1. Introduction
Cemented carbides, commonly known as hard metals (HM), are advanced composite materials consisting of hard tungsten carbide (WC) particles embedded within a ductile metallic binder, typically cobalt (Co) [
1,
2,
3]. HMs are renowned for their exceptional hardness and wear resistance. These are indispensable in metal machining, cutting tools, and wear applications. Their unique combination of hardness and toughness is primarily governed by the ratio of carbide to the binder and the particle size of the WC phase, typically ranging from the submicron level to several micrometers [
4,
5,
6]. Cemented carbide manufacturing primarily involves powder metallurgy, which involves powder dispersion, forming, and sintering at high temperatures close to the eutectic point (~1320 °C) [
7,
8,
9].
A critical stage in the production process is the dispersion of WC and binder powders, traditionally achieved through wet milling in organic solvents such as alcohols or alkanes [
10,
11,
12]. These solvents not only prevent overheating and oxidation but also enhance the homogeneity of the powder mixture. However, the reliance on organic solvents presents significant challenges, including their high flammability, toxicity, and environmental impact. Organic solvent usage in the hard metal industry contributes up to 8% of total processing CO
2 emissions [
13], with acetone and ethanol alone accounting for an estimated 2.5 tons of VOCs annually per production line. Organic solvents can account for up to 15% of consumable costs in WC-Co powder preparation, with disposal and recovery costs further increasing the operational burden [
14]. As a result, water-based processing has emerged as an eco-friendly alternative, addressing safety and environmental concerns [
15,
16,
17]. Despite its potential, aqueous processing faces issues such as powder oxidation and the formation of hard agglomerates, which require careful optimization of suspension stability through pH adjustment or dispersant addition [
12,
18].
Spray drying is another critical step in HM production, transforming the liquid suspension into granulated powders ready for pressing and sintering. Traditionally, this process relies on organic solvent-based suspensions, amplifying the associated environmental and safety concerns. Replacing organic solvents with water in spray drying presents a promising path toward sustainable manufacturing, reducing carbon emissions, improving workplace safety, and compliance with stricter environmental regulations. However, this shift necessitates a thorough evaluation of process scalability and energy efficiency, mainly when applied across different spray-dryer configurations, such as the lab-scale equipment [
19] (1 m diameter) and production equipment (2/12 m diameter) [
20]. Larger spray dryers demand higher energy inputs for evaporation and offer significantly higher evaporation capacities, enabling greater productivity [
3,
15]. This enhanced capability makes them more suitable for industrial-scale operations, as they can process larger volumes of suspension within shorter time frames. However, their operational efficiency remains critical in determining the feasibility of adopting water-based spray drying at such scales, balancing the trade-offs between energy consumption, production throughput, and environmental impact. In our previous studies, we have highlighted the challenges of water milling of WC-Co, as well as all of our approaches on tackling these risks [
3,
8,
12]. In this study, we turn our attention to highlighting the aspects regarding the environmental, safety, and energy implications of substituting organic solvents with water in spray drying across three spray-dryer setups. The goal of this study was to highlight that the use of water-based spray drying results in properties that are effectively identical to those achieved with conventional solvent-based processing. By analyzing carbon emissions, energy consumption, and process performance, this work highlights the potential for sustainable advancements in HM production. Our findings contribute to ongoing efforts to minimize the environmental footprint of industrial manufacturing processes and align with global sustainability goals.
3. Results and Discussions
The first part of this study offers a theoretical evaluation of different spray-dryer capacities and different industrially used suspensions. Critical insights into their environmental impact and productivity in the spray-drying processes can be gathered by evaluating spray dryers with lab-scale dimensions and production installations.
