures
2.6. Assessment of Anti-Genotoxic Activity of Leaves of S. scabrum Using the Comet Assay
2.7. Determination of Anti-Oxidant Activity of S. scabrum
2.8. Assessing Induction of Nrf2 Antioxidant Pathway by S. scabrum
2.9. Measurement of Cytotoxicity and Cytostatic Activity
2.10. Data Analysis
3. Results
3.1. Phytochemical Composition of Raw, Processed and UV-C Treated S. scabrum
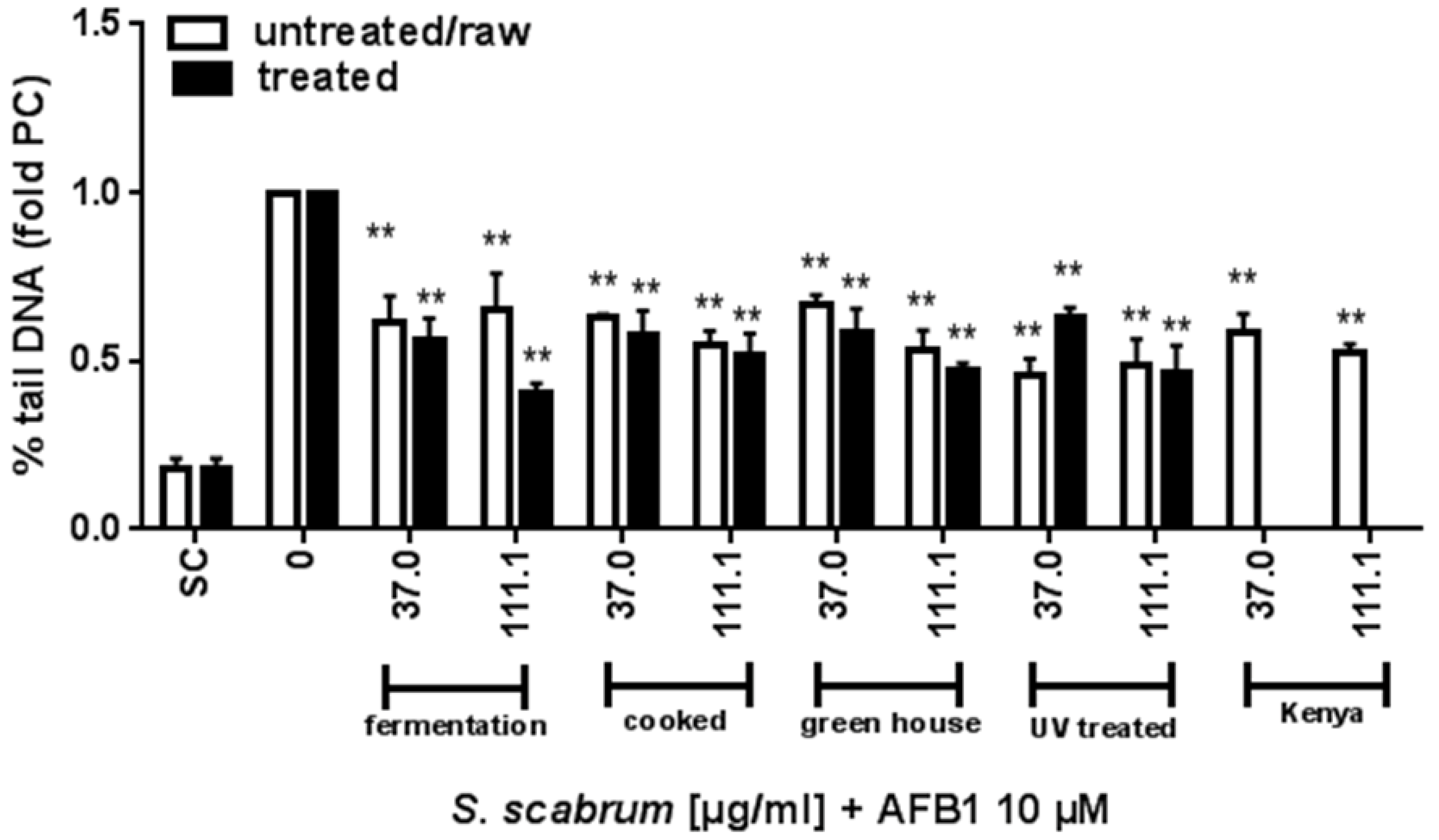
3.2. Effect of Cultivation and Processing on the Protective Potential of S. scabrum against AFB1 Induced DNA Damage
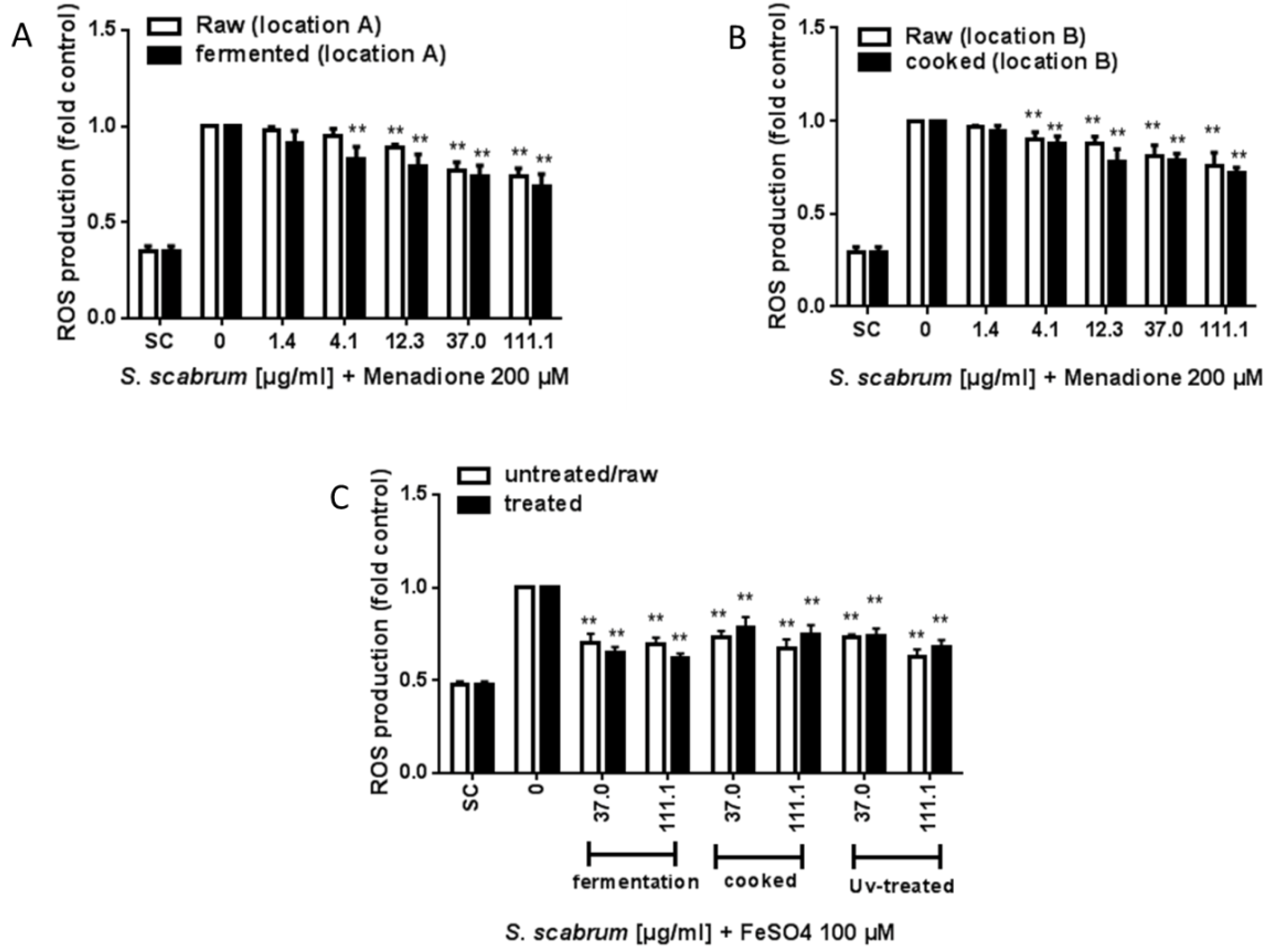
3.3. ROS Scavenging Activity and Induction of ARE/Nrf2-Mediated Gene Expression
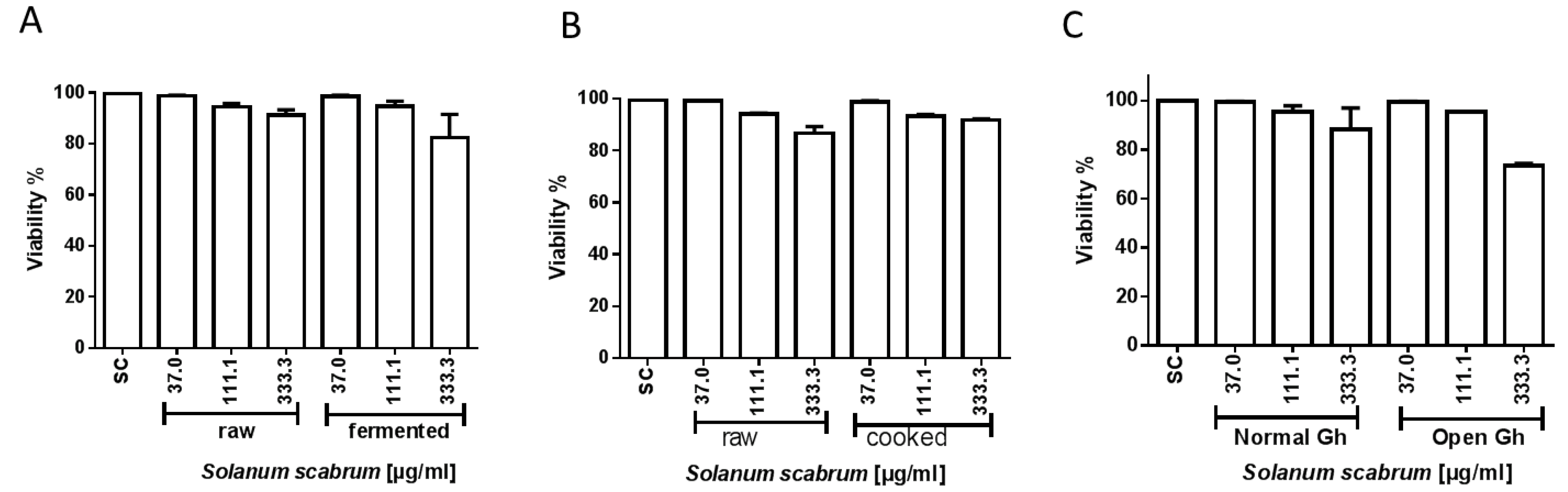
3.4. Induction of Cytotoxicity by Ethanolic S. scabrum Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manoko, M.L.K.; van den Berg, R.G.; Feron, R.M.C.; van der Weerden, G.M.; Mariani, C. Genetic diversity of the African hexaploid species Solanum scabrum Mill. and Solanum nigrum L. (Solanaceae). Genet. Resour. Crop. Evol. 2008, 55, 409–418. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Evaluation of minerals, phytochemical compounds and antioxidant activity of Mexican, Central American, and African green leafy vegetables. Plant Foods Hum. Nutr. 2015, 70, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Baldermann, S.; Ngwene, B.; Wesonga, J.; Schreiner, M. Indigenous leafy vegetables of Eastern Africa—A source of extraordinary secondary plant metabolites. Food Res. Int. 2017, 100, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Byrnes, D.; Giurleo, D.; Villani, T.; Simon, J.E.; Wu, Q. Rapid screening of toxic glycoalkaloids and micronutrients in edible nightshades (Solanum spp.). JFDA 2018, 26, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Laher, F.; Aremu, A.O.; Van Staden, J.; Finnie, J.F. Evaluating the effect of storage on the biological activity and chemical composition of three South African medicinal plants. S. Afr. J. Bot. 2013, 88, 414–418. [Google Scholar] [CrossRef]
- Michalska, A.; Łysiak, G. Bioactive Compounds of blueberries: Post-harvest factors influencing the nutritional value of products. IJMS 2015, 16, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Luthria, D. Instrumental Methods for the Analysis and Identification of Bioactive Molecules, 3th ed.; American Chemical Society: Beltsville, MD, USA, 2014; pp. 3–31. [Google Scholar]
- Chao, J.; Dai, Y.; Cheng, H.Y.; Lam, W.; Cheng, Y.C.; Li, K.; Peng, W.H.; Pao, L.H.; Hsieh, M.T.; Qin, X.M. Improving the concentrations of the active components in the herbal tea ingredient, uraria crinita: The effect of post-harvest oven-drying processing. Sci. Rep. 2017, 7, 38763. [Google Scholar] [CrossRef] [PubMed]
