Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review
Abstract
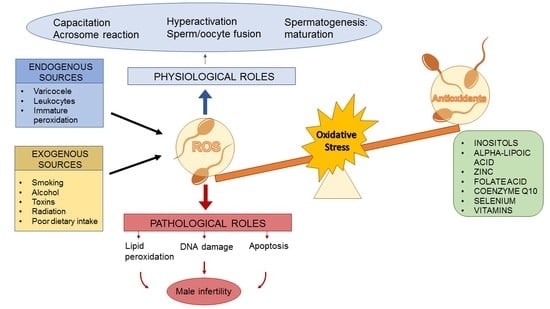
:1. Introduction
2. Materials and Methods
3. Inositols
4. Alpha-Lipoic Acid
5. Zinc
Zinc and Folic Acid
6. Coenzyme Q10
7. Selenium
8. Vitamins
8.1. Vitamin E
8.2. Vitamin C
8.3. Vitamin B12
9. About the Combination of Antioxidants
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Professionals S-O. EAU Guidelines: Male Sexual Dysfunction. Uroweb. Available online: https://uroweb.org/guideline/male-sexual-dysfunction/#3_2 (accessed on 23 March 2021).
- Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 23 December 2021).
- ICD-11. Available online: https://icd.who.int/en (accessed on 23 December 2021).
- Johnson, L. Efficiency of spermatogenesis. Microsc. Res. Tech. 1995, 32, 385–422. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosti, E.; Menezo, Y. Gamete activation: Basic knowledge and clinical applications. Hum. Reprod. Updat. 2016, 22, 420–439. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Boni, R.; Tosti, E. Gamete quality in a multistressor environment. Environ. Int. 2020, 138, 105627. [Google Scholar] [CrossRef]
- WHO Laboratory Manual for the Examination and Processing of Human Semen. Available online: https://www.who.int/publications-detail-redirect/9789240030787 (accessed on 15 January 2022).
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Updat. 2008, 14, 243–258. [Google Scholar] [CrossRef]
- MacLeod, J. The rôle of oxygen in the metabolism and motility of human spermatozoa. Am. J. Physiol. Leg. Content 1943, 138, 512–518. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, C. Reactive Oxygen Species and Male Fertility. Antioxidants 2020, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Ursini, F.; Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014, 73, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Menezo, Y.; Dale, B.; Coppola, G.; Dattilo, M.; Tosti, E.; Boni, R. Metabolic enhancers supporting 1-carbon cycle affect sperm functionality: An in vitro comparative study. Sci. Rep. 2018, 8, 11769. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Milostić-Srb, A.; Včev, A.; Tandara, M.; Marić, S.; Kuić-Vadlja, V.; Srb, N.; Holik, D. Importance of Zinc Concentration in Seminal Fluid of Men Diagnosed with Infertility. Acta Clin. Croat. 2020, 59, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Governini, L.; Ponchia, R.; Artini, P.G.; Casarosa, E.; Marzi, I.; Capaldo, A.; Luddi, A.; Piomboni, P. Respiratory Mitochondrial Efficiency and DNA Oxidation in Human Sperm after In Vitro Myo-Inositol Treatment. J. Clin. Med. 2020, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Capece, M.; Romeo, G.; Ruffo, A.; Romis, L.; Mordente, S.; Di Lauro, G. A Phytotherapic Approach to Reduce Sperm DNA Fragmentation in Patients with Male Infertility. Urol. J. 2016, 84, 79–82. [Google Scholar] [CrossRef]
- Manochantr, S.; Chiamchanya, C.; Sobhon, P. Relationship between chromatin condensation, DNA integrity and quality of ejaculated spermatozoa from infertile men. Andrologia 2011, 44, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Mens Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- Asadi, A.; Ghahremani, R.; Abdolmaleki, A.; Rajaei, F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. Int. J. Reprod. Biomed. 2021, 19, 493–504. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Chauvin, T.R.; Griswold, M.D. Characterization of the Expression and Regulation of Genes Necessary for myo-Inositol Biosynthesis and Transport in the Seminiferous Epithelium1. Biol. Reprod. 2004, 70, 744–751. [Google Scholar] [CrossRef]
