Prolonged Cold Ischemia Did Not Impair Mitochondrial Oxygen Consumption or Reactive Oxygen Species Production in Human Uterine Fundus and Horn Myometrium
Abstract
:1. Introduction
2. Population and Methods
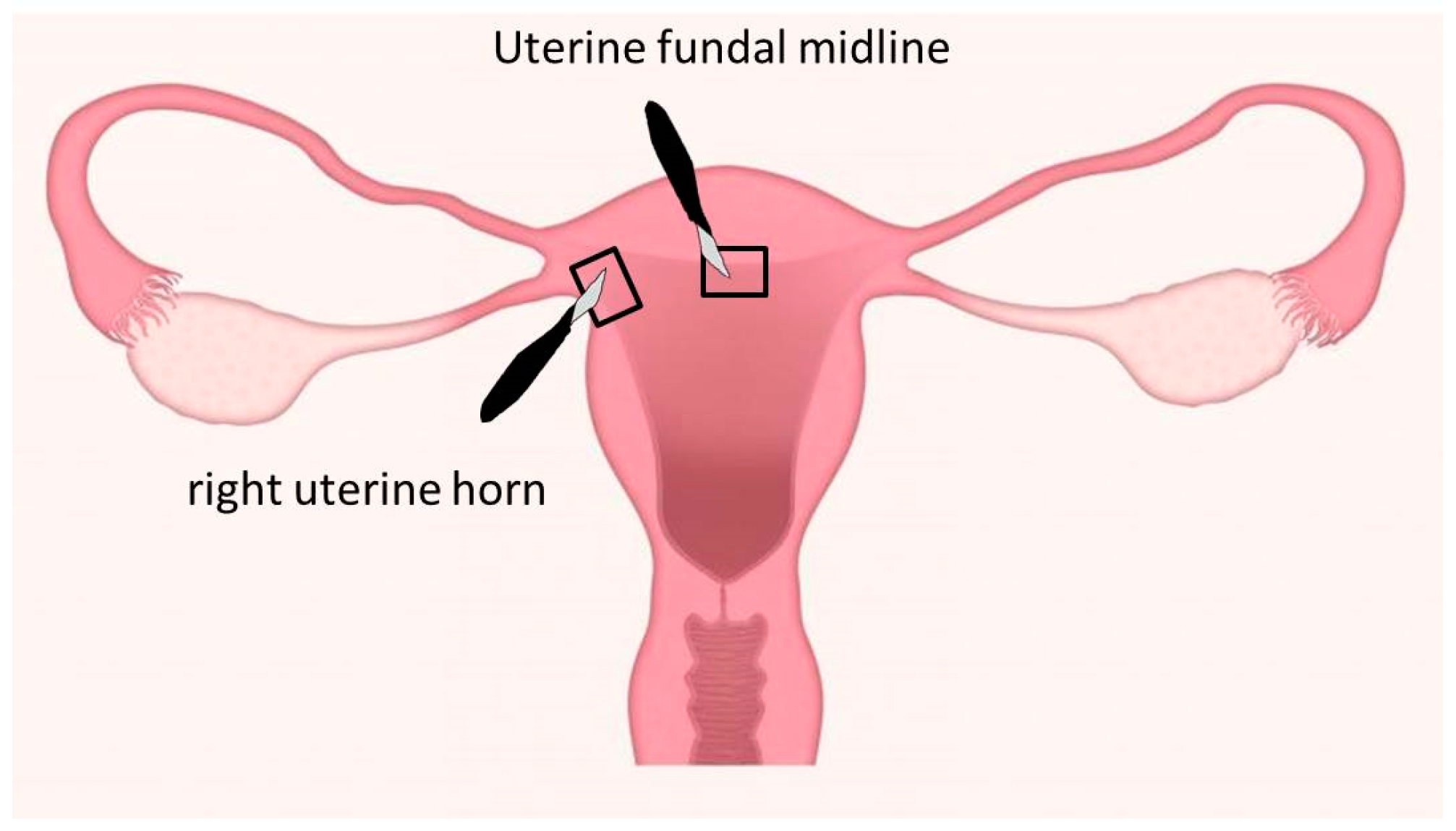
2.1. Study Design
2.2. Parameters Determined
2.2.1. Mitochondrial Use of Oxygen
2.2.2. Reactive Oxygen Species Production
2.2.3. Free Radical Leak
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Patients
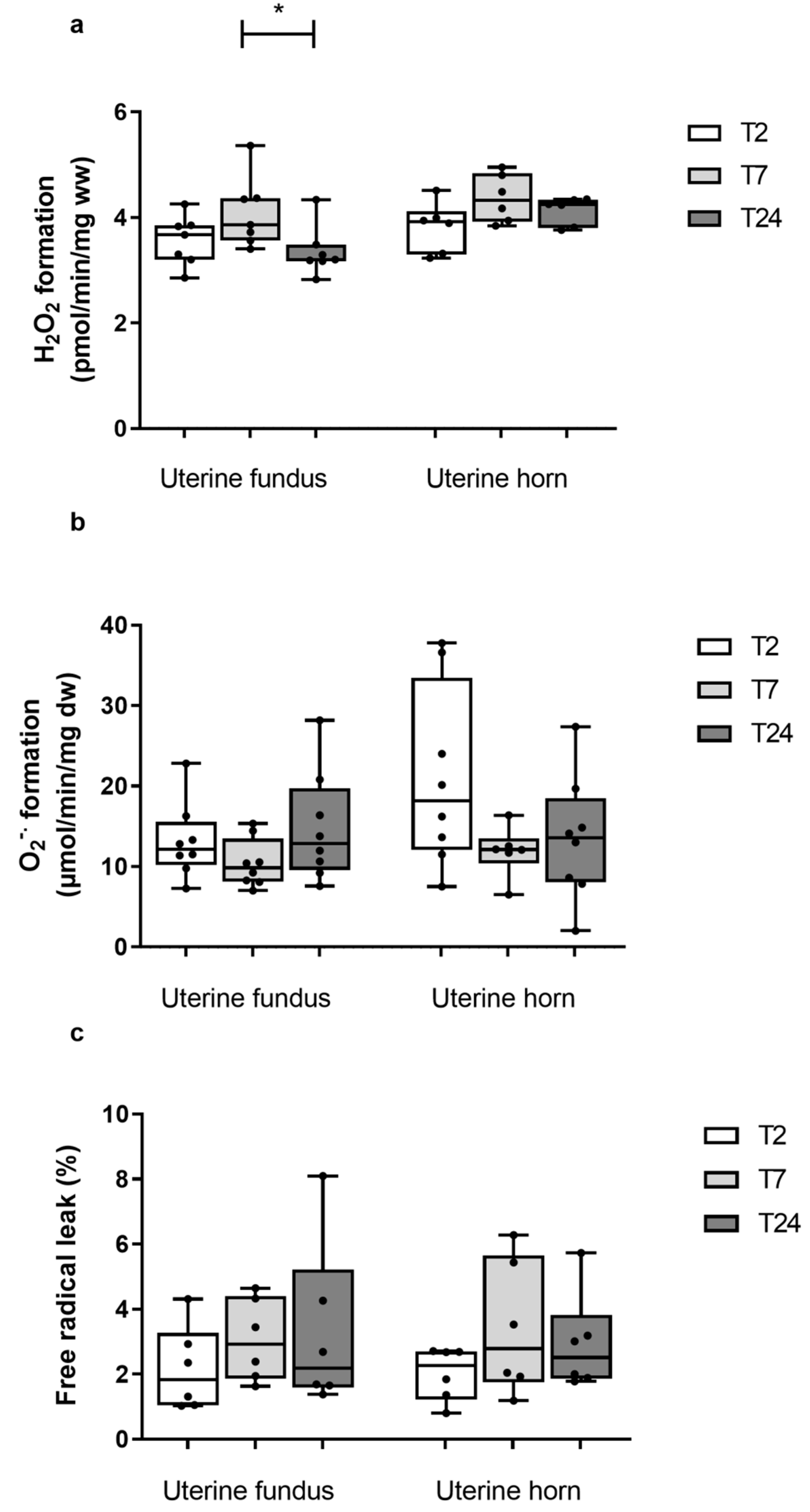
3.2. Mitochondrial Oxygen Consumption in Uterine Fundus and Horn after Two, Seven, and Twenty-Four Hours of Cold Ischemia
3.2.1. Uterine Fundus
3.2.2. Uterine Horn
3.3. ROS Production at the Uterine Fundus and Horn after Two, Seven, and Twenty-Four Hours of Cold Ischemia
3.3.1. Uterine Fundus
3.3.2. Uterine Horn
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milliez, J. Uterine Transplantation: FIGO Committee for the Ethical Aspects of Human Reproduction and Women’s Health. Int. J. Gynecol. Obstet. 2009, 106, 270. [Google Scholar] [CrossRef] [PubMed]
- Fageeh, W.; Raffa, H.; Jabbad, H.; Marzouki, A. Transplantation of the Human Uterus. Int. J. Gynecol. Obstet. 2002, 76, 245–251. [Google Scholar] [CrossRef]
- Ozkan, O.; Akar, M.E.; Ozkan, O.; Erdogan, O.; Hadimioglu, N.; Yilmaz, M.; Gunseren, F.; Cincik, M.; Pestereli, E.; Kocak, H.; et al. Preliminary Results of the First Human Uterus Transplantation from a Multiorgan Donor. Fertil. Steril. 2013, 99, 470–476.e5. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Johannesson, L.; Dahm-Kähler, P.; Enskog, A.; Mölne, J.; Kvarnström, N.; Diaz-Garcia, C.; Hanafy, A.; Lundmark, C.; Marcickiewicz, J.; et al. First Clinical Uterus Transplantation Trial: A Six-Month Report. Fertil. Steril. 2014, 101, 1228–1236. [Google Scholar] [CrossRef]
- Flyckt, R.; Kotlyar, A.; Arian, S.; Eghtesad, B.; Falcone, T.; Tzakis, A. Deceased Donor Uterine Transplantation. Fertil. Steril. 2017, 107, e13. [Google Scholar] [CrossRef] [Green Version]
- Testa, G.; Koon, E.C.; Johannesson, L.; McKenna, G.J.; Anthony, T.; Klintmalm, G.B.; Gunby, R.T.; Warren, A.M.; Putman, J.M.; de Prisco, G.; et al. Living Donor Uterus Transplantation: A Single Center’s Observations and Lessons Learned from Early Setbacks to Technical Success. Am. J. Transpl. 2017, 17, 2901–2910. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Xue, T.; Tao, K.-S.; Zhang, G.; Zhao, G.-Y.; Yu, S.-Q.; Cheng, L.; Yang, Z.-X.; Zheng, M.-J.; Li, F.; et al. Modified Human Uterus Transplantation Using Ovarian Veins for Venous Drainage: The First Report of Surgically Successful Robotic-Assisted Uterus Procurement and Follow-up for 12 Months. Fertil. Steril. 2017, 108, 346–356.e1. [Google Scholar] [CrossRef] [Green Version]