- Gogo, E.O.; Förster, N.; Dannehl, D.; Frommherz, L.; Trierweiler, B.; Opiyo, A.M.; Ulrichs, C.; Huyskens-Keil, S. Postharvest UV-C application to improve health promoting secondary plant compound pattern in vegetable amaranth. Innov. Food Sci. Emerg. Technol. 2018, 45, 426–437. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Cho, G.S.; Trierweiler, B.; Kabisch, J.; Rösch, N.; Neve, H.; Bockelmann, W.; Frommherz, L.; Nielsen, D.S.; Krych, L.; et al. Fermentation of African kale (Brassica carinata) using L. plantarum BFE 5092 and L. fermentum BFE 6620 starter strains. Int. J. Food Microbiol. 2016, 238, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Oguntoyinbo, F.A.; Fusco, V.; Cho, G.S.; Kabisch, J.; Neve, H.; Bockelmann, W.; Huch, M.; Frommherz, L.; Trierweiler, B.; Becker, B.; et al. Produce from Africa’s gardens, potential for leafy vegetable and fruit fermentations. Front Microbiol. 2016, 7, 981. [Google Scholar] [CrossRef] [PubMed]
- Odongo, G.A.; Schlotz, N.; Herz, C.; Hanschen, F.S.; Baldermann, S.; Neugart, S.; Trierweiler, B.; Frommherz, L.; Franz, C.M.; Ngwene, B.; et al. The role of plant processing for the cancer preventive potential of Ethiopian kale (Brassica carinata). Food Nutr. Res. 2017, 61, 1271527. [Google Scholar] [CrossRef] [PubMed]
- Schroter, D.; Baldermann, S.; Schreiner, M.; Witzel, K.; Maul, R.; Rohn, S.; Neugart, S. Natural diversity of hydroxycinnamic acid derivatives, flavonoid glycosides, carotenoids and chlorophylls in leaves of six different amaranth species. Food Chem. 2018, 267, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Kassie, F.; Gminski, R.; Schmeiser, H.H.; Mersch-Sundermann, V. 3-Nitrobenzanthrone (3-NBA) induced micronucleus formation and DNA damage in human hepatoma (HepG2) cells. Toxicol. Lett. 2004, 146, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Herz, C.; Lutz-Bonengel, S.; Hertrampf, A.; Marton, M.R.; Mersch-Sundermann, V. The MAPK pathway signals telomerase modulation in response to isothiocyanate-induced DNA damage of human liver cancer cells. PLoS ONE 2013, 8, e53240. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.M.; Costa, P.M.; Ribeiro e Silva, C.; Chen-Chen, L. Assessment of the genotoxic, antigenotoxic, and cytotoxic activities of the ethanolic fruit extract of Solanum lycocarpum A. St. Hill. (Solanaceae) by micronucleus test in mice. J. Med. Food 2010, 13, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.M.; Paula, J.R.; Chen-Chen, L. Solanum paniculatum L. leaf and fruit extracts: Assessment of modulation of cytotoxicity and genotoxicity by micronucleus test in mice. J. Med. Food 2010, 13, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Tai, C.J.; Wang, C.W.; Choong, C.Y.; Lee, B.H.; Shi, Y.C.; Tai, C.J. Anti-cancer activity of solanum nigrum (AESN) through suppression of mitochondrial function and epithelial-mesenchymal transition (EMT) in breast cancer cells. Molecules 2016, 21, 553. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Michel, D.; Sakharkar, M.K.; Yang, J. Evaluating the cytotoxic effects of the water extracts of four anticancer herbs against human malignant melanoma cells. Drug Des. Dev. Ther. 2016, 10, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, A.; Madeo, M.; Speranza, G.; Cocucci, M. Influence of environmental factors on composition of phenolic antioxidants of Achillea collina Becker ex Rchb. Nat. Prod. Res. 2010, 24, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Tatar, Ö.; Konakchiev, A.; Tsonev, T.; Velikova, V.; Gesheva, E.; Bayram, E.; Vitkova, A.; Edreva, A. Plant-soil water status-induced changes in physiological and biochemical properties of yarrow. J. Essent. Oil Bear. 2016, 19, 1776–1787. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Ghisoni, S.; Baccolo, G.; Blasi, F.; Montesano, D.; Trevisan, M.; Lucini, L. UHPLC-ESI-QTOF-MS profile of polyphenols in Goji berries (Lycium barbarum L.) and its dynamics during in vitro gastrointestinal digestion and fermentation. J. Funct. Foods 2018, 40, 564–572. [Google Scholar] [CrossRef]
- Corona, G.; Coman, M.M.; Guo, Y.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Effect of simulated gastrointestinal digestion and fermentation on polyphenolic content and bioactivity of brown seaweed phlorotannin-rich extracts. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet 2014, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Kiemlian Kwee, J. Yin and Yang of polyphenols in cancer prevention: A short review. Anticancer Agents Med. Chem. 2016, 16, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Eggler, A.L.; Savinov, S.N. Chemical and biological mechanisms of phytochemical activation of Nrf2 and importance in disease prevention. Recent Adv. Phytochem. 2013, 43, 121–155. [Google Scholar] [PubMed]
- Jadeja, R.N.; Upadhyay, K.K.; Devkar, R.V.; Khurana, S. Naturally occurring nrf2 activators: Potential in treatment of liver injury. Oxid. Med. Cell Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress––implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, Y.; Niu, Q.; Xu, S.Z.; Pang, L.J.; Ma, R.L.; Jing, M.X.; Feng, G.L.; Tang, J.X.; Zhang, Q.; et al. Lutein has a protective effect on hepatotoxicity induced by arsenic via Nrf2 signaling. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Wu, Y.; Zhang, Y.; Wang, Z.; Liu, X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-kB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol. Nutr. Food Res. 2015, 59, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in arabidopsis leaves and functions independent of binding to psii antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef] [PubMed]