- Rose, A.F.D.; Baldi, M.; Gallo, F.; Rossi, P.; Gattuccio, I.; Marino, A.; Canepa, P.; Mantica, G.; Paraboschi, I.; Gattuccio, F. The management of male infertility: From nutraceuticals to diagnostics. Int. J. Med. Device Adjuv. Treat. 2018, 1, e110. [Google Scholar]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does It Improve Sperm Mitochondrial Function and Sperm Motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef]
- Calogero, A.E.; Gullo, G.; La Vignera, S.; Condorelli, R.A.; Vaiarelli, A. Myoinositol improves sperm parameters and serum reproductive hormones in patients with idiopathic infertility: A prospective double-blind randomized placebo-controlled study. Andrology 2015, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Levin, M.H.; Verón, G.L. Myo-inositol in health and disease: Its impact on semen parameters and male fertility. Andrology 2020, 8, 277–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, S.; Cofini, M.; Rigante, D.; Leonardi, A.; Lucchetti, L.; Cipolla, C.; Lanciotti, L.; Penta, L. Inhibin B in healthy and cryptorchid boys. Ital. J. Pediatr. 2018, 44, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Boni, R.; Gallo, A.; Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 2016, 5, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Casarosa, E.; Carletti, E.; Monteleone, P.; Di Noia, A.; Di Berardino, O.M. In vitro effect of myo-inositol on sperm motility in normal and oligoasthenospermia patients undergoing in vitro fertilization. Gynecol. Endocrinol. 2016, 33, 109–112. [Google Scholar] [CrossRef]
- Brahim, S.F.; Osman, K.; Das, S.; Othman, A.M.; Majid, N.A.; Rahman, M.P.A. A study of the antioxidant effect of alpha lipoic acids on sperm quality. Clinics 2008, 63, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Bingham, P.M.; Stuart, S.D.; Zachar, Z. Lipoic acid and lipoic acid analogs in cancer metabolism and chemotherapy. Expert Rev. Clin. Pharmacol. 2014, 7, 837–846. [Google Scholar] [CrossRef]
- Ali, Y.F.; Desouky, O.S.; Selim, N.S.; Ereiba, K.M. Assessment of the role of α-lipoic acid against the oxidative stress of induced iron overload. J. Radiat. Res. Appl. Sci. 2015, 8, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Haghighian, H.K.; Haidari, F.; Mohammadi-Asl, J.; Dadfar, M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil. Steril. 2015, 104, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Taherian, S.S.; Khayamabed, R.; Tavalaee, M.; Nasr-Esfahani, M.H. Alpha-lipoic acid minimises reactive oxygen species-induced damages during sperm processing. Andrologia 2019, 51, e13314. [Google Scholar] [CrossRef] [PubMed]
- Di Tucci, C.; Galati, G.; Mattei, G.; Bonanni, V.; Capri, O.; D’Amelio, R.; Muzii, L.; Benedetti Panici, P. The role of alpha lipoic acid in female and male infertility: A systematic review. Gynecol. Endocrinol. 2021, 37, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol. Life Sci. 2019, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Molavi, N.; Tavalaee, M.; Abbasi, H.; Nasr-Esfahani, M.H. Alpha-lipoic acid improves sperm motility in infertile men after varicocelectomy: A triple-blind randomized controlled trial. Reprod. Biomed. Online 2020, 41, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Asa, E.; Ahmadi, R.; Mahmoodi, M.; Mohammadniya, A. Supplementation of freezing media with alpha lipoic acid preserves the structural and functional characteristics of sperm against cryodamage in infertile men with asthenoteratozoospermia. Cryobiology 2020, 96, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Zaman, S.; Sajjad, M.; Shoaib, M.; Gilani, G. Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int. J. Appl. Basic Med. Res. 2011, 1, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.R.; Khadivi, F.; Hashemi, F.; Bakhtiari, A. Effect of Zinc on Spermatogenesis and Sperm Chromatin Condensation in Bleomycin, Etoposide, Cisplatin Treated Rats. Cell J. Yakhteh 2019, 20, 521–526. [Google Scholar]
- Ishizuka, M.; Ohtsuka, E.; Inoue, A.; Odaka, M.; Ohshima, H.; Tamura, N.; Yoshida, K.; Sako, N.; Baba, T.; Kashiwabara, S.; et al. Abnormal spermatogenesis and male infertility in testicular zinc finger protein Zfp318 -knockout mice. Dev. Growth Differ. 2016, 58, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Björndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2009, 16, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Kothari, R.P.; Chaudhari, A.R. Zinc Levels in Seminal Fluid in Infertile Males and its Relation with Serum Free Testosterone. J. Clin. Diagn. Res. JCDR 2016, 10, CC05–CC08. [Google Scholar] [CrossRef] [PubMed]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Alsalman, A.R.S.; Almashhedy, L.A.; Alta’ee, A.H.; Hadwan, M.H. Effect of Zinc Supplementation on Urate Pathway Enzymes in Spermatozoa and Seminal Plasma of Iraqi Asthenozoospermic Patients: A Randomized Controlled Trial. Int. J. Fertil. Steril. 2019, 13, 315–323. [Google Scholar] [PubMed]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Nematollahi-Mahani, S.N.; Azizollahi, G.H.; Baneshi, M.R.; Safari, Z.; Azizollahi, S. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia 2014, 46, 240–245. [Google Scholar] [CrossRef]
- De Luca, M.N.; Colone, M.; Gambioli, R.; Stringaro, A.; Unfer, V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants 2021, 10, 1283. [Google Scholar] [CrossRef]
- Raigani, M.; Yaghmaei, B.; Amirjannti, N.; Lakpour, N.; Akhondi, M.M.; Zeraati, H.; Hajihosseinal, M.; Sadeghi, M.R. The micronutrient supplements, zinc sulphate and folic acid, did not ameliorate sperm functional parameters in oligoasthenoteratozoospermic men. Andrologia 2014, 46, 956–962. [Google Scholar] [CrossRef]
- Irani, M.; Amirian, M.; Sadeghi, R.; Le Lez, J.; Roudsari, R.L. The Effect of Folate and Folate Plus Zinc Supplementation on Endocrine Parameters and Sperm Characteristics in Sub-Fertile Men: A Systematic Review and Meta-Analysis. Urol. J. 2017, 14, 4069–4078. [Google Scholar] [CrossRef]
- Salvio, G.; Cutini, M.; Ciarloni, A.; Giovannini, L.; Perrone, M.; Balercia, G. Coenzyme Q10 and Male Infertility: A Systematic Review. Antioxidants 2021, 10, 874. [Google Scholar] [CrossRef]
- Lafuente, R.; González-Comadrán, M.; Solà, I.; López, G.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Fortification methods of coenzyme Q10 in yogurt and its health functionality—A review. Front Biosci. Sch. 2021, 13, 131. [CrossRef] [PubMed]
- Festa, R.; Giacchi, E.; Raimondo, S.; Tiano, L.; Zuccarelli, P.; Silvestrini, A.; Meucci, E.; Littarru, G.P.; Mancini, A. Coenzyme Q10 supplementation in infertile men with low-grade varicocele: An open, uncontrolled pilot study. Andrologia 2014, 46, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin. Exp. Reprod. Med. 2021, 48, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Nadjarzadeh, A.; Shidfar, F.; Amirjannati, N.; Vafa, M.; Motevalian, S.A.; Gohari, M.R.; Kakhki, S.A.N.; Akhondi, M.M.; Sadeghi, M.R. Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: A double-blind randomised clinical trial. Andrologia 2013, 46, 177–183. [Google Scholar] [CrossRef]
