Evaluating the Contribution of Sporosarcina to Carbonate Precipitation in Anaerobic Soils: A Microbial Community and Quantitative Analysis
Abstract
1. Introduction
2. Materials and Methods
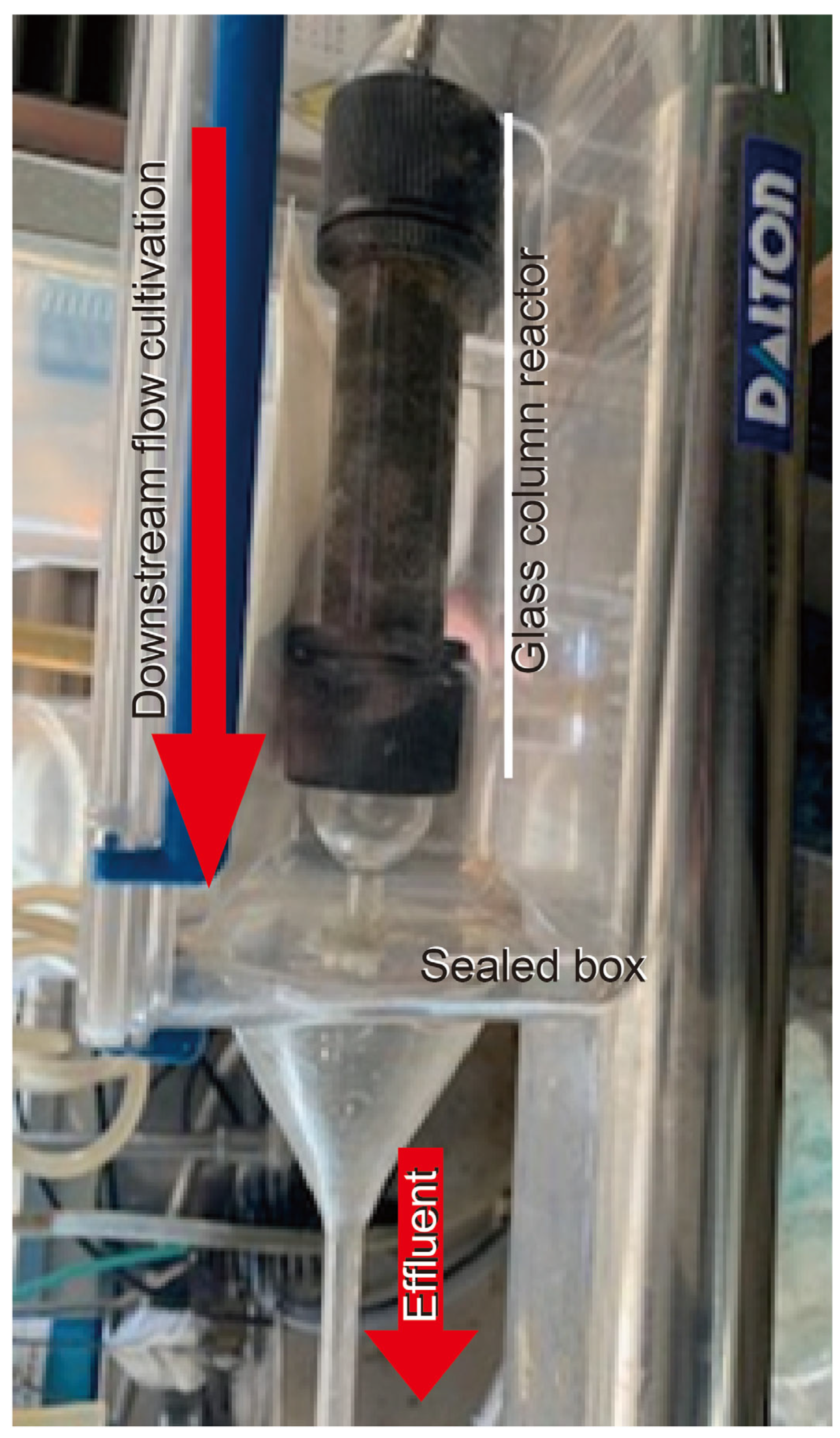
2.1. Reactor Operational Conditions
2.1.1. Continuous Reactors
2.1.2. Batch Reactors
2.2. DNA Extraction and Community Structure Analysis
2.3. qPCR Conditions
2.4. Declaration of Generative AI and AI-Assisted Technologies in the Writing Process
3. Results and Discussions
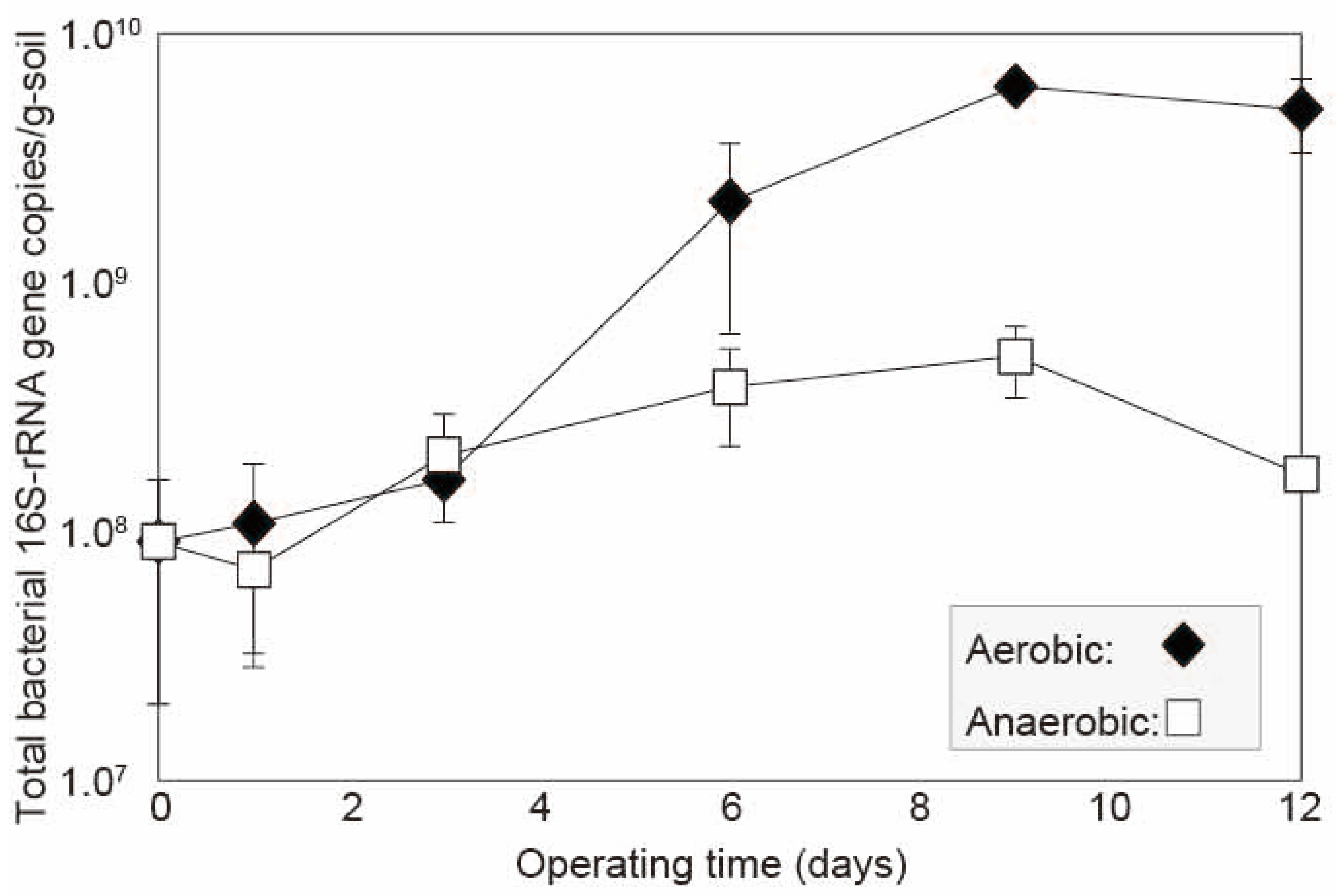
3.1. Comparative Dynamics of Aerobic and Anaerobic Bacterial Populations
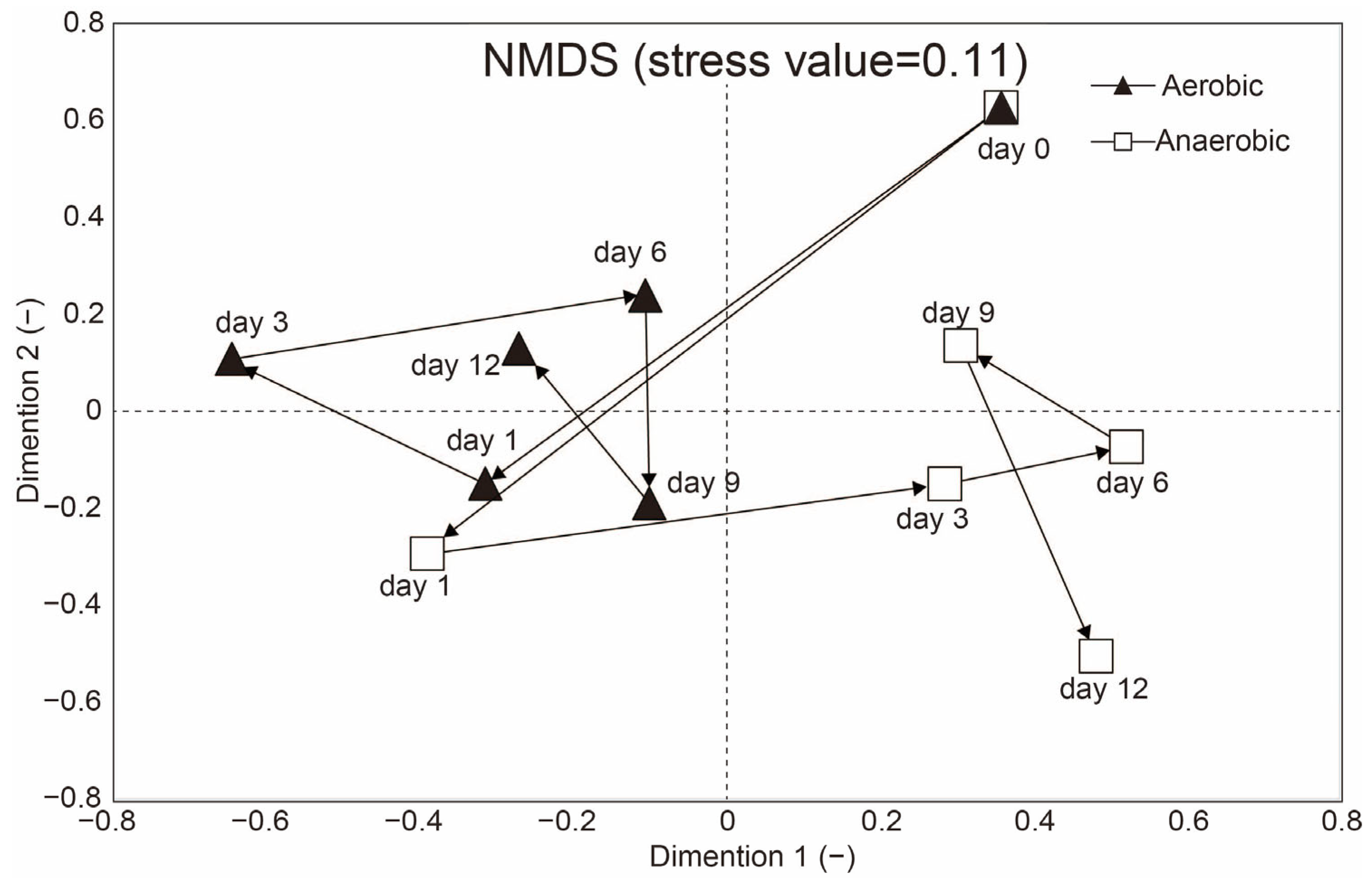
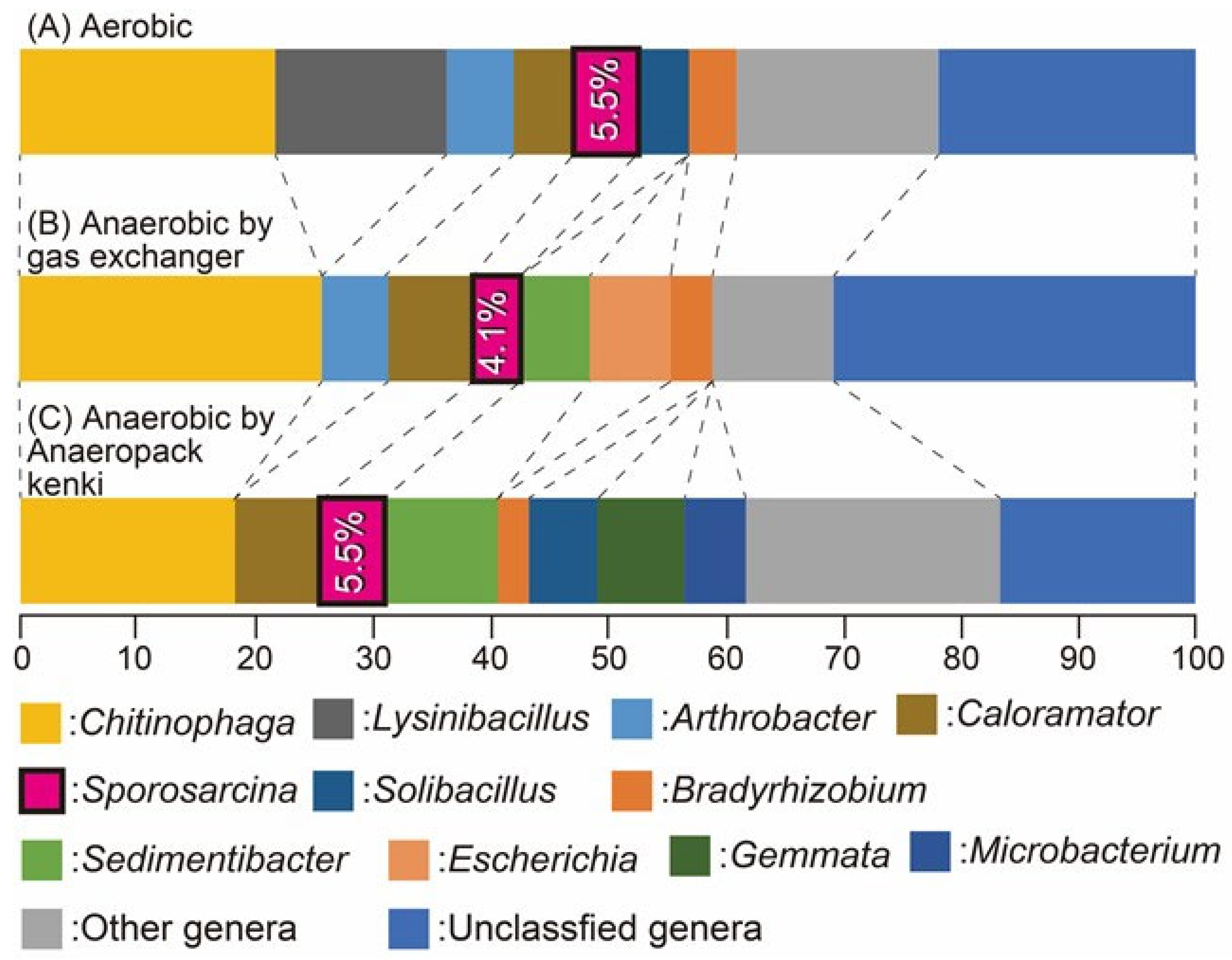
3.2. Microbial Community Shifts and Sporosarcina Dominance Under Aerobic and Anaerobic Reactor Conditions
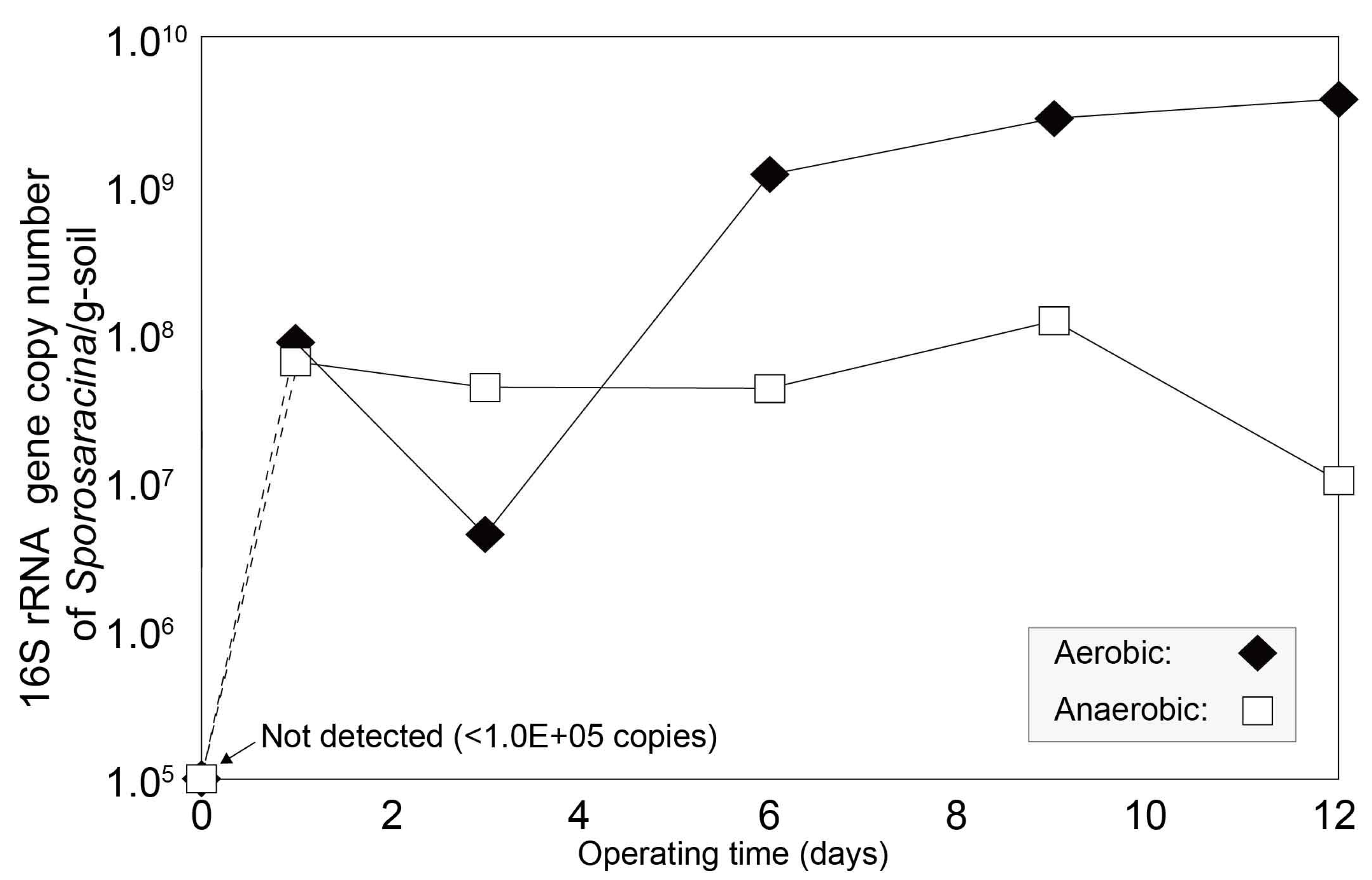
3.3. Impact of Oxygen Availability on Sporosarcina Proliferation and MICP Potential
3.4. Impact of Oxygen Availability on Sporosarcina Proliferation and MICP Potential in Saturated Soil
3.5. Integrating Present Results with Previous Knowledge and Applications
3.5.1. Reconciling with Previous Research on Sporosarcina Under Oxygen-Limited Conditions
3.5.2. Implications for Biostimulation Strategies in Soil Stabilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| MICP | Microbially induced calcite precipitation |
References
- Loi, D.H.; Jayakody, S.; Sassa, K.; Konagai, K.; Hirota, K.; Ono, A.; Takanaka, T.; Oki, T.; Minamitani, T. Landslides triggered by the 2024 Noto Peninsula earthquake. 202 Landslides triggered by the 2024 Noto Peninsula earthquake. Landslides 2024, 21, 2583–2590. [Google Scholar] [CrossRef]
