Abstract
The objective of this study was to examine if diets differing in crude protein (CP) to metabolizable energy (ME) ratio (CP:ME) pre-weaning altered peripheral quantitative computed tomography (pQCT) measures of bone mass and strength in lambs. The left hind leg of lambs were available at the completion of a trial designed to examine the effect that altering the CP:ME ratio in milk replacer had on growth and body composition of pre-weaned lambs reared artificially. Treatments consisted of either normal commercial milk replacer (CMR, n = 10) containing 240 g/kg CP and 21.89 MJ/kg ME, high protein milk replacer (HPM, n = 9) containing CMR with additional milk protein concentrate to reach 478.7 g/kg CP and 19.15 MJ/kg ME or a mix of normal milk replacer and milk protein concentrate adjusted twice-weekly to match optimal CP:ME requirements (MB, n = 8) based on maintenance plus 300 g/d liveweight gain. At 22 kg live weight, lambs were euthanized and the tibia including the surrounding muscle was collected and scanned using pQCT at the mid-diaphysis. Lambs on the HPM and MB diets had a greater average daily gain (p < 0.01). There were limited differences in bone morphology and muscle mass, though notably the higher protein diets (MB and HPM) were associated with greater cortical thickness (p < 0.05) and, therefore, potentially greater peak bone mass at maturity This finding demonstrates that pre-weaning diets, and the protein content in particular, may influence the developmental potential of long bones and attainment of peak bone mass at maturity.
1. Introduction
In mammals, most growth occurs prior to puberty and, in many species, the period of most rapid growth and development occurs in the infantile growth phase, which in lambs represents the period up to weaning. Optimal bone growth requires significant protein intake as protein (in the form of collagen) constitutes 50% of bone volume and approximately one third of bone weight [1]. Adequate dietary protein also stimulates the release of insulin-like growth factor (IGF-1) resulting in an increase in muscle mass [2]. Increased muscle mass in turn stimulates appositional bone growth via increased strain (the mechanostat theorem and Wolff’s law) [3]. Muscle also secretes IGF-1 and other growth factors which have an anabolic effect on bone. This inherent synchronization of bone and muscle development has led to the concept of the “bone-muscle unit” [4].
In contrast to the anabolic effect of an adequate protein diet, a low protein diet results in a lower muscle and bone mass. In times of severe nutrient deficiency, body mass is reduced and hormones that control bone material properties are disrupted [5]. Times of severe inadequate nutrition result in a decrease in IGF-1 and decreased stimulation and proliferation of chondrocytes in the physis. Longitudinal bone growth is halted whilst there is a persistent nutritional deficit. If inadequate nutrition is overcome, longitudinal bone growth can resume but occurs at an accelerated rate. However, the effect on areal bone mineral density can be permanent such that 34.6% of adolescents affected with anorexia nervosa will present with osteoporosis and 30% will have a history of fractures [6].
Protein deficiency in cattle as a result of protein or energy malnutrition has been identified as a risk factor for spontaneous humeral fractures. Humeri from cattle affected by spontaneous humeral fractures have a reduced cortical bone thickness and signs of abnormal bone absorption [7,8]. Additionally, bones from affected cows have been shown to have decreased collagen content; with type 1 collagen making up 90% of bone protein or matrix content [9]. In cattle and sheep, the use of fodder beet (Beta vulgaris), a winter feed known to be low in protein and phosphorus, has been linked to metabolic bone disease [10,11]. Studies in pigs fed low protein rations have also resulted in the development of osteoporosis and retarded growth [12]. Interestingly, replacing the protein in the ration resulted in rapid increase in growth that was similar to that of controls. It is not surprising that there is such consistency of the response of bone to protein deficiency within mammals (such as cattle, sheep and humans) and this reflects why ovine models are used within human orthopedic research [13].
A common anatomical location to assess the bone muscle relationship in humans using peripheral quantitative computed tomography (pQCT) is the mid-tibia [14,15]. Mid-tibia sites have been used previously to describe muscle and bone growth [14], and investigate the effects of diet and exercise on fracture risk [15]. Previous studies in sheep have also used the tibia to describe bone morphology as it is easily accessible with the surrounding muscle intact enabling the potential for scans in live animals [16].