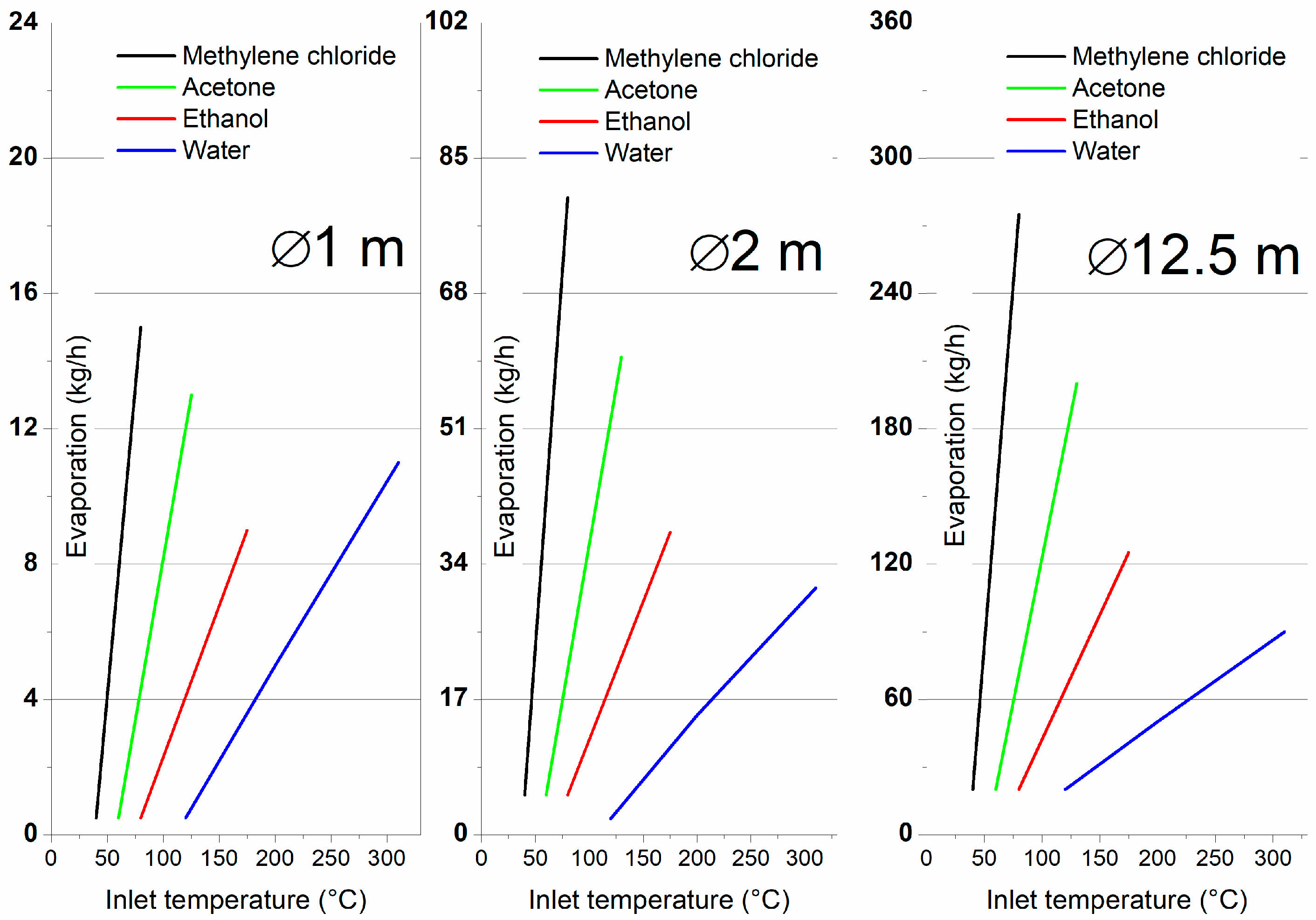
Figure 1 highlights the evaporation rates based on each solvent required inlet temperature [
3,
19,
20].
As can be seen, and as expected, higher inlet temperatures result in increased productivity due to faster and more efficient evaporation rates, enabling greater throughput in a given time [
22]. However, the inlet temperature plays a critical role in productivity and determining the quality of the final product. Excessively high inlet temperatures can lead to improper drying dynamics, potentially affecting residual humidity levels and compromising particle morphology [
3,
18]. Maintaining the correct balance is essential to ensure that the powders exhibit desirable characteristics, such as optimal flowability, uniform particle size, and minimal agglomeration, which are crucial for downstream processes such as pressing and sintering [
10,
11,
23].
Starting from these values, the energy required to heat solvents, evaporation rates, and equivalent CO2 emissions per kilogram of hard metal (HM) powder produced are analyzed. The calculus was performed considering a 60% solids content in the suspension.