- Hashimoto, R.; Tsuchida, T.; Moriwaki, T.; Kano, S. Hiroshima Prefecture geo-disasters due to Western Japan Torrential rainfall in July 2018. Soils Found. 2020, 60, 283–299. [Google Scholar] [CrossRef]
- Kameda, J.; Kamiya, H.; Masumoto, H.; Morisaki, T.; Hiratsuka, T.; Inaoi, C. Fluidized landslides triggered by the liquefaction of subsurface volcanic deposits during the 2018 Iburi–Tobu earthquake, Hokkaido. Sci. Rep. 2019, 9, 13119. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, S.; Huat, B.B.; Arun, P.; Barghchi, M. A review of stabilization of soft soils by injection of chemical grouting. Aust. J. Basic Appl. Sci. 2010, 4, 5862–5868. [Google Scholar]
- Fondjo, S.A.A.; Theron, E.; Ray, R. Stabilization of Expansive Soils Using Mechanical and Chemical Methods: A Comprehensive Review. Civ. Eng. Archit. 2021, 9, 1295–1308. [Google Scholar] [CrossRef]
- Gallagher, P.M.; Pamuk, A.; Abdoun, T. Stabilization of liquefiable soils using colloidal silica grout. J. Mater. Civ. Eng. 2007, 19, 33–40. [Google Scholar] [CrossRef]
- Naeimi, M.; Haddad, A. Environmental impacts of chemical and microbial grouting. Environ. Sci. Pollut. Res. 2020, 27, 2264–2272. [Google Scholar] [CrossRef]
- Indraratna, B.; Medawela, S.K.; Athuraliya, S.; Heitor, A.; Baral, P. Chemical clogging of granular media under acidic groundwater conditions. Environ. Geotech. 2019, 9, 450–462. [Google Scholar] [CrossRef]
- Baidya, P.; Dahal, B.; Pandit, A.; Joshi, D. Bacteria-Induced Calcite Precipitation for Engineering and Environmental Applications. Adv. Mater. Sci. Eng. 2023, 2023, 2613209. [Google Scholar] [CrossRef]
- Hadi, S.; Abbas, H.; Almajed, A.; Binyahya, A.; Al-Salloum, Y. Biocementation by Sporosarcina pasteurii ATCC6453 under simulated conditions in sand columns. J. Mater. Res. Technol. 2022, 18, 4375–4384. [Google Scholar] [CrossRef]
