Hungry Caterpillars: Massive Outbreaks of Achaea lienardi in Hluhluwe-iMfolozi Park, South Africa
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
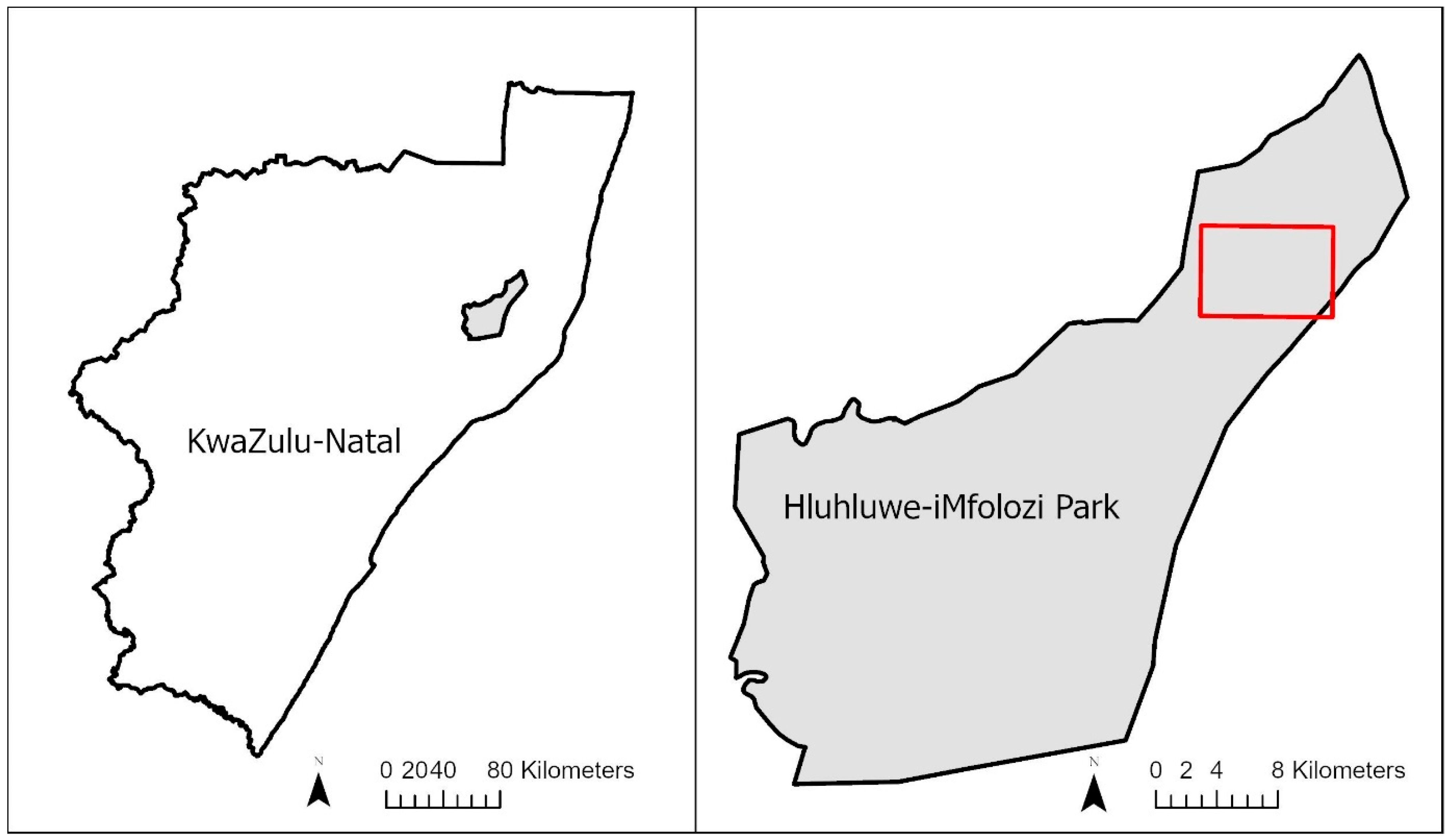
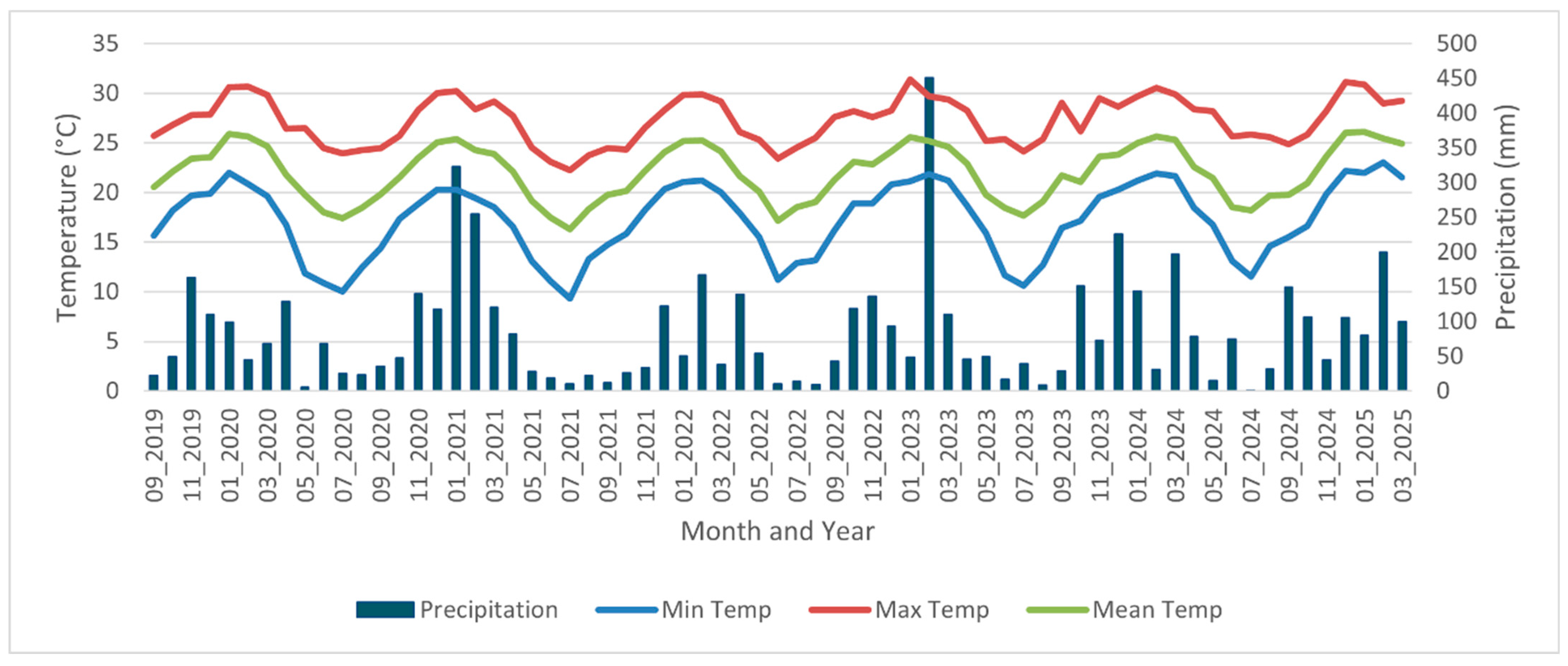
2.1. Study Area
2.2. A. lienardi Biology
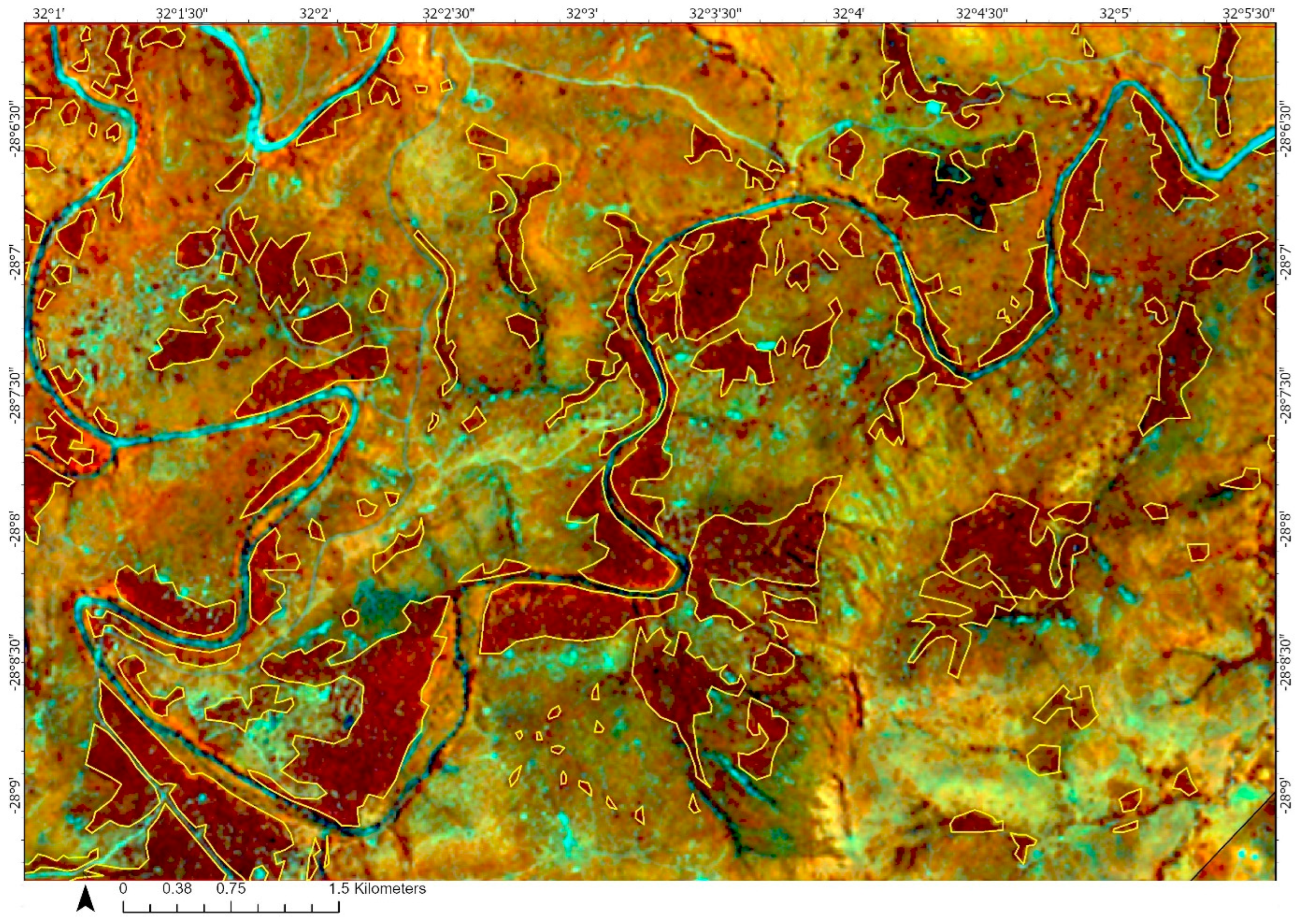
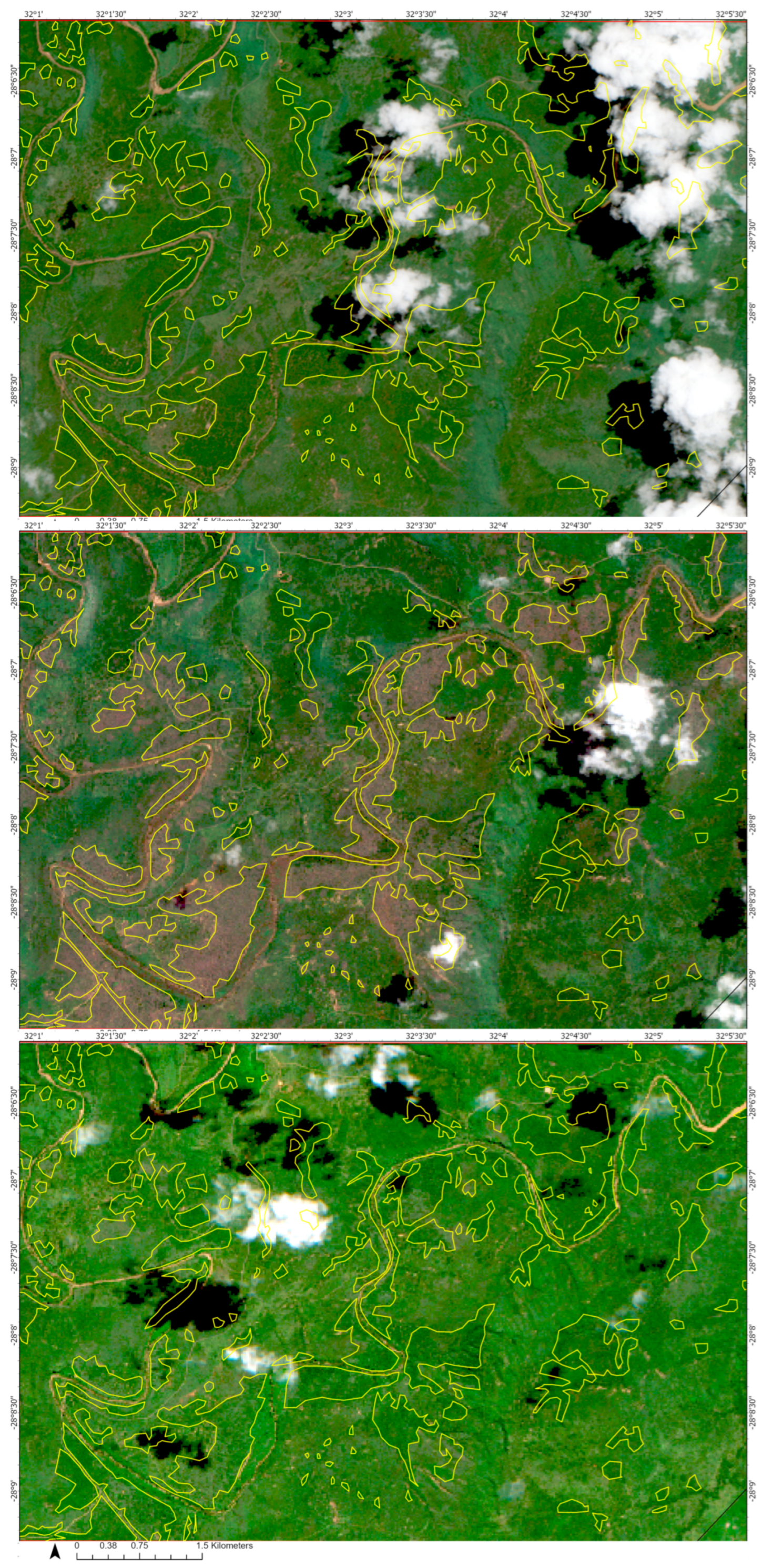
2.3. Methods
3. Results
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HiP | Hluhluwe-iMfolozi Park |
| KZN | KwaZulu-Natal |
| SA | South Africa |
References
- African Moths. Achaea lienardi. Available online: https://africanmoths.com/pages/EREBIDAE/EREBINAE/achaea%20lienardi.htm (accessed on 16 May 2025).