- Brucker, S.Y.; Brännström, M.; Taran, F.-A.; Nadalin, S.; Königsrainer, A.; Rall, K.; Schöller, D.; Henes, M.; Bösmüller, H.; Fend, F.; et al. Selecting Living Donors for Uterus Transplantation: Lessons Learned from Two Transplantations Resulting in Menstrual Functionality and Another Attempt, Aborted after Organ Retrieval. Arch. Gynecol. Obs. 2018, 297, 675–684. [Google Scholar] [CrossRef]
- Puntambekar, S.; Telang, M.; Kulkarni, P.; Jadhav, S.; Sathe, R.; Warty, N.; Puntambekar, S.; Kade, S.; Panse, M.; Agarkhedkar, N.; et al. Laparoscopic-Assisted Uterus Retrieval From Live Organ Donors for Uterine Transplant. J. Minim. Invasive Gynecol. 2018, 25, 571–572. [Google Scholar] [CrossRef]
- Puntambekar, S.; Puntambekar, S.; Telang, M.; Kulkarni, P.; Date, S.; Panse, M.; Sathe, R.; Agarkhedkar, N.; Warty, N.; Kade, S.; et al. Novel Anastomotic Technique for Uterine Transplant Using Utero-Ovarian Veins for Venous Drainage and Internal Iliac Arteries for Perfusion in Two Laparoscopically Harvested Uteri. J. Minim. Invasive Gynecol. 2019, 26, 628–635. [Google Scholar] [CrossRef]
- Brännström, M.; Enskog, A.; Kvarnström, N.; Ayoubi, J.M.; Dahm-Kähler, P. Global Results of Human Uterus Transplantation and Strategies for Pre-Transplantation Screening of Donors. Fertil. Steril. 2019, 112, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chmel, R.; Novackova, M.; Janousek, L.; Matecha, J.; Pastor, Z.; Maluskova, J.; Cekal, M.; Kristek, J.; Olausson, M.; Fronek, J. Revaluation and Lessons Learned from the First 9 Cases of a Czech Uterus Transplantation Trial: Four Deceased Donor and 5 Living Donor Uterus Transplantations. Am. J. Transpl. 2019, 19, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Saso, S.; Bracewell-Milnes, T.; Thum, M.; Nicopoullos, J.; Diaz-Garcia, C.; Friend, P.; Ghaem-Maghami, S.; Testa, G.; Johannesson, L.; et al. Human Uterine Transplantation: A Review of Outcomes from the First 45 Cases. BJOG Int. J. Obs. Gy. 2019, 126, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Koon, E.C.; Bayer, J.; McKenna, G.J.; Wall, A.; Fernandez, H.; Martinez, E.J.; Gupta, A.; Ruiz, R.; Onaca, N.; et al. Dallas UtErus Transplant Study: Early Outcomes and Complications of Robot-Assisted Hysterectomy for Living Uterus Donors. Transplantation 2021, 105, 225–230. [Google Scholar] [CrossRef]