The energy required to heat each kilogram of solvent is a critical factor in the sustainability of the spray-drying process. The heat energy, Q, required can be approximated as follows:
where m is the mass of the solvent, c denotes the specific heat capacity, and ΔT stands for temperature increase (inlet temperature minus ambient). Water has a specific heat capacity of approximately 4.18 kJ/kg·°C [
24]. In contrast, for organic solvents like ethanol and acetone, with lower specific heat capacities (~2.4 kJ/kg·°C), the energy required is proportionally lower, but the environmental costs (e.g., CO
2 emissions) remain significantly higher due to their combustion profiles [
24]. The comparison of energy consumption and CO
2 emissions across different spray-dryer sizes and solvents reveals key insights into the environmental efficiency and sustainability of the spray-drying process for hard metal (HM) powders. Water-based spray drying consistently demonstrates superior environmental performance to organic solvents like methylene chloride, acetone, and ethanol, with differences becoming even more pronounced as the dryer size increases.
The comparative evaluation of water and industrial solvents such as ethanol and acetone in spray-drying processes highlights environmental-impact and productivity insights. While water-based spray drying generally requires more energy for evaporation, it ultimately proves to be more environmentally friendly, particularly when considering CO
2 emissions and long-term sustainability, especially in larger spray dryers. Ethanol and acetone, commonly used in industrial processes [
18], display significantly higher environmental costs regarding CO
2 emissions, though they require less energy for evaporation than water. Water-based spray-drying demands more energy across all spray-dryer sizes, with the lowest (1 m diameter) requirement of approximately 0.5 kWh per kilogram of HM powder at 120 °C. In comparison, ethanol and acetone require half the energy for evaporation due to their lower evaporation temperature (acetone consuming 0.33 kWh per kilogram of powder at 60 °C and ethanol using 0.2 kWh per kilogram at 80 °C). As dryer size increases, these differences in energy consumption become less pronounced, particularly in the larger 12.5 m diameter dryer, where the system’s efficiency increases, and energy consumption per unit of HM powder decreases. For example, water requires 0.53 kWh per kilogram of HM powder in this larger dryer, while acetone requires 0.44 kWh per kilogram. Despite water’s higher energy demand, the difference becomes smaller with increased evaporation capacity, which enhances the thermal efficiency of larger dryers.
However, when comparing the CO2 emissions associated with the solvents, water shows a clear advantage regarding environmental impact. For instance, water evaporation at 200 °C in the 1 m diameter dryer produces only 0.32 kg CO2 per kilogram of HM powder, whereas acetone generates 1.1 kg CO2 and ethanol 0.6 kg CO2 under similar conditions. In the 12.5 m diameter dryer, water’s CO2 emissions drop to 0.25 kg per kilogram of HM powder, while acetone and ethanol still exhibit significantly higher emissions—11 kg CO2 and 25 kg CO2 per kilogram of powder, respectively. This demonstrates that, while ethanol and acetone may offer slightly lower energy requirements, their higher CO2 emissions make them less sustainable than water, especially in larger systems with higher productivity.
From the perspective of the working hypothesis, the results confirm that water-based spray drying is a viable alternative to organic solvents, mainly when employed in larger dryers. These findings extend the current understanding of solvent use in powder metallurgy by demonstrating the scalability of water-based systems without compromising productivity [
7]. Furthermore, this study supports the hypothesis that larger dryers offer operational benefits and reduced environmental costs, making them suitable for meeting increasing industrial demands while adhering to sustainability standards. The wider consequences of these findings extend beyond the hard metal industry. The demonstrated efficiency gains with larger dryers and water-based processing apply to other sectors where spray drying is utilized, such as pharmaceuticals and food processing. The reduced reliance on organic solvents not only decreases CO
2 emissions but also addresses growing regulatory pressures to minimize the use of hazardous chemicals in manufacturing.
The evaluation of SEM images in
Figure 2 of the spray-dried WC-Co granules from water- and solvent-based processes reveals a striking similarity in their morphology, underscoring the effectiveness of optimizing spray-drying parameters.
Both granules, produced under carefully controlled conditions, exhibit comparable spherical shapes and sizes, highlighting that the difference in solvent type does not inherently dictate granule quality when appropriate drying temperatures and conditions are employed. The spherical morphology, a critical feature for ensuring proper flowability, packing density, and uniform sintering behavior in cemented carbide manufacturing, was successfully achieved in both cases [
3,
18,
23]. This finding challenges the traditional notion that solvent-based processes yield superior granule quality. Instead, it demonstrates that achieving similar outcomes with water-based spray drying is feasible, provided the inlet temperature and other drying parameters are precisely tuned. For example, by using higher inlet temperatures for the water-based process to compensate for its higher latent heat of evaporation, the resultant granules achieved the same shrinkage uniformity and particle cohesion level as those obtained from solvent-based methods. This similarity suggests that the challenges typically associated with water-based drying, such as the potential for hard agglomerates and irregular particle formation due to its higher surface tension, can be mitigated by carefully tailoring the drying conditions.