The pre-weaning diet of lambs may alter the pattern of growth and the developmental potential of the long bones associated with height. In an earlier study artificially reared lambs were reported to have greater stature than ewe-reared lambs but did not differ in other measures of bone morphology. However, the ewe-reared lambs had a greater cortical bone content to muscle area ratio, possibly due to differences in the crude protein (CP) to metabolizable energy (ME) ratio and the frequency of feeding [16]. Milk replacers tend to have a CP to ME ratio that may be inadequate for higher lamb growth rates in early life stage (~5 kg), but theoretically provide excess CP for requirements in later stages (~18 kg) [17]. Although ewe milk also has a CP:ME ratio lower than lamb requirements, the deficit is potentially compensated by the ability to consume more milk, or the presence of other growth stimulants in the milk [18].
The aim of this experiment was to examine if diets differing in CP:ME ratio fed pre-weaning alter the relationship of muscle mass with peripheral quantitative computed tomography (pQCT) measures of bone mass and strength in lambs.
2. Materials and Methods
2.1. Experimental Design and Animal Management
Hindlimbs of lambs that had been collected and stored as frozen samples at the completion of an earlier study examining the effects of differing CP:ME ratio fed on growth and body composition in pre-weaned lambs formed the sample construct for this study [19]. Thus, the sample size was dictated by the primary objective of the earlier study examining the effects of differing CP:ME ratio fed on growth and body composition rather than differentiation of the bone muscle unit between nutritional treatment groups.
Twenty-seven male twin-born Romney lambs (Ovis aries) were separated from their dam 24 h post-partum, housed indoors and fed milk replacer as previously described in detail by Herath, et al. [19]. Dams were grazed together, and lambs used were all born naturally. Briefly, the lambs were randomly assigned to one of three treatments with varying dietary CP:ME. These diets were, normal commercial milk replacer (CMR, n = 10) containing 240 g/kg CP and 21.89 MJ/kg ME (Milligan’s Feed Ltd., Oamaru, New Zealand), high protein milk replacer (HPM, n = 9) containing CMR with additional milk protein concentrate to reach 478.7 g/kg CP and 19.15 MJ/kg ME (Fonterra, Auckland, New Zealand) at a 62:38 ratio, or a mix of normal milk replacer and milk protein concentrate adjusted twice-weekly to match optimal CP:ME requirements (MB, n = 8) based on maintenance plus 300 g/d liveweight (LW) gain. All lambs were fed milk replacer at 2.1 times their maintenance energy requirement calculated using MEm = 0.40 MJ/kgLW0.75d−1 [17], and pellets (composed of barley, broll, soya bean and molasses containing 180 g/kg CP and 11.5 MJ/kg ME) were fed ad libitum from two weeks of age. Each feed, milk powder for each lamb was mixed with warm tap water at a 1:4 (w/w) ratio. Lambs were bottle fed five times a day (at 7.00 a.m., 10.30 a.m., 2.30 p.m., 6.30 p.m. and 9.00 p.m.) until two weeks of age where they were fed four times a day (7.00 a.m., 10.30 a.m., 2.30 p.m. and 7.00 p.m.) until four weeks of age and finally three times a day (7.00 a.m., 1.00 p.m. and 7.00 p.m.) until slaughter. All lambs had free access to water.
2.2. Slaughter
When lambs reached a target live weight of 22 kg (regardless of age), they were humanely euthanized via captive bolt and exsanguination. The left hind legs were dissected from the skinned carcass at the proximal end of the femur, and the distal end of the tibia (through the tarsal bones distal to the calcaneus and proximal to the metatarsal bones). The legs were double wrapped in plastic wrap and stored at −20 °C until pQCT scanning. For the purposes of this study, data were obtained from the previous study for live weight at day one and at slaughter, and hot carcass weight (HCW) [19].
2.3. Peripheral Quantitative Computed Tomography (pQCT)
Each limb was defrosted to allow palpation of the femorotibial joint, and tibia length was measured from the distal end of the lateral malleolus to the proximal edge of the tibial tuberosity. An XCT-2000 scanner (pQCT; XCT 2000, Stratec Medical, Pforzheim, Germany) was used to scan the mid-diaphysis of the tibia at 50% of the total bone length with a voxel size of 0.3 mm3. All parameters were extracted from the pQCT software as an Excel file using contour mode 1 and peel mode 1. The total cross-sectional area for muscle at the mid-diaphysis of the tibia was determined in the parameter using a threshold for muscle of 40 mg/cm3, for bone of 280 mg/cm3, and cortical bone as >710 mg/cm3. The pQCT measurements of bone included total bone area, total bone content, total bone density, cortical bone area, cortical bone density, cortical bone thickness, periosteal circumference, endosteal circumference and stress-strain index.