- Perez-Galvez, A.; Viera, I.; Roca, M. Chemistry in the bioactivity of chlorophylls: An overview. Curr. Med. Chem. 2017, 24, 4515–4536. [Google Scholar] [CrossRef] [PubMed]
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino vesuviano” tomatoes. J. Food Compos. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Criddle, D.N.; Gillies, S.; Baumgartner-Wilson, H.K.; Jaffar, M.; Chinje, E.C.; Passmore, S.; Chvanov, M.; Barrow, S.; Gerasimenko, O.V.; Tepikin, A.V.; et al. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J. Biol. Chem. 2006, 281, 40485–40492. [Google Scholar] [CrossRef] [PubMed]
- Bresgen, N.; Eckl, P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, L.M.; Guzman, M.L.; Rivella, S. Iron and reactive oxygen species: Friends or foes of cancer cells? Antioxid. Redox Signal. 2014, 20, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Odongo, G.A.; Schlotz, N.; Baldermann, S.; Neugart, S.; Ngwene, B.; Schreiner, M.; Lamy, E. Effects of Amaranthus cruentus L. on aflatoxin B1- and oxidative stress-induced DNA damage in human liver (HepG2) cells. Food Biosci. 2018, 26, 42–48. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, G.; Tang, Y.; Yao, P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem. Toxicol. 2018, 114, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Yamaguchi, T.; Takamura, H.; Atoba, T.M. Effects of thermal treatment on radical-scavenging activity of single and mixed polyphenolic compounds. J. Food Sci. 2004, 69, FCT7–FCT10. [Google Scholar] [CrossRef]
- Fiol, M.; Weckmuller, A.; Neugart, S.; Schreiner, M.; Rohn, S.; Krumbein, A.; Kroh, L.W. Thermal-induced changes of kale’s antioxidant activity analyzed by HPLC-UV/Vis-online-TEAC detection. Food chem. 2013, 138, 857–865. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Joubert, E.; Marais, J.; Manley, M. Unravelling the total antioxidant capacity of pinotage wines: Contribution of phenolic compounds. J. Agric. Food Chem. 2006, 54, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Kolniak-Ostek, J.; Wojdylo, A. Characterization of phenolic compounds and antioxidant activity of Solanum scabrum and Solanum burbankii berries. J. Agric. Food Chem. 2014, 62, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Airao, V.; Panara, N.; Vaishnav, D.; Ranpariya, V.; Sheth, N.; Parmar, S. Solasodine protects rat brain against ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol. 2014, 725, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Ijaz, S.; Mohammad, I.S.; Muhammad, K.S.; Akhtar, N.; Khan, H.M.S. Aglycone solanidine and solasodine derivatives: A natural approach towards cancer. Biomed. Pharmacother. 