Furthermore, this equivalence in granule morphology has significant implications for industrial practice. It indicates that water-based spray drying can produce granules of comparable quality to solvent-based methods, thus eliminating the necessity for organic solvents in many cases. This shift can lead to reduced safety hazards; lower environmental impact; and a significant decrease in operational costs related to solvent handling, storage, and recovery.
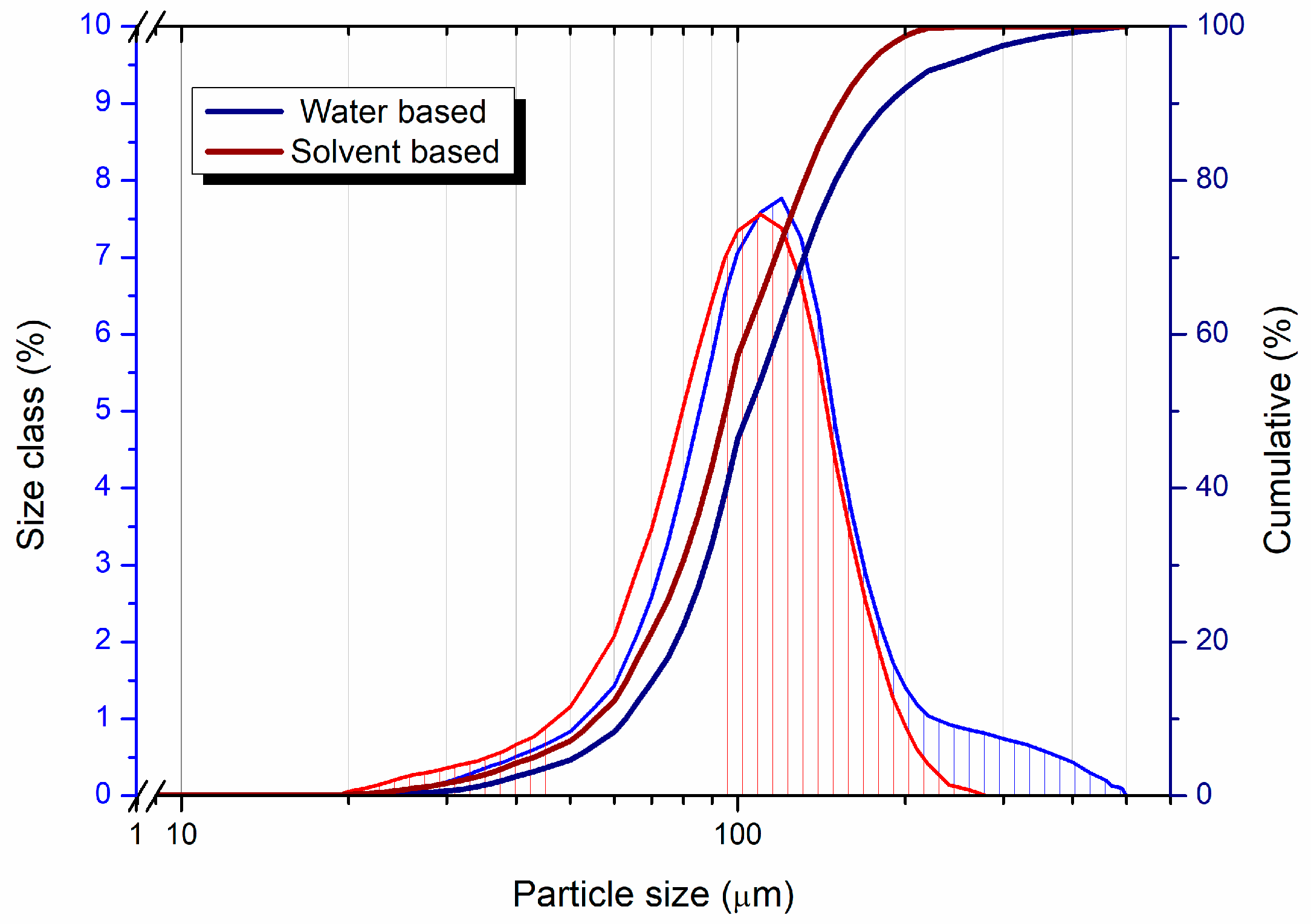
Figure 3 presents the particle size distributions of the water- and solvent-based granules.