- García-Díaz, E.C.; Gómez-Quiroz, L.E.; Arenas-Ríos, E.; Aragón-Martínez, A.; Ibarra-Arias, J.A.; Retana-Márquez, M.D.S.I. Oxidative status in testis and epididymal sperm parameters after acute and chronic stress by cold-water immersion in the adult rat. Syst. Biol. Reprod. Med. 2015, 61, 150–160. [Google Scholar] [CrossRef]
- Thakur, A.S.; Littarru, G.P.; Funahashi, I.; Painkara, U.S.; Dange, N.S.; Chauhan, P. Effect of Ubiquinol Therapy on Sperm Parameters and Serum Testosterone Levels in Oligoasthenozoospermic Infertile Men. J. Clin. Diagn. Res. JCDR 2015, 9, BC01–BC03. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Grajeta, H. The role of selenium in human conception and pregnancy. J. Trace Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef]
- Mintziori, G.; Mousiolis, A.; Duntas, L.; Goulis, D.G. Evidence for a manifold role of selenium in infertility. Hormones 2019, 19, 55–59. [Google Scholar] [CrossRef]
- Foresta, C.; Flohé, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [Green Version]
- Michaelis, M.; Gralla, O.; Behrends, T.; Scharpf, M.; Endermann, T.; Rijntjes, E.; Pietschmann, N.; Hollenbach, B.; Schomburg, L. Selenoprotein P in seminal fluid is a novel biomarker of sperm quality. Biochem. Biophys. Res. Commun. 2014, 443, 905–910. [Google Scholar] [CrossRef]
- Mistry, H.D.; Pipkin, F.B.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talebi, S.; Arab, A.; Sorraya, N. The Association between Dietary Antioxidants and Semen Parameters: A Cross-Sectional Study among Iranian Infertile Men. Biol. Trace Elem. Res. 2021, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Mahmood, Z.; Shahid, M.; Saeed, M.U.Q.; Tahir, I.M.; Shah, S.A.; Munir, N.; El-Ghorab, A. Impact of reactive oxygen species on antioxidant capacity of male reproductive system. Int. J. Immunopathol. Pharmacol. 2015, 29, 421–425. [Google Scholar] [CrossRef] [Green Version]
- Cunha, L.M.S.C.P.D.; Teixeira, M.Y.P.; Daltro, A.F.C.S.; Torquato Filho, S.E.; de Assis, R.C.; Celedonio, R.F.; Pires, L.V.; Maia, C.S.C.; Guedes, M.I.F. Unbalance of Se and nutritional status in male infertility. JBRA Assist Reprod. 2021, 25, 202–208. [Google Scholar] [CrossRef]
- Ener, K.; Aldemir, M.; Işık, E.; Okulu, E.; Özcan, M.F.; Uğurlu, M.; Tangal, S.; Özayar, A. The impact of vitamin E supplementation on semen parameters and pregnancy rates after varicocelectomy: A randomised controlled study. Andrologia 2016, 48, 829–834. [Google Scholar] [CrossRef]
- Rengaraj, D.; Hong, Y.H. Effects of Dietary Vitamin E on Fertility Functions in Poultry Species. Int. J. Mol. Sci. 2015, 16, 9910–9921. [Google Scholar] [CrossRef] [Green Version]
- Cyrus, A.; Kabir, A.; Goodarzi, D.; Moghimi, M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: A double blind placebo controlled clinical trial. Int. Braz J Urol 2015, 41, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Hajjar, T.; Soleymani, F.; Vatanchian, M. Protective Effect of Vitamin C and Zinc as an Antioxidant against Chemotherapy-Induced Male Reproductive Toxicity. J. Med. Life 2020, 13, 138–143. [Google Scholar]
- Office of Dietary Supplements-Vitamin B12. Available online: https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/ (accessed on 19 December 2020).