- Mortensen, B.M.; Haber, M.J.; DeJong, J.T.; Caslake, L.F.; Nelson, D.C. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Dodds, K.; Ngwenya, B.T.; Butler, I.B.; Elphick, S.C. Inhibition of Sporosarcina pasteurii under Anoxic Conditions: Implications for Subsurface Carbonate Precipitation and Remediation via Ureolysis. Environ. Sci. Technol. 2012, 46, 8351–8355. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-W.; Kim, B.-Y.; Song, J.; Weon, H.-Y.; Schumann, P.; Tindall, B.J.; Stackebrandt, E.; Fritze, D. Sporosarcina koreensis sp. nov. and Sporosarcina soli sp. nov., isolated from soil in Korea. Int. J. Syst. Evol. Microbiol. 2007, 57, 1694–1698. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, S.; Zhou, J. Strategy of nitrate removal in anaerobic ammonia oxidation-dependent processes. Chemosphere 2023, 313, 137586. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Ji, J.; Shi, L.; Gao, R.; Li, X. Partial denitrification providing nitrite: Opportunities of extending application for anammox. Environ. Int. 2019, 131, 105001. [Google Scholar] [CrossRef]
- Wright, C.L.; Lehtovirta-Morley, L.E. Nitrification and beyond: Metabolic versatility of ammonia oxidising archaea. ISME J. 2023, 17, 1358–1368. [Google Scholar] [CrossRef]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Kobayashi, A.; Sano, D.; Taniuchi, A.; Ishii, S.; Okabe, S. Use of a genetically-engineered Escherichia coli strain as a sample process control for quantification of the host-specific bacterial genetic markers. Appl. Microbiol. Biotechnol. 2013, 97, 9165–9173. [Google Scholar] [CrossRef]