Ultimately, these findings emphasize that the choice of solvent becomes less critical when drying conditions are carefully optimized. This opens the door for broader adoption of water-based spray drying in cemented carbide manufacturing and potentially other fields where spherical, uniform granules are required, thereby contributing to greener and more sustainable production practices [
11,
15]. The comparison of SEM images and particle size distributions (PSDs) of spray-dried WC-Co granules from water-based and solvent-based processes demonstrates the feasibility of achieving comparable granule characteristics, irrespective of the solvent used when appropriate drying conditions are applied. Both granules exhibit d50 values around 93 µm and 99 µm, as can be noticed in
Table 1, respectively, with similar spherical morphologies and smooth surfaces, as observed in the SEM images. These findings underscore the potential for water-based spray drying to produce granules of equal quality to solvent-based methods, provided that processing parameters are optimized. The similarity in PSDs indicates that the granule formation mechanisms are consistent across both processes. The narrow distribution and similar median sizes reflect well-controlled atomization and drying, essential for uniform granule behavior during subsequent pressing and sintering steps. The slight difference in d50 values (approximately 6%) is negligible from a practical perspective, suggesting that solvent minimally influences granule size when processing conditions are appropriately managed. The SEM images corroborate the PSD results, showing granules with smooth, defect-free surfaces and spherical shapes. Such features are crucial for achieving high packing density and minimizing voids during pressing, ensuring optimal mechanical properties in the final sintered product. The similar morphologies of the granules further support the hypothesis that the type of solvent used—whether water or an organic solvent—does not inherently determine granule quality. Achieving these results in the water-based process required careful optimization of spray-drying parameters, particularly the inlet temperature. The drying process effectively minimized differences between the two methods by tailoring the temperature to account for water’s higher latent heat of evaporation and surface tension. This finding is significant, as it demonstrates that the historical reliance on organic solvents for spray drying WC-Co powders is not necessarily justified by superior granule quality but rather by historical processing preferences and perceptions.
From an industrial perspective, the results hold considerable promise for transitioning toward more sustainable production methods. Water-based spray drying reduces dependency on hazardous organic solvents, lowering health and safety risks, environmental impact, and costs associated with solvent handling and recovery systems. Additionally, the comparable PSDs and SEM morphologies suggest that water-based processes can seamlessly replace solvent-based ones without compromising product performance or quality. In conclusion, the analysis of SEM images and particle size distributions highlights that, with optimized spray-drying parameters, water-based processes can match solvent-based methods in producing high-quality WC-Co granules. This equivalence provides a strong argument for re-evaluating traditional solvent-based practices, supporting a shift toward more sustainable, water-based manufacturing processes in the hard metal industry.