2017, 94, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Kalalinia, F.; Karimi-Sani, I. Anticancer properties of solamargine: A systematic review. Phytother. Res. 2017, 31, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Hassenberg, K.; Huyskens-Keil, S.; Herppich, W.B. Impact of postharvest UV-C and ozone treatments on microbiological properties of white asparagus (Asparagus offificinalis L.). J. Appl. Bot. Food Qual. 2012, 85, 174–181. [Google Scholar]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huyskens-Keil, S.; Hassenberg, K.; Herppich, W.B. Impact of postharvest UV-C and ozone treatment on textural properties of white asparagus (Asparagus officinalis L.). J. Appl. Bot. Food Qual. 2011, 84, 229–234. [Google Scholar]
- Jansen, M.A.K.; van den Noort, R.E.; Tan, M.Y.A.; Prinsen, E.; Lagrimini, L.M. Thorneley RNF. Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiol. 2001, 126, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; O’Brien, N.; Krumbein, A. UV-B-induced secondary plant metabolites-potential benefits for plant and human health. CRC Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Wilkening, S.; Stahl, F.; Bader, A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003, 31, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Gogo, E.O.; Opiyo, A.M.; Hassenberg, K.; Ulrichs, C.; Huyskens-Keil, S. Postharvest UV-C treatment for extending shelf life and improving nutritional quality of African indigenous leafy vegetables. Postharvest Biol. Technol. 2017, 129, 107–117. [Google Scholar] [CrossRef]
- Ercolini, D.; Fogliano, V. Food design to feed the human gut microbiota. J. Agric. Food Chem. 2018, 66, 3754–3758. [Google Scholar] [CrossRef] [PubMed]



| Polyphenols | Raw Ethanol | Raw Water | Fermented Ethanol | Fermented Water |
|---|---|---|---|---|
| tentative structure | ||||
| 3-caffeoylquinic acid | 1314 ± 26 | 828 ± 41 | 1186 ± 24 | 1493 ± 30 |
| 5-caffeoylquinic acid | 1023 ± 20 | 1365 ± 27 | 1490 ± 30 | 2315 ± 46 |
| 4-caffeoylquinic acid | 30,839 ± 617 | 8311 ± 166 | 5394 ± 108 | 15,032 ± 301 |
| caffeoylmalate | n.d. | 1574 ± 31 | 3688 ± 74 | 1418 ± 28 |
| caffeoylmalate | 52,628 ± 1053 | 34,750 ± 695 | 12,527 ± 251 | 35,211 ± 704 |
| quercetin-3-glucosylrhamnogalcatoside | 185,237 ± 3704 | 2083 ± 42 | 479 ± 24 | 994 ± 50 |
| coumaric acid | 1622 ± 32 | 653 ± 33 | 850 ± 43 | 533 ± 27 |
| quercetin-3-rhamnogalactoside | 422 ± 21 | 438 ± 22 | n.d. | n.d. |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 2) | 3326 ± 67 | 5475 ± 110 | 1776 ± 36 | 5428 ± 109 |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 3) | 1649 ± 33 | 2396 ± 48 | 932 ± 47 | 2930 ± 59 |
| sinapoylmalate | 608 ± 30 | 644 ± 32 | 566 ± 28 | 809 ± 40 |