- Jones, B.P.; Kasaven, L.; Vali, S.; Saso, S.; Jalmbrant, M.; Bracewell-Milnes, T.; Thum, M.-Y.; Quiroga, I.; Friend, P.; Diaz-Garcia, C.; et al. Uterine Transplantation: Review of Livebirths and Reproductive Implications. Transplantation 2021, 105, 1695–1707. [Google Scholar] [CrossRef]
- Tummers, P.; Göker, M.; Dahm-Kahler, P.; Brännström, M.; Tullius, S.G.; Rogiers, X.; Van Laecke, S.; Weyers, S. Meeting Report: First State-of-the-Art Meeting on Uterus Transplantation. Transplantation 2019, 103, 455–458. [Google Scholar] [CrossRef]
- Ejzenberg, D.; Andraus, W.; Baratelli Carelli Mendes, L.R.; Ducatti, L.; Song, A.; Tanigawa, R.; Rocha-Santos, V.; Macedo Arantes, R.; Soares, J.M.; Serafini, P.C.; et al. Livebirth after Uterus Transplantation from a Deceased Donor in a Recipient with Uterine Infertility. Lancet 2018, 392, 2697–2704. [Google Scholar] [CrossRef]
- Chmel, R.; Cekal, M.; Pastor, Z.; Chmel, R.; Paulasova, P.; Havlovicova, M.; Macek, M.; Novackova, M. Assisted Reproductive Techniques and Pregnancy Results in Women with Mayer-Rokitansky-Küster-Hauser Syndrome Undergoing Uterus Transplantation: The Czech Experience. J. Pediatric Adolesc. Gynecol. 2020, 33, 410–414. [Google Scholar] [CrossRef]
- Flyckt, R.; Falcone, T.; Quintini, C.; Perni, U.; Eghtesad, B.; Richards, E.G.; Farrell, R.M.; Hashimoto, K.; Miller, C.; Ricci, S.; et al. First Birth from a Deceased Donor Uterus in the United States: From Severe Graft Rejection to Successful Cesarean Delivery. Am. J. Obstet. Gynecol. 2020, 223, 143–151. [Google Scholar] [CrossRef]
- Brännström, M.; Bokström, H.; Dahm-Kähler, P.; Diaz-Garcia, C.; Ekberg, J.; Enskog, A.; Hagberg, H.; Johannesson, L.; Kvarnström, N.; Mölne, J.; et al. One Uterus Bridging Three Generations: First Live Birth after Mother-to-Daughter Uterus Transplantation. Fertil. Steril. 2016, 106, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, T.; Piver, P.; Pichon, N.; Bibes, R.; Guillaudeau, A.; Piccardo, A.; Pesteil, F.; Tricard, J.; Gardet, E.; Laskar, M.; et al. Uterus Retrieval Process from Brain Dead Donors. Fertil. Steril. 