The comparison of water-based and solvent-based WC-Co granules shows similar performance in key metrics, including flowability and apparent density. The solvent-based granules, with a d50 of 93 µm, exhibit a flow time of 28.4 s and an apparent density of 3.14 g/cm
3, while the water-based granules, with a slightly larger d50 of 99 µm, achieve a faster flow time of 27.8 s and a higher density of 3.18 g/cm
3. These minor differences highlight that, with optimized spray-drying conditions, the water-based process can produce granules that match or even surpass those made with solvents. The slightly higher density and comparable flowability of water-based granules suggest improved packing efficiency, offering potential benefits in the final sintered product. These results and water-based processes’ environmental and safety advantages underscore their suitability as a sustainable alternative to traditional solvent-based methods. In
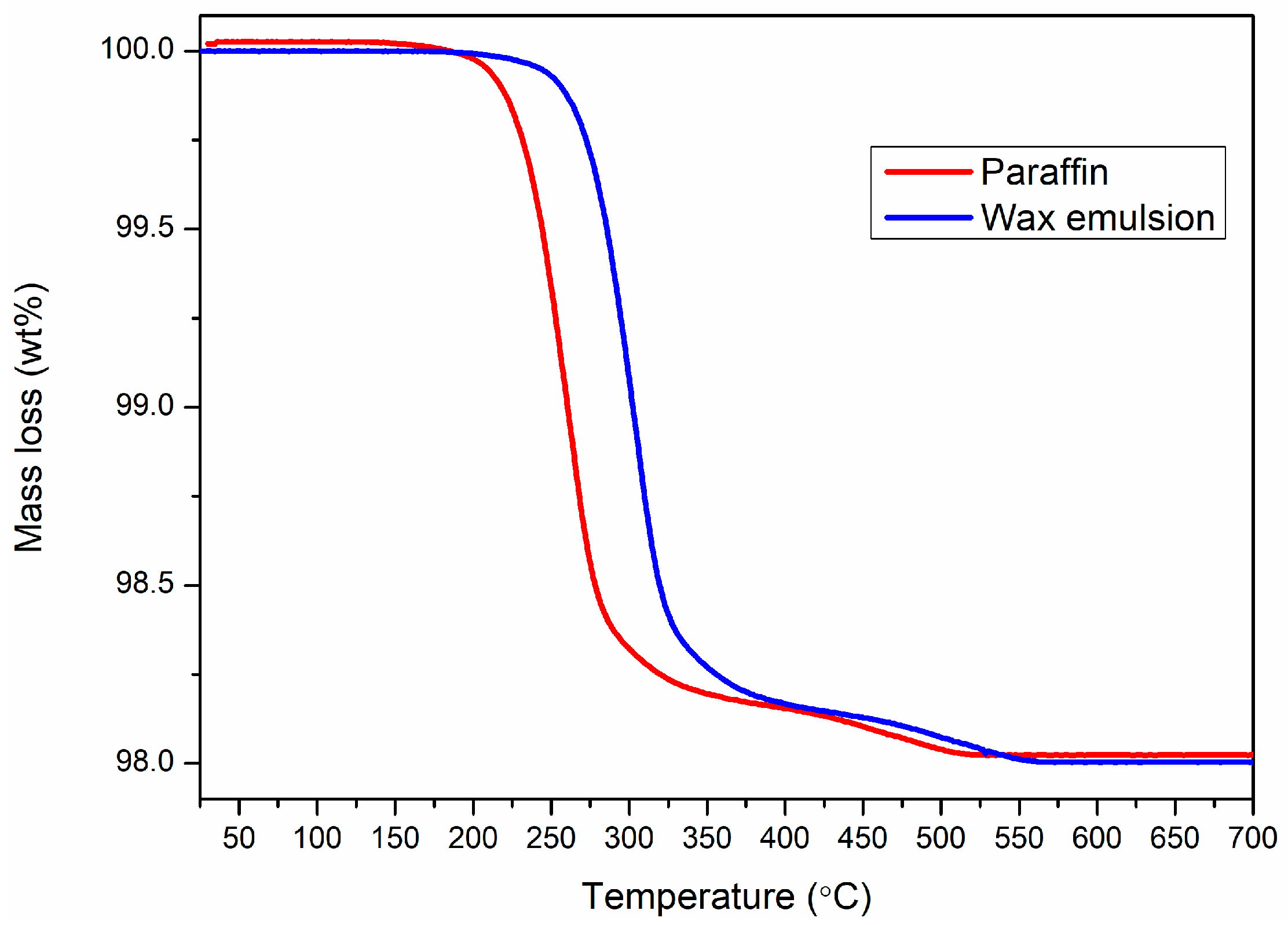
Figure 4 are given the TG curves corresponding to the two types of powders, highlighting that their debinding takes place in the 200–350-degree temperature range and that the plasticizer content after spraying is around 2%, a typical value for the granules in this field [
3,
16].
The 200–350 temperature range is typical for paraffin-based systems, with the decomposition temperature dictated by the molecular mass’s length [
17,
25]. High-molecular-mass paraffin wax decomposes in the 200–300 temperature range, whereas the low-molecular-mass paraffin decomposes at more significant temperatures, up to 350 [
26]. The slight shift in the thermal decomposition behavior can be dictated by the particle size distribution, as well as by the presence of emulsifiers.
The powder granules described above were directly pressed into cylindrical shapes and sintered using an industrially relevant sintering cycle, which included a H
2 overpressure during the debinding stage. This specific sintering approach ensures optimal binder removal and prevents oxidation, maintaining the integrity of the WC-Co composition. The results, presented in
Table 2, provide insights into the sintered samples’ densification, microstructure, and mechanical properties. These outcomes further validate the efficacy of the spray-dried powders, particularly those from water-based processes, in meeting the stringent performance criteria required for industrial applications.
As can be seen, the properties of the obtained samples are similar, and no noticeable difference between the two types of processing can be observed. The saturation magnetization values are consistent with the two-phase region of the WC-Co system, while the HV30 hardness and fracture toughness indicate a submicronic microstructure [
8]. The microstructures given in
Figure 5, obtained by optical microscopy, highlight the similarities between the two powders, which contain a homogeneous distribution of the two elements, without any cobalt pooling or porosity.