- Owen, D.F.; Whiteley, D. Reflexive selection: Moment’s hypothesis resurrected. Oikos 1986, 47, 117–120. Available online: www.jstor.org/stable/3565927 (accessed on 14 May 2025). [CrossRef]
- Staude, H.S.; Maclean, M.; Mecenero, S.; Pretorius, R.J.; Oberprieler, R.G.; Van Nort, S.; Sharp, A.; Sharp, I.; Balona, J.; Bradley, S. An overview of Lepidoptera-host-parasitoid associations for southern Africa, including an illustrated report on 2 370 African Lepidoptera-host and 119 parasitoid-Lepidoptera associations. Metamorphosis 2020, 31, 1–394. [Google Scholar] [CrossRef]
- Webb, D.v.V. Ekologiese Studie van die Wattelspanrusper. Boerdery Suid Afr. 1953, 385–390. [Google Scholar]
- Moore, S. Fruit-feeding moths in the Eastern Cape: Experiences and control options. SAFJ 2010, 9, 49–52. [Google Scholar]
- Goddard, M.; Hill, M.P.; Moore, S.D. An analysis of the fruit-sucking and fruit-piercing moth complex in citrus orchards in South Africa. Afr. Entomol. 2019, 27, 1–9. [Google Scholar] [CrossRef]
- Taylor, J.S. The fruit-piercing moth, Achaea lienardi Boisduvalo (Lepidoptera: Noctuidae), in the Eastern Cape Province. J. Ent. Soc. S. Afr. 1965, 28, 50–56. [Google Scholar]
- Leston, D. The natural history of some West African insects. Part 8. The annual invasion of Accra by noctuid moths (Lep., Noctuidae). Entomol. Mon. Mag. 1980, 115, 35–36. [Google Scholar]
- Henschel, J.R.; Duncan, F.D.; du Toit, J.C.O.; Milton, S.J.; van der Merwe, H. The brown locust refocussed—Knowns, unknowns and the relevance of Locustana pardalina (Walker) to Karoo ecosystems and rangeland management. J. Arid Environ. 2023, 215, 105104. [Google Scholar] [CrossRef]
- Agricultural Research Council. African Armyworm Outbreak. Available online: https://www.arc.agric.za/arc-ppri/Pages/African-Armyworm-Outbreak.aspx (accessed on 9 May 2025).
- Farmers Weekly. SA Experiences Worst African Armyworm Infestation on Record. Available online: https://www.farmersweekly.co.za/agri-news/south-africa/sa-experiences-worst-african-armyworm-infestation-on-record/ (accessed on 9 May 2025).