2014, 102, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Tricard, J.; Ponsonnard, S.; Tholance, Y.; Mesturoux, L.; Lachatre, D.; Couquet, C.; Terro, F.; Yardin, C.; Marquet, P.; Piccardo, A.; et al. Uterus Tolerance to Extended Cold Ischemic Storage after Auto-Transplantation in Ewes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 214, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Kisu, I.; Banno, K.; Matoba, Y.; Adachi, M.; Aoki, D. Basic Research on Uterus Transplantation in Nonhuman Primates in Japan: Uterus Transplant Research in Japan. J. Obstet. Gynaecol. Res. 2018, 44, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Pottecher, J.; Guillot, M.; Belaidi, E.; Charles, A.-L.; Lejay, A.; Gharib, A.; Diemunsch, P.; Geny, B. Cyclosporine A Normalizes Mitochondrial Coupling, Reactive Oxygen Species Production, and Inflammation and Partially Restores Skeletal Muscle Maximal Oxidative Capacity in Experimental Aortic Cross-Clamping. J. Vasc. Surg. 2013, 57, 1100–1108.e2. [Google Scholar] [CrossRef] [Green Version]
- Pizzimenti, M.; Riou, M.; Charles, A.-L.; Talha, S.; Meyer, A.; Andres, E.; Chakfé, N.; Lejay, A.; Geny, B. The Rise of Mitochondria in Peripheral Arterial Disease Physiopathology: Experimental and Clinical Data. JCM 2019, 8, 2125. [Google Scholar] [CrossRef] [Green Version]
- Paradis, S.; Charles, A.-L.; Meyer, A.; Lejay, A.; Scholey, J.W.; Chakfé, N.; Zoll, J.; Geny, B. Chronology of Mitochondrial and Cellular Events during Skeletal Muscle Ischemia-Reperfusion. Am. J. Physiol.-Cell Physiol. 2016, 310, C968–C982. [Google Scholar] [CrossRef] [Green Version]
- Charles, A.-L.; Guilbert, A.-S.; Guillot, M.; Talha, S.; Lejay, A.; Meyer, A.; Kindo, M.; Wolff, V.; Bouitbir, J.; Zoll, J.; et al. Muscles Susceptibility to Ischemia-Reperfusion Injuries Depends on Fiber Type Specific Antioxidant Level. Front. Physiol. 2017, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Diana, M.; Noll, E.; Diemunsch, P.; Dallemagne, B.; Benahmed, M.A.; Agnus, V.; Soler, L.; Barry, B.; Namer, I.J.; Demartines, N.; et al. Enhanced-Reality Video Fluorescence: A Real-Time Assessment of Intestinal Viability. Ann. Surg. 2014, 259, 700–707. [Google Scholar] [CrossRef]