| kaempferol-3-diglucoside | 423 ± 21 | 643 ± 32 | 538 ± 27 | 1036 ± 21 |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 528 ± 26 | 868 ± 43 | 564 ± 28 | 589 ± 29 |
| quercetin-3-pentosylrutinoside | 1787 ±36 | 2090 ± 42 | 1024 ± 20 | 2661 ± 53 |
| sinapic acid | 1705± 34 | 539 ± 27 | 1131 ± 23 | 2742 ± 55 |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 892 ± 45 | 688 ± 34 | 610 ± 31 | 1004 ± 20 |
| quercetin-3-rutinoside | 1346 ± 27 | 709 ± 35 | 911 ± 46 | 1737 ± 35 |
| Total | 101,964 ± 2039 | 64,054 ± 1281 | 33,666 ± 673 | 75,343 ± 1507 |
| Carotenoids | ||||
| β-carotene | 1 ± 0.1 | n.d. | 1 ± 0.1 | n.d. |
| zeaxanthin | 70 ± 7.0 | 1 ± 0.1 | 1326 ± 132.6 | 4 ± 0.4 |
| lutein | 515 ± 51.5 | 1 ± 0.1 | 462 ± 46.2 | n.d. |
| Total | 586 ± 58.6 | 2 ± 0.2 | 1789 ± 178.9 | 4 ± 0.4 |
| Chlorophylls | ||||
| chlorophyll a | 3500 ± 350.0 | n.d. | n.d. | n.d. |
| chlorophyll b | 1076 ± 107.6 | n.d. | 50 ± 5.0 | n.d. |
| Total | 4576 ± 457.6 | n.d. | 50 ± 5.0 | n.d. |
| Polyphenols | Raw Ethanol | Raw Water | Cooked Ethanol | Cooked Water |
|---|---|---|---|---|
| tentative structure | ||||
| 3-caffeoylquinic acid | 1390 ± 28 | 1626 ± 33 | 1314 ± 26 | 1879 ± 38 |
| 5-caffeoylquinic acid | 1231 ± 25 | 1637 ± 33 | 1409 ± 28 | 2273 ± 45 |
| 4-caffeoylquinic acid | 4946 ± 99 | 4630 ± 93 | 2460 ± 49 | 4567 ± 91 |
| caffeoylmalate | 800 ± 40 | 3226 ± 65 | 702 ± 35 | 2513 ± 52 |
| caffeoylmalate | 31,178 ± 624 | 65,157 ± 1303 | 22,048 ± 441 | 58,559 ± 1171 |
| quercetin-3-glucosylrhamnogalcatoside | 611 ± 31 | 734 ± 37 | 548 ± 27 | 611 ± 31 |
| coumaric acid | 598 ± 30 | 913 ± 46 | 650 ± 33 | 811 ± 41 |
| quercetin-3-rhamnogalactoside | 367 ± 18 | 247 ± 12 | n.d. | n.d. |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 2) | 901 ± 18 | 1198 ± 24 | n.d. | 856 ± 43 |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 3) | 541 ± 27 | 796 ± 40 | n.d. | 629 ± 31 |
| sinapoylmalate | 520 ± 26 | 540 ± 27 | n.d. | n.d. |
| kaempferol-3-diglucoside | n.d. | 423 ± 21 | n.d. | n.d. |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 430 ± 22 | 506 ± 25 | 398 ± 20 | 445 ± 22 |
| quercetin-3-pentosylrutinoside | 319 ± 16 | 369 ± 18 | 272 ± 14 | 309 ± 15 |
| sinapic acid | n.d. | n.d. | n.d. | n.d. |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 376 ± 8 | 387 ± 8 | n.d. | n.d. |
| quercetin-3-rutinoside | 518 ± 26 | 497 ± 25 | 441 ± 22 | 393 ± 20 |
| Total | 44,726 ± 895 | 82,886 ± 1658 | 30,242 ± 605 | 73,845 ± 1477 |
| Carotenoids | ||||
| β-carotene | 1 ± 0.1 | n.d. | 1 ± 0.1 | 1 ± 0.1 |
| zeaxanthin | 50 ± 5.0 | n.d. | 41 ± 4.1 | 1 ± 0.1 |
| lutein | 640 ± 64.0 | n.d. | 355±35.5 | 6 ± 0.6 |
| Total | 691 ± 69.1 | n.d. | 397 ± 39.7 | 8 ± 0.8 |
| Chlorophylls | ||||
| chlorophyll a | 1991 ± 199.1 | n.d. | 898 ± 89.8 | 17 ± 1.7 |
| chlorophyll b | 1272 ± 127.2 | n.d. | 583 ± 58.3 | n.d. |