2.4. Statistical Analysis
The integrity of the data was assessed using histograms and density plots, and box and whisker plots to identify potential outliers. Data were tested for normality using the Shapiro–Wilks test with a threshold of p > 0.05. Means and standard error for bone parameters were calculated using a general linear model with the fixed effect of treatment. When treatment (diet) was significant (p < 0.05), the respective pQCT bone measurement was plotted against hot carcass weight. Statistical analysis was conducted using R version 4.2.0 and RStudio (R Core Team, 2022; R Studio Team, 2022, Vienna, Austria). Average daily gain was calculated as: (final weight-birth weight)/age.
3. Results
Relevant slaughter data as presented in Herath, et al. [19] and tibia length are summarized in Table 1. Average daily gain was greatest in lambs fed the HPM and MB diets (Table 1 and Figure 1) resulting in a tendency for lambs to be younger at the 22 kg slaughter weight. Tibia length was not significantly different between treatments (p = 0.37).

Table 1.
Measures of lambs fed milk replacers with variable crude protein to metabolizable energy ratio (mean ± SE, commercial milk replacer (CMR), high protein milk (HPM), milk blend (MB)) and slaughtered at 22 kg live weight.
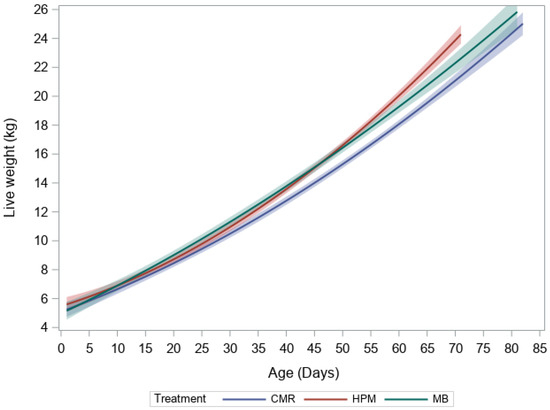
Figure 1.
Second order polynomial with 95% confidence interval for live weight and age of lambs fed commercial milk replacer (blue), high protein milk (red), milk blend (green) diets and slaughtered at 22 kg live weight.
Lambs fed the HPM and MB diets had a greater cortical bone thickness (~6%) and greater ratio of cortical bone thickness (10%) to tibia length than lambs on the CMR diet (p ≤ 0.05, Table 2). There was no significant effect of diet on measures of bone area/circumference and muscle area surrounding the tibia.

Table 2.
The peripheral quantitative computed tomography parameters at the mid-diaphyseal site of the tibia for lambs fed milk replacers with variable crude protein to metabolizable energy ratio (mean ± SE, commercial milk replacer (CMR), high protein milk (HPM), milk blend (MB)) and slaughtered at 22 kg live weight.
There was a positive linear relationship between hot carcass weight with cortical bone thickness and cortical bone thickness to tibia bone length ratio (Figure 2). Treatment remained significant when hot carcass weight was fitted as a covariate (p < 0.05)
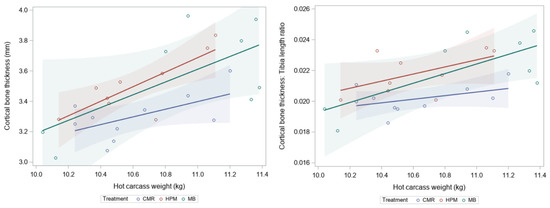
Figure 2.
Scatterplot with regression lines and 95% confidence intervals for cortical bone thickness (left) and the ratio of cortical bone thickness to tibial bone length (right) in the mid-diaphysis against hot carcass weight of lambs fed commercial milk replacer (blue), high protein milk (red), milk blend (green) diets and slaughtered at 22 kg live weight.