- Lejay, A.; Charles, A.-L.; Georg, I.; Goupilleau, F.; Delay, C.; Talha, S.; Thaveau, F.; Chakfé, N.; Geny, B. Critical Limb Ischaemia Exacerbates Mitochondrial Dysfunction in ApoE−/− Mice Compared with ApoE+/+ Mice, but N-Acetyl Cysteine Still Confers Protection. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 576–582. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a Therapeutic Target for Common Pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Kamarauskaite, J.; Baniene, R.; Trumbeckas, D.; Strazdauskas, A.; Trumbeckaite, S. Increased Succinate Accumulation Induces ROS Generation in In Vivo Ischemia/Reperfusion-Affected Rat Kidney Mitochondria. BioMed Res. Int. 2020, 2020, 8855585. [Google Scholar] [CrossRef] [PubMed]
- Go, K.L.; Lee, S.; Zendejas, I.; Behrns, K.E.; Kim, J.-S. Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury. BioMed Res. Int. 2015, 2015, 183469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, Z.; Charles, A.L.; Kindo, M.; Pottecher, J.; Chamaraux-Tran, T.N.; Lejay, A.; Zoll, J.; Mazzucotelli, J.P.; Geny, B. Remote Effects of Lower Limb Ischemia-Reperfusion: Impaired Lung, Unchanged Liver, and Stimulated Kidney Oxidative Capacities. BioMed Res. Int. 2014, 2014, 392390. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Kisu, I.; Nagai, T.; Emoto, K.; Banno, K.; Umene, K.; Nogami, Y.; Tsuchiya, H.; Itagaki, I.; Kawamoto, I.; et al. Evaluation of Allowable Time and Histopathological Changes in Warm Ischemia of the Uterus in Cynomolgus Monkey as a Model for Uterus Transplantation. Acta Obs. Gynecol Scand. 2016, 95, 991–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardieu, A.; Dion, L.; Lavoué, V.; Chazelas, P.; Marquet, P.; Piver, P.; Sallée, C.; Aubard, Y.; Barin-Le Guellec, C.; Favreau, F.; et al. The Key Role of Warm and Cold Ischemia in Uterus Transplantation: A Review. JCM 2019, 8, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowska, J.; Wąsowska, B.; Gilun, P. Expression of Hypoxia Inducible Factor 1α and Antioxidant Enzymes: Superoxide Dismutases-1 and -2 in Ischemic Porcine Endometrium. Reprod. Biol. 2017, 17, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Del Priore, G.; Stega, J.; Sieunarine, K.; Ungar, L.; Smith, J.R. Human Uterus Retrieval From a Multi-Organ Donor. Obstet. Gynecol. 2007, 109, 101–104. [Google Scholar] [CrossRef]
- Guillot, M.; Charles, A.L.; Chamaraux-Tran, T.N.; Bouitbir, J.; Meyer, A.; Zoll, J.; Schneider, F.; Geny, B. Oxidative stress precedes skeletal muscle mitochondrial dysfunction during experimental aortic cross-clamping but is not associated with early lung, heart, brain, liver, or kidney mitochondrial impairment. J. Vasc. Surg. 2014, 60, 1043–1051. [Google Scholar] [CrossRef] [Green Version]