| Total | 3263 ± 326.3 | n.d. | 1481 ± 148.1 | 17 ± 1.7 |
| Polyphenols | Untreated Ethanol | UV-C Treated Ethanol |
|---|---|---|
| tentative structure | ||
| 3-caffeoylquinic acid | 1658 ± 33 | 1042 ± 21 |
| 5-caffeoylquinic acid | 4009 ± 80 | 4715 ± 94 |
| 4-caffeoylquinic acid | 34,633 ± 693 | 38,998 ± 780 |
| caffeoylmalate | 820 ± 16 | 775 ± 39 |
| caffeoylmalate | 18,594 ± 372 | 26,400 ± 528 |
| quercetin-3-glucosylrhamnogalcatoside | 1928 ± 39 | 2490 ± 50 |
| coumaric acid | 1215 ± 24 | 784 ± 39 |
| quercetin-3-rhamnogalactoside | 388 ± 19 | 439 ± 22 |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 2) | 2912 ± 58 | 3436 ± 69 |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 3) | 1546 ± 31 | 1832 ± 37 |
| sinapoylmalate | 571 ± 29 | 612 ± 31 |
| kaempferol-3-diglucoside | 433 ± 22 | 475 ± 24 |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 574 ± 29 | 594 ± 30 |
| quercetin-3-pentosylrutinoside | 1126 ± 23 | 1332 ± 27 |
| sinapic acid | 1431 ± 29 | 1329 ± 27 |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 651 ± 33 | 628 ± 31 |
| quercetin-3-rutinoside | 1045 ± 21 | 1103 ± 22 |
| Total | 73,534 ± 1471 | 86,984 ± 1740 |
| Carotenoids | ||
| β-carotene | 1 ± 0.1 | 1 ± 0.1 |
| zeaxanthin | 74 ± 7.4 | 111 ± 11.1 |
| lutein | 557 ± 55.7 | 541 ± 54.1 |
| Total | 632 ± 63.2 | 653 ± 65.3 |
| Chlorophylls | ||
| chlorophyll a | 3250 ± 325.0 | 1929 ± 192.9 |
| chlorophyll b | 1390 ± 139.0 | 1015 ± 101.5 |
| Total | 4640 ± 464.0 | 2944 ± 294.4 |
| Polyphenols | Ng Ethanol | Og Ethanol |
|---|---|---|
| tentative structure | ||
| 3-caffeoylquinic acid | 3815 ± 76 | 3636 ± 73 |
| 5-caffeoylquinic acid | 6669 ± 133 | 15,526 ± 311 |
| 4-caffeoylquinic acid | 28,790 ± 576 | 114,700 ± 2294 |
| caffeoylmalate | 773 ± 15 | n.d. |
| caffeoylmalate | 31,704 ± 634 | 42,237 ± 845 |
| quercetin-3-glucosylrhamnogalcatoside | 2035 ± 41 | 3096 ± 62 |
| coumaric acid | 1501 ± 30 | 1603 ± 32 |
| quercetin-3-rhamnogalactoside | 377 ± 19 | 627 ± 31 |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 2) | 3705 ± 74 | 4464 ± 89 |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 3) | 1620 ± 32 | 1555 ± 31 |
| sinapoylmalate | 522 ± 26 | 550 ± 28 |
| kaempferol-3-diglucoside | 457 ± 9 | 541 ± 11 |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 611 ± 31 | 550 ± 28 |
| quercetin-3-pentosylrutinoside | 1037 ± 52 | 788 ± 39 |
| sinapic acid | 1113 ± 22 | 1106 ± 22 |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 968 ± 19 | 1337 ± 27 |
| quercetin-3-rutinoside | 2393 ± 48 | 4128 ± 83 |
| Total | 88,090 ± 1762 | 196,444 ± 3929 |
| Carotenoids | ||
| β-carotene | 1 ± 0.1 | 1 ± 0.1 |
| zeaxanthin | 24 ± 2.4 | 65 ± 6.5 |
| lutein | 187 ± 18.7 | 104 ± 10.4 |
| Total | 212 ± 21.2 | 170 ± 17.0 |