- Bravo, C.; De Nobili, M.; Gambi, A.; Martin-Neto, L.; Nascimento, O.R.; Toniolo, R. Kinetics of electron transfer reactions by humic substances: Implications for their biogeochemical roles and determination of their electron donating capacity. Chemosphere 2022, 286, 131755. [Google Scholar] [CrossRef]
- Li, T.; Li, R.; Zhou, Q. The application and progress of bioelectrochemical systems (BESs) in soil remediation: A review. Green Energy Environ. 2021, 6, 50–65. [Google Scholar] [CrossRef]
- Butler, G.; Hsu, S.-B.; Waltman, P. A mathematical model of the chemostat with periodic washout rate. SIAM J. Appl. Math. 1985, 45, 435–449. [Google Scholar] [CrossRef]
- Oh, M.-K.; Rohlin, L.; Kao, K.C.; Liao, J.C. Global expression profiling of acetate-grown Escherichia coli. J. Biol. Chem. 2002, 277, 13175–13183. [Google Scholar] [CrossRef]
- Lee, M.; Woo, S.-G.; Chae, M.; Shin, M.-C.; Jung, H.-M.; Ten, L.N. Stenotrophomonas daejeonensis sp. nov., isolated from sewage. Int. J. Syst. Evol. 2011, 61, 598–604. [Google Scholar] [CrossRef]
- Jung, H.S.; Yun, J.U.; Jung, M.-J.; Song, H.S.; Kim, Y.B.; Kim, Y.; Kim, J.-G.; Jeon, C.O.; Roh, S.W.; Whon, T.W. Peptoniphilus equinus sp. nov., a novel Gram-stain-positive anaerobic coccus isolated from the faeces of a thoroughbred racehorse. Int. J. Syst. Evol. Microbiol. 2023, 73, 006053. [Google Scholar] [CrossRef]
- Tomazetto, G.; Hahnke, S.; Langer, T.; Wibberg, D.; Blom, J.; Maus, I.; Pühler, A.; Klocke, M.; Schlüter, A. The completely annotated genome and comparative genomics of the Peptoniphilaceae bacterium str. ING2-D1G, a novel acidogenic bacterium isolated from a mesophilic biogas reactor. J. Biotechnol. 2017, 257, 178–186. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Yutin, N.; Wolf, Y.I.; Vera Alvarez, R.; Koonin, E.V. Conservation and evolution of the sporulation gene set in diverse members of the Firmicutes. J. Bacteriol. 2022, 204, e00079-22. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Espinosa-Ortiz, E.J.; Parks, S.L.; Phillips, A.J.; Cunningham, A.B.; Gerlach, R. Kinetics of calcite precipitation by ureolytic bacteria under aerobic and anaerobic conditions. Biogeosciences 2019, 16, 2147–2161. [Google Scholar] [CrossRef]
- Scott, A.; Evans, D. Dissolved oxygen in saturated soil. Soil Sci. Soc. Am. J. 1955, 19, 7–12. [Google Scholar] [CrossRef]
- Gallagher, T.M.; Breecker, D.O. The obscuring effects of calcite dissolution and formation on quantifying soil respiration. Glob. Biogeochem. Cycles 2020, 34, e2020GB006584. [Google Scholar] [CrossRef]
- Dupraz, S.; Parmentier, M.; Ménez, B.; Guyot, F. Experimental and numerical modeling of bacterially induced pH increase and calcite precipitation in saline aquifers. Chem. Geol. 2009, 265, 44–53. [Google Scholar] [CrossRef]
- Henze, J.; Randall, D.G. Microbial induced calcium carbonate precipitation at elevated pH values (> 11) using Sporosarcina pasteurii. J. Environ. Chem. Eng. 2018, 6, 5008–5013. [Google Scholar] [CrossRef]
- Tobler, D.J.; Cuthbert, M.O.; Greswell, R.B.; Riley, M.S.; Renshaw, J.C.; Handley-Sidhu, S.; Phoenix, V.R. Comparison of rates of ureolysis between Sporosarcina pasteurii and an indigenous groundwater community under conditions required to precipitate large volumes of calcite. Geochim. Cosmochim. Acta 2011, 75, 3290–3301. [Google Scholar] [CrossRef]