- Wranning, C.A.; Mölne, J.; El-Akouri, R.R.; Kurlberg, G.; Brännström, M. Short-Term Ischaemic Storage of Human Uterine Myometrium—Basic Studies towards Uterine Transplantation. Hum. Reprod. 2005, 20, 2736–2744. [Google Scholar] [CrossRef] [Green Version]
- Tardieu, A.; Chazelas, P.; Faye, P.-A.; Favreau, F.; Nadal-Desbarats, L.; Sallée, C.; Margueritte, F.; Couquet, C.-Y.; Marquet, P.; Guellec, C.B.-L.; et al. Changes in the Metabolic Composition of Storage Solution with Prolonged Cold Ischemia of the Uterus. J. Assist. Reprod. Genet. 2019, 36, 1169–1178. [Google Scholar] [CrossRef]

| Clinical Data | Mean ± SD, Min–Max |
|---|---|
| Age (mean ± SD, min–max) | 47.6 ± 3.9 (44–57) |
| Gravidity (mean ± SD, min–max) * | 2.37 ± 1.49 (0–5) |
| Parity (mean ± SD, min–max) ** | 2 ± 1.1 (0–4) |
| Smoker (n/8) | 1 |
| Post–menopausal (n/8) | 0 |
| Cardiovascular disease (n/8) | 2 |
| Indication (n/8) | 4 myomas–3 adenomyosis–1 prolapse |
| Type of hysterectomy (n/8) | 5 laparoscopic–3 vaginal–0 laparotomy |
| Histology(n/8) | 2 myomas–3 adenomyosis 1 sarcoma–1 normal tissue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pélissié, M.; Charles, A.-L.; Goupilleau, F.; Georg, I.; Bryand, A.; Geny, B.; Garbin, O. Prolonged Cold Ischemia Did Not Impair Mitochondrial Oxygen Consumption or Reactive Oxygen Species Production in Human Uterine Fundus and Horn Myometrium. Oxygen 2022, 2, 12-21. https://doi.org/10.3390/oxygen2010002
Pélissié M, Charles A-L, Goupilleau F, Georg I, Bryand A, Geny B, Garbin O. Prolonged Cold Ischemia Did Not Impair Mitochondrial Oxygen Consumption or Reactive Oxygen Species Production in Human Uterine Fundus and Horn Myometrium. Oxygen. 2022; 2(1):12-21. https://doi.org/10.3390/oxygen2010002
Chicago/Turabian StylePélissié, Mathilde, Anne-Laure Charles, Fabienne Goupilleau, Isabelle Georg, Angélique Bryand, Bernard Geny, and Olivier Garbin. 2022. "Prolonged Cold Ischemia Did Not Impair Mitochondrial Oxygen Consumption or Reactive Oxygen Species Production in Human Uterine Fundus and Horn Myometrium" Oxygen 2, no. 1: 12-21. https://doi.org/10.3390/oxygen2010002
APA StylePélissié, M., Charles, A.-L., Goupilleau, F., Georg, I., Bryand, A., Geny, B., & Garbin, O. (2022). Prolonged Cold Ischemia Did Not Impair Mitochondrial Oxygen Consumption or Reactive Oxygen Species Production in Human Uterine Fundus and Horn Myometrium. Oxygen, 2(1), 12-21. https://doi.org/10.3390/oxygen2010002