| Chlorophylls | ||
| chlorophyll a | 2870 ± 287.0 | 1884 ± 188.4 |
| chlorophyll b | 856 ± 85.6 | 539 ± 53.9 |
| Total | 3726 ± 372.6 | 2424 ± 242.4 |
| Polyphenols | Raw_K Ethanol |
|---|---|
| tentative structure | |
| 3-caffeoylquinic acid | 1899 ± 38 |
| 5-caffeoylquinic acid | 1064 ± 21 |
| 4-caffeoylquinic acid | 7826 ± 157 |
| caffeoylmalate | 6163 ± 123 |
| caffeoylmalate | 33,424 ± 668 |
| quercetin-3-glucosylrhamnogalcatoside | 3007 ± 60 |
| coumaric acid | 2118 ± 42 |
| quercetin-3-rhamnogalactoside | 639 ± 32 |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 2) | 5749 ± 115 |
| quercetin-3-rhamnosylrhamnogalactoside (isomer 3) | 2722 ± 54 |
| sinapoylmalate | n.d. |
| kaempferol-3-diglucoside | 514 ± 26 |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 821 ± 41 |
| quercetin-3-pentosylrutinoside | 1177 ± 24 |
| sinapic acid | 1049 ± 21 |
| kaempferol-3-rhamnosylrhamnogalactoside (isomer 1) | 1972 ± 39 |
| quercetin-3-rutinoside | 8318 ± 166 |
| Total | 78,462 ± 1569 |
| Carotenoids | |
| β-carotene | 1 ± 0.1 |
| zeaxanthin | 51 ± 5.1 |
| lutein | 141 ± 14.1 |
| Total | 193 ± 19.3 |
| Chlorophylls | |
| chlorophyll a | 2152 ± 215.2 |
| chlorophyll b | 672 ± 67.2 |
| Total | 2824 ± 282.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odongo, G.A.; Schlotz, N.; Baldermann, S.; Neugart, S.; Huyskens-Keil, S.; Ngwene, B.; Trierweiler, B.; Schreiner, M.; Lamy, E. African Nightshade (Solanum scabrum Mill.): Impact of Cultivation and Plant Processing on Its Health Promoting Potential as Determined in a Human Liver Cell Model. Nutrients 2018, 10, 1532. https://doi.org/10.3390/nu10101532
Odongo GA, Schlotz N, Baldermann S, Neugart S, Huyskens-Keil S, Ngwene B, Trierweiler B, Schreiner M, Lamy E. African Nightshade (Solanum scabrum Mill.): Impact of Cultivation and Plant Processing on Its Health Promoting Potential as Determined in a Human Liver Cell Model. Nutrients. 2018; 10(10):1532. https://doi.org/10.3390/nu10101532
Chicago/Turabian StyleOdongo, Grace Akinyi, Nina Schlotz, Susanne Baldermann, Susanne Neugart, Susanne Huyskens-Keil, Benard Ngwene, Bernhard Trierweiler, Monika Schreiner, and Evelyn Lamy. 2018. "African Nightshade (Solanum scabrum Mill.): Impact of Cultivation and Plant Processing on Its Health Promoting Potential as Determined in a Human Liver Cell Model" Nutrients 10, no. 10: 1532. https://doi.org/10.3390/nu10101532
APA StyleOdongo, G. A., Schlotz, N., Baldermann, S., Neugart, S., Huyskens-Keil, S., Ngwene, B., Trierweiler, B., Schreiner, M., & Lamy, E. (2018). African Nightshade (Solanum scabrum Mill.): Impact of Cultivation and Plant Processing on Its Health Promoting Potential as Determined in a Human Liver Cell Model. Nutrients, 10(10), 1532. https://doi.org/10.3390/nu10101532