4. Discussion
To achieve target slaughter weights, the CMR lambs with the lower growth rate were approximately 5 days older than the MB and HPM lambs. The tendency for a longer tibia in the CMR lambs reflected that these lambs were older at slaughter. There was no difference in the periosteal circumference to tibia length ratio between diets indicating that periosteal circumference may be highly conserved relative to bone length irrespective of diet [20]. This was not unexpected due to the major contribution periosteal circumference has in the resistance to bending strain in long bones. The greater cortical bone thickness and ratio of cortical bone thickness to tibia length in lambs on HPM and MB diets may reflect the effect of increased dietary protein on bone development. The provision of a high protein diet stimulates an increase in bone deposition on the endosteal surface. Although preweaning growth is heavily constrained [21], the difference in cortical bone thickness was significant demonstrating the biological importance of adequate dietary protein on bone material properties in young mammals [22]. Human models have suggested a similar mechanism whereby poor nutrition offered to children due to low socioeconomic backgrounds results in a lower cortical bone thickness and delayed bone age than children raised in higher socioeconomic backgrounds with access to better nutrition [23,24]. The effect of socioeconomic background on bone length and diameter was not observed indicating that cortical bone thickness may be a better indicator of nutritional status [24]. In dairy cattle periods of restricted nutrition, particularly protein intake, during the first and second winter before the first lactation have been associated with reduced cortical thickness of long bones [8]. These data indicate that cortical thickness in long bones may be a sensitive measure of adequate nutrition, particularly protein intake across the different stages of growth.
Differences in diet composition resulted in a greater average daily gain for lambs raised on HPM and MB diets. Approximately two thirds of average daily gain was contributed to by increases in carcass and viscera plus blood weight as previously reported by Herath, et al. [19]. Greater protein in the HPM and MB diet allowed for a greater growth rate than lambs on the CMR diet. However, the additional protein in the HPM diet compared to the MB did not result in a greater growth rate compared to the MB diet. It was suggested in Herath, et al. [19], that additional metabolizable energy would be required in the HPM diet to permit lambs to efficiently utilise the additional dietary protein. However, diet did not appear to have an effect on carcass characteristics such as carcass weight and dressing out percentage [19]. In addition, differences between groups in average daily gain did not affect the relationship of bone morphology with muscle area in the mid-diaphysis of the tibia, with the same muscle to bone ratio and muscle area surrounding the tibia between treatments. This reflects that bone development and its relationship to muscle mass is heavily conserved [16,21]. Bone and muscle are highly related such that an increase in muscle mass or activity of muscles (loading) will result in greater bone strength [3]. Therefore, as the lambs were all slaughtered at the same weight, and the close relationship between liveweight and bone morphology, it is perhaps unsurprising that limited changes in bone morphology were observed and reinforces this critical relationship between liveweight and bone morphology measures. It is interesting, that despite different average daily gains and differences in daily protein intake, the amount of muscle laid down by the lambs was consistent at a given carcass weight, albeit at a later age in the CMR group, again suggesting a highly conserved growth pattern.
The limited differences in bone morphology and measures of strength agrees with previous lamb [16] and calf studies [20], where alterations in milk intake or rearing system did not affect bone morphology prior to weaning. However, it has been suggested that inadequate pre-weaning nutrition can have detrimental effects on future growth and affect how bone responds to future nutritional and environmental events [21]. The increased cortical thickness, while not greatly contributing to bone strength, does represent greater reserves available for future growth, and potentially greater peak bone mass at maturity. These results from ruminant studies are consistent with findings from prospective cohort studies within the human literature where pre-weaning milk intake did not grossly affect bone morphology at the time but can affect how bone responds to future challenges and ultimately future growth [25,26].
The material used in this study was obtained from samples stored after the completion of the earlier trial. The opportunistic nature of this material collected restricted the sample size and meant reliance on growth parameters previous collected with a focus on body composition (soft tissue) rather than bone growth and development [19]. Examination of the growth curves indicated that an additional week of growth as the lambs progressed into the late pre-weaning growth period may have been required to be able to identify differences between treatment groups for a number of pQCT parameters. The termination of the trial when the animals obtained a set live weight did provide a confounding factor when examining the effect of the diet on the timing of growth. Previous research indicated that diet (milk replacers vs ewes milk) did not affect bone morphology in the tibia, but affected stature growth [16]. This observation and the data presented here demonstrates that the pattern of pre-weaning growth is heavily constrained. Previous work by our group identified differences between ewe-reared and CMR lambs for bone:muscle ratio [16]. The inability to identify differences in this parameter between treatment may be related to the conservation of the pattern of growth and slaughter at a set carcass weight. In addition, there was variation in the way the leg was disarticulated from the body. Although the muscle surrounding the tibia was intact, muscles surrounding the lateral malleolus were cut unevenly and measures of leg weight could not be taken.
In order to scan the legs they had to be defrosted and straightened to provide consistency in positioning with scanning. As a disarticulated limb the muscles in the leg may be in a different position and tension compared with an intact limb. Therefore, measures of total muscle area may not accurately reflect the muscularity of the lamb and the muscle to bone ratio observed in vivo. However, the limbs were placed in a consistent position within the scanner and this limitation may only impact comparison of absolute muscle circumference values between intact and disarticulated limbs and not the relative ranking of values.