- Gat, D.; Ronen, Z.; Tsesarsky, M. Soil bacteria population dynamics following stimulation for ureolytic microbial-induced CaCO3 precipitation. Environ. Sci. Technol. 2016, 50, 616–624. [Google Scholar] [CrossRef]
- Shibai, A.; Furusawa, C. Development of Specialized Devices for Microbial Experimental Evolution. Dev. Growth Differ. 2024, 66, 372–380. [Google Scholar] [CrossRef]
| Factor | F-Value | p-Value | Interpretation |
|---|---|---|---|
| Condition (Aerobic vs. Anaerobic) | 78.18 | 1.33 × 10⁻9 | Significant: Ct values (i.e., bacterial counts) differ by oxygen conditions |
| Time (days) | 45.88 | 1.27 × 10⁻9 | Significant: Ct values change over time |
| Interaction (Condition × Time) | 5.81 | 6.40 × 10⁻4 | Significant: Temporal patterns of bacterial growth differ by condition |
| Day 0 | % total reads | ||
| Unclassified at genus level | 56.08% | ||
| Niabella | 20.20% | ||
| Sphingomonas | 12.57% | ||
| Sediminibacterium | 3.71% | ||
| Staphylococcus | 3.64% | ||
| Bartonella | 1.46% | ||
| Propionibacterium | 1.33% | ||
| Candidatus Rhabdochlamydia | 0.11% | ||
| Other genera | 0.90% | ||
| Day 1 aerobic | % total reads | Day 1 anaerobic | % total reads |
| Sporosarcina | 77.95% | Sporosarcina | 88.68% |
| Unclassified at genus level | 6.66% | Unclassified at genus level | 4.57% |
| Bacillus | 3.24% | Bacillus | 1.32% |
| Escherichia | 2.44% | Planococcus | 1.08% |
| Enterobacter | 2.20% | Paenibacillus | 0.59% |
| Brevibacillus | 1.96% | PaeniSporosarcina | 0.54% |
| Serratia | 0.77% | Achromobacter | 0.39% |
| Planococcus | 0.67% | Lysobacter | 0.37% |
| Other genera | 4.11% | Other genera | 2.46% |
| Day 3 aerobic | % total reads | Day 3 anaerobic | % total reads |
| Escherichia | 21.20% | Unclassified at genus level | 34.58% |
| Stenotrophomonas | 14.40% | Sporosarcina | 20.55% |