- Sokame, B.M.; Kipkorir, B.; Agboka, K.M.; Niassy, S.; Belayneh, Y.; Elkahky, M.; Tonnang, H.E.Z. A system dynamics model for predicting African armyworm occurrence and population dynamics. Agric. Ecosyst. Environ. 2025, 380, 109378. [Google Scholar] [CrossRef]
- Golding, F.D. Fruit-piercing Lepidoptera in Nigeria. Bull. Entomol. Res. 1945, 36, 181–184. [Google Scholar] [CrossRef]
- Copernicus Browser. Available online: https://browser.dataspace.copernicus.eu/ (accessed on 14 March 2025).
- Visual Crossing Corporation. Visual Crossing Weather (2019–2025). Available online: https://www.visualcrossing.com/ (accessed on 6 May 2025).
- Farmers Weekly. Eastern Cape ‘Moth Plague’ No Threat to Crop Farmers. 7 May 2009. Available online: https://www.farmersweekly.co.za/archive/eastern-cape-moth-plague-no-threat-to-crop-farmers/#:~:text=Prof%20Villet%20said%20the%20recent,late%20availability%20of%20prey%20depressed (accessed on 28 May 2025).
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Rutschmann, A.; Dupoué, A.; Miles, D.B.; Megia-Palma, R.; Lauden, C.; Richard, M.; Badiane, A.; Rozen-Rechels, D.; Brevet, M.; Blaimont, P.; et al. Intense nocturnal warming alters growth strategies, colouration and parasite load in a diurnal lizard. J. Anim. Ecol. 2021, 90, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Danks, H.V. Winter habitats and ecological adaptations for winter survival. In Insects at Low Temperature; Lee, R.E., Denlinger, D.L., Eds.; Springer: Boston, MA, USA, 1991; pp. 231–259. [Google Scholar] [CrossRef]
- Musolin, D. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Glob. Change Biol. 2007, 13, 1565–1585. [Google Scholar] [CrossRef]
- Cooperative Governance and Traditional Affairs. Government Declares National State of Disaster to Enable Intensive Response to Widespread Floods. Available online: https://www.cogta.gov.za/index.php/2023/02/13/government-declares-national-state-of-disaster-to-enable-intensive-response-to-widespread-floods/#:~:text=Forecasts%20indicate%20this%20weather%20pattern,structures%20have%20responded%20to%20date (accessed on 28 May 2025).
- Ossowski, L.L.J. Problems of forest entomology in the distribution area of Black Wattle, A. mollissima, in the Union of South Africa. Anz. Schadlingskunde 1957, 30, 133–137. [Google Scholar] [CrossRef]
- Asogwa, E.U.; Hammed, L.A.; Ndubuaku, T.C.N. Integrated production and protection practices of cashew (Anacardium occidentale) in Nigeria. Afr. J. Biotechnol. 2008, 7, 4868–4873. Available online: http://www.academicjournals.org/AJB (accessed on 23 May 2025).
- Ossowski, L.L.J. Notes on insects observed during the period 1949–1952. Rep. Wattle Res. Inst. Univ. Natal 1952, 12, 81–88. [Google Scholar]
- Govender, P. Management of insect pests: Have the goalposts changed with certification? S. Afr. For. J. 2002, 195, 39–45. [Google Scholar] [CrossRef]
- Cotterell, G.S. Citrus fruit-piercing moths—Summary of information and progress. Pap. 3rd W. Afr. Agric. Conf. Nigeria 1938 Sect. Gold Coast 1940, 1, 11–24. [Google Scholar]
- Williams, K.A. Museum collections—Resources for biological monitoring. Afr. Invertebr. 2010, 51, 219–221. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jewitt, D. Hungry Caterpillars: Massive Outbreaks of Achaea lienardi in Hluhluwe-iMfolozi Park, South Africa. Wild 2025, 2, 34. https://doi.org/10.3390/wild2030034
Jewitt D. Hungry Caterpillars: Massive Outbreaks of Achaea lienardi in Hluhluwe-iMfolozi Park, South Africa. Wild. 2025; 2(3):34. https://doi.org/10.3390/wild2030034
Chicago/Turabian StyleJewitt, Debbie. 2025. "Hungry Caterpillars: Massive Outbreaks of Achaea lienardi in Hluhluwe-iMfolozi Park, South Africa" Wild 2, no. 3: 34. https://doi.org/10.3390/wild2030034
APA StyleJewitt, D. (2025). Hungry Caterpillars: Massive Outbreaks of Achaea lienardi in Hluhluwe-iMfolozi Park, South Africa. Wild, 2(3), 34. https://doi.org/10.3390/wild2030034