In addition, the study was limited in the number of animals in each treatment group which reduced the power of this study. Retrospective power analysis identified that in order to detect a significant difference between diets in the observed means for total bone content, approximately 28 lambs would be needed in each group. However, the sample size was sufficient to detect the more sensitive measures of protein content of the diet via measurement of cortical thickness despite the heavy conservation of the growth and development profile in mammals. Therefore, the current study demonstrates the need for a larger scale trial examining the effects of pre-weaning diet on subsequent bone morphology.
5. Conclusions
Although a higher CP:ME diet resulted in a greater growth rate in lambs reared on commercial milk replacer, there were limited differences in bone morphology and heavy conservation of the relationship of bone parameters with muscle area. The greater cortical bone thickness with high protein diets emphasizes the importance of adequate protein intake for bone deposition in young mammals and the influence of early nutrition on the attainment of peak bone mass. It is possible that, despite limited gross changes in morphology observed pre-weaning that the developmental potential and response of bone to future challenges may still have been altered. Future research should examine how the pre-weaning diet affects future growth post weaning and how this moderates bones response to nutritional challenges.
Author Contributions
Conceptualization, C.W.R. and M.J.G. methodology, C.W.R., M.J.G. and B.R.A.; formal analysis, B.R.A. and C.W.R.; investigation, B.R.A. and H.M.G.P.H.; data curation, B.R.A.; writing—original draft preparation, B.R.A., M.J.G. and C.W.R.; writing—review and editing M.J.G., B.R.A., P.J.B., K.E.D., S.J.P., H.T.B., P.C.H.M., P.R.K., C.W.R. and H.M.G.P.H.; funding acquisition, C.W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Gravida (National Centre for Growth and Development) and The New Zealand Equine Trust, grant number “9/2017”.
Institutional Review Board Statement
The experiment was carried out at Massey University, Palmerston North, New Zealand, with approval from the Massey University Animal Ethics Committee (MUAEC 17/48).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request from first author.
Acknowledgments
The authors acknowledge the technical assistance of Catriona Jenkinson.
Conflicts of Interest
The authors declare no real or perceived conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wallace, T.C. Optimizing dietary protein for lifelong bone health: A paradox unraveled. Nutr. Today 2019, 54, 107–115. [Google Scholar] [CrossRef]
- Schüler, R.; Markova, M.; Osterhoff, M.A.; Arafat, A.; Pivovarova, O.; Machann, J.; Hierholzer, J.; Hornemann, S.; Rohn, S.; Pfeiffer, A.F. Similar dietary regulation of IGF-1-and IGF-binding proteins by animal and plant protein in subjects with type 2 diabetes. Eur. J. Nutr. 2021, 60, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.-J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [PubMed]
- Turner, J.; Bulsara, M.; McDermott, B.; Byrne, G.; Prince, R.; Forbes, D. Predictors of low bone density in young adolescent females with anorexia nervosa and other dieting disorders. Int. J. Eat. Disord. 2001, 30, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.K.; Grinspoon, S.K.; Ciampa, J.; Hier, J.; Herzog, D.; Klibanski, A. Medical Findings in Outpatients With Anorexia Nervosa. Arch. Intern. Med. 2005, 165, 561–566. [Google Scholar] [CrossRef]
- Gibson, M.; Dittmer, K.; Hickson, R.; Back, P.; Wehrle-Martinez, A.; Rogers, C. The Mid-Diaphysis Is a Poor Predictor of Humeral Fracture Risk Indicating That Predisposing Factors Are Recent. Ruminants 2021, 1, 23–30. [Google Scholar] [CrossRef]
- Wehrle-Martinez, A.; Lawrence, K.; Back, P.J.; Rogers, C.W.; Gibson, M.; Dittmer, K.E. Osteoporosis is the cause of spontaneous humeral fracture in dairy cows from New Zealand. Vet. Pathol. 2022; ahead of print. [Google Scholar]