| Unclassified genus level | 14.30% | Bacillus | 6.36% |
| Xanthomonas | 13.70% | Peptoniphilus | 3.92% |
| Enterobacter | 11.98% | Dolichospermum | 3.10% |
| Achromobacter | 8.67% | Serratia | 3.10% |
| Brevibacillus | 5.02% | Escherichia | 2.61% |
| Sporosarcina | 2.60% | Brevibacillus | 2.12% |
| Other genera | 8.13% | Other genera | 23.66% |
| Day 6 aerobic | % total reads | Day 6 anaerobic | % total reads |
| Sporosarcina | 54.56% | Unclassified at genus level | 39.35% |
| Achromobacter | 9.18% | Sporosarcina | 10.75% |
| Unclassified at genus level | 7.78% | Bacillus | 8.11% |
| Peptoniphilus | 5.15% | Peptoniphilus | 3.25% |
| Natronincola | 4.74% | Xanthomonas | 2.43% |
| Enterobacter | 3.93% | Serratia | 2.43% |
| Xanthomonas | 2.81% | Alkaliphilus | 2.43% |
| Serratia | 2.35% | Natronincola | 1.83% |
| Other genera | 9.50% | Other genera | 29.42% |
| Day 9 aerobic | % total reads | Day 9 anaerobic | % total reads |
| Sporosarcina | 45.06% | Sporosarcina | 23.29% |
| Unclassified at genus level | 26.01% | Unclassified at genus level | 18.35% |
| Achromobacter | 6.89% | Bacillus | 18.20% |
| Pullulanibacillus | 5.80% | Peptoniphilus | 15.38% |
| Peptoniphilus | 3.91% | Virgibacillus | 6.44% |
| Bacillus | 2.12% | Natronincola | 4.49% |
| Natronincola | 1.92% | Achromobacter | 2.77% |
| Enterobacter | 1.46% | Desulfurispora | 1.50% |
| Other genera | 6.83% | Other genera | 9.58% |
| Day 12 aerobic | % total reads | Day 12 anaerobic | % total reads |
| Sporosarcina | 75.33% | Unclassified at genus level | 25.33% |
| Unclassified at genus level | 6.30% | Virgibacillus | 16.90% |
| Peptoniphilus | 5.10% | Peptoniphilus | 15.87% |