- Banihani, S.A. Vitamin B12 and Semen Quality. Biomolecules 2017, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Hosseinabadi, F.; Jenabi, M.; Ghafarizadeh, A.A.; Yazdanikhah, S. The effect of vitamin B12 supplement on post-thaw motility, viability and DNA damage of human sperm. Andrologia 2020, 52, e13877. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.; Khoury, V.E.; Malakieh, R. Can Nutrition Help in the Treatment of Infertility? Prev. Nutr. Food Sci. 2021, 26, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Terai, K.; Horie, S.; Fukuhara, S.; Miyagawa, Y.; Kobayashi, K.; Tsujimura, A. Combination therapy with antioxidants improves total motile sperm counts: A Preliminary Study. Reprod. Med. Biol. 2019, 19, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Aquila, S.; Russo, G. Sperm performance in oligoasthenoteratozoospermic patients is induced by a nutraceuticals mix, containing mainly myo-inositol. Syst. Biol. Reprod. Med. 2020, 67, 50–63. [Google Scholar] [CrossRef]
- Scaruffi, P.; Licata, E.; Maccarini, E.; Massarotti, C.; Bovis, F.; Sozzi, F.; Stigliani, S.; Lago, A.D.; Casciano, I.; Rago, R.; et al. Oral Antioxidant Treatment of Men Significantly Improves the Reproductive Outcome of IVF Cycles. J. Clin. Med. 2021, 10, 3254. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.Z.; Hansen, K.R.; Barnhart, K.T.; Cedars, M.I.; Legro, R.S.; Diamond, M.P.; Krawetz, S.A.; Usadi, R.; Baker, V.L.; Coward, R.M.; et al. The Effect of Antioxidants on Male Factor Infertility: The MOXI Randomized Clinical Trial. Fertil. Steril. 2020, 113, 552–560.e3. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Ozer, C. Antioxidant Treatment of Increased Sperm DNA Fragmentation: Complex Combinations Are Not More Successful. Arch. Ital. Urol. E Androl. 2020, 92, 4. [Google Scholar] [CrossRef]
- Scroppo, F.I.; Costantini, E.; Zucchi, A.; Illiano, E.; Trama, F.; Brancorsini, S.; Crocetto, F.; Gismondo, M.R.; Dehò, F.; Mercuriali, A.; et al. COVID-19 disease in clinical setting: Impact on gonadal function, transmission risk, and sperm quality in young males. J. Basic Clin. Physiol. Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cilio, S.; Rienzo, M.; Villano, G.; Mirto, B.F.; Giampaglia, G.; Capone, F.; Ferretti, G.; Di Zazzo, E.; Crocetto, F. Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review. Oxygen 2022, 2, 1-11. https://doi.org/10.3390/oxygen2010001
Cilio S, Rienzo M, Villano G, Mirto BF, Giampaglia G, Capone F, Ferretti G, Di Zazzo E, Crocetto F. Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review. Oxygen. 2022; 2(1):1-11. https://doi.org/10.3390/oxygen2010001
Chicago/Turabian StyleCilio, Simone, Monica Rienzo, Gianluca Villano, Benito Fabio Mirto, Gaetano Giampaglia, Federico Capone, Gianpiero Ferretti, Erika Di Zazzo, and Felice Crocetto. 2022. "Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review" Oxygen 2, no. 1: 1-11. https://doi.org/10.3390/oxygen2010001
APA StyleCilio, S., Rienzo, M., Villano, G., Mirto, B. F., Giampaglia, G., Capone, F., Ferretti, G., Di Zazzo, E., & Crocetto, F. (2022). Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review. Oxygen, 2(1), 1-11. https://doi.org/10.3390/oxygen2010001