- Wehrle-Martinez, A.; Naffa, R.; Back, P.; Rogers, C.W.; Lawrence, K.; Loo, T.; Sutherland-Smith, A.; Dittmer, K. Novel Assessment of Collagen and Its Crosslink Content in the Humerus from Primiparous Dairy Cows with Spontaneous Humeral Fractures Due to Osteoporosis from New Zealand. Biology 2022, 11, 1387. [Google Scholar] [CrossRef]
- Rickets in heifers grazing fodder beet. Vet. Rec. 2022, 190, 407–410. [CrossRef]
- Bones in Protein Deficiency. Nutr. Rev. 1963, 21, 242–244. [CrossRef]
- Stewart, R.J.C.; Platt, B.S. Dietary protein deficiency and growth and mineralisation of bones of pig. In Proceedings of the Nutrition Society; CAB International: Wallingford, UK, 1961; Volume 20. [Google Scholar]
- Cabrera, D.; Wolber, F.M.; Dittmer, K.; Rogers, C.; Ridler, A.; Aberdein, D.; Parkinson, T.; Chambers, P.; Fraser, K.; Roy, N.C. Glucocorticoids affect bone mineral density and bone remodelling in OVX sheep: A pilot study. Bone Rep. 2018, 9, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nicholson, P.; Wang, Q.; Alén, M.; Cheng, S. Bone and muscle development during puberty in girls: A seven-year longitudinal study. J. Bone Miner. Res. 2009, 24, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Frølich, J.; Hansen, S.; Winkler, L.A.-D.; Andresen, A.K.; Hermann, A.P.; Støving, R.K. The role of body weight on bone in anorexia nervosa: A HR-pQCT study. Calcif. Tissue Int. 2017, 101, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.J.; Rogers, C.W.; Pettigrew, E.J.; Pain, S.J.; Dittmer, K.E.; Herath, H.M.G.P.; Back, P.J. The Effect of Artificial Rearing on Live Weight Gain and Bone Morphology of the Tibia in Lambs Prior to Weaning. Ruminants 2022, 2, 101–111. [Google Scholar] [CrossRef]
- Danso, A.; Morel, P.; Kenyon, P.; Blair, H. Effect of different feeding regimens on energy and protein utilization and partitioning for maintenance and growth in pre-weaned lambs reared artificially. J. Anim. Sci. 2016, 94, 5359–5371. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Khan, M.; Lee, W.; Yang, S.; Kim, S.; Ki, K.; Kim, H.; Ha, J.; Choi, Y. Influence of equalizing the gross composition of milk replacer to that of whole milk on the performance of Holstein calves. J. Anim. Sci. 2009, 87, 1129–1137. [Google Scholar] [CrossRef]
- Herath, H.; Pain, S.; Kenyon, P.; Blair, H.; Morel, P. Effect of dietary protein to energy ratio of milk replacer on growth and body composition of pre-weaned lambs reared artificially. Anim. Feed Sci. Technol. 2020, 264, 114478. [Google Scholar] [CrossRef]
- Gibson, M.; Dittmer, K.; Hickson, R.; Back, P.; Rogers, C. Bone Morphology and Strength in the Mid-Diaphysis of the Humerus and Metacarpus in Dairy Calves Prior to Weaning. Animals 2020, 10, 1422. [Google Scholar] [CrossRef]
- Moallem, U.; Werner, D.; Lehrer, H.; Zachut, M.; Livshitz, L.; Yakoby, S.; Shamay, A. Long-term effects of ad libitum whole milk prior to weaning and prepubertal protein supplementation on skeletal growth rate and first-lactation milk production. J. Dairy Sci. 2010, 93, 2639–2650. [Google Scholar] [CrossRef]
- Vatanparast, H.; Bailey, D.A.; Baxter-Jones, A.D.; Whiting, S.J. The effects of dietary protein on bone mineral mass in young adults may be modulated by adolescent calcium intake. J. Nutr. 2007, 137, 2674–2679. [Google Scholar] [CrossRef]
- Yayha, Z.; Millward, D.J. Dietary protein and the regulation of long-bone and muscle growth in the rat. Clin. Sci. 1994, 87, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Mays, S.; Ives, R.; Brickley, M. The effects of socioeconomic status on endochondral and appositional bone growth, and acquisition of cortical bone in children from 19th century Birmingham, England. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2009, 140, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Cawley, M.; Bhalla, A.; Egger, P.; Ring, F.; Morton, L.; Barker, D. Childhood growth, physical activity, and peak bone mass in women. J. Bone Miner. Res. 1995, 10, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Robinson, S.M.; Crozier, S.R.; Marriott, L.D.; Gale, C.R.; Cole, Z.A.; Inskip, H.M.; Godfrey, K.M.; Cooper, C. Breast-feeding and adherence to infant feeding guidelines do not influence bone mass at age 4 years. Br. J. Nutr. 2009, 102, 915–920. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).