| Achromobacter | 3.48% | Bacillus | 15.55% |
| Natronincola | 1.83% | Sporosarcina | 5.74% |
| Pullulanibacillus | 1.31% | Natronincola | 5.18% |
| Planococcus | 1.06% | Lentibacillus | 1.63% |
| Enterobacter | 0.72% | Lactobacillus | 1.49% |
| Other genera | 4.87% | Other genera | 12.31% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, Z.-i.; Kirihara, K.-s.; Komoto, S.; Sera, W.; Kojima, R.; Ihara, S.; Itoiri, Y.; Tanikawa, D.; Iwasaki, Y. Evaluating the Contribution of Sporosarcina to Carbonate Precipitation in Anaerobic Soils: A Microbial Community and Quantitative Analysis. Appl. Microbiol. 2025, 5, 53. https://doi.org/10.3390/applmicrobiol5020053
Kimura Z-i, Kirihara K-s, Komoto S, Sera W, Kojima R, Ihara S, Itoiri Y, Tanikawa D, Iwasaki Y. Evaluating the Contribution of Sporosarcina to Carbonate Precipitation in Anaerobic Soils: A Microbial Community and Quantitative Analysis. Applied Microbiology. 2025; 5(2):53. https://doi.org/10.3390/applmicrobiol5020053
Chicago/Turabian StyleKimura, Zen-ichiro, Ko-shiro Kirihara, Saki Komoto, Wataru Sera, Ryota Kojima, Sota Ihara, Yuya Itoiri, Daisuke Tanikawa, and Yuki Iwasaki. 2025. "Evaluating the Contribution of Sporosarcina to Carbonate Precipitation in Anaerobic Soils: A Microbial Community and Quantitative Analysis" Applied Microbiology 5, no. 2: 53. https://doi.org/10.3390/applmicrobiol5020053
APA StyleKimura, Z.-i., Kirihara, K.-s., Komoto, S., Sera, W., Kojima, R., Ihara, S., Itoiri, Y., Tanikawa, D., & Iwasaki, Y. (2025). Evaluating the Contribution of Sporosarcina to Carbonate Precipitation in Anaerobic Soils: A Microbial Community and Quantitative Analysis. Applied Microbiology, 5(2), 53. https://doi.org/10.3390/applmicrobiol5020053






