Effect of r-Human Insulin (Humulin®) and Sugars on Escherichia coli K-12 Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Bacterial Growth Phase
2.3. Latex Adherence
2.4. Hydrophobic Biofilm Assay
2.5. Statistics
3. Results
3.1. Binding to Latex
3.1.1. Effects of Insulin with 6-Carbon Aldo Sugars on Log and Stat E. coli Adherence
3.1.2. Effects of Insulin with 6-Carbon Keto Sugars on Log and Stat E. coli Binding
3.1.3. Effects of Insulin with 5-Carbon Sugars on Log and Stat E. coli Binding
3.1.4. Effects of Insulin with Disaccharides on Log and Stat E. coli Binding
3.1.5. Effects of Insulin with Altered Glucose Compounds on Log and Stat E. coli Binding
3.2. Effect of Insulin and Sugars on E. coli Biofilm Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Barrios, A.; Zuo, R.; Hashimoto, Y.; Yang, L.; Bentley, W.; Wood, T. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022). J. Bacteriol. 2006, 188, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Konaklieva, M.; Plotkin, B. Chemical communication—Do we have a quorum? Mini-Rev. Med. Chem. 2006, 6, 817–825. [Google Scholar] [CrossRef]
- Reading, N.; Sperandio, V. Quorum sensing: The many languages of bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11. [Google Scholar] [CrossRef]
- Klosowska, K.; Plotkin, B. Human insulin modulation of Escherichia coli adherence and chemotaxis. Am. J. Infect. Dis. 2006, 2, 197–200. [Google Scholar] [CrossRef]
- Plotkin, B.; Wu, Z.; Ward, K.; Nadella, S.; Green, J.; Rummani, B. Effect of human insulin on the formation of catheter-associated E. coli biofilms. Open J. Urol. 2014, 4, 49–56. [Google Scholar] [CrossRef]
- Patel, N.; Curtis, J.C.; Plotkin, B.J. Insulin Regulation of Escherichia coli Abiotic Biofilm Formation: Effect of Nutrients and Growth Conditions. Antibiotics 2021, 10, 1349. [Google Scholar] [CrossRef]
- LeRoith, D.; Shiloach, J.; Roth, J.; Lesniak, M. Evolutionary origins of vertebrate hormones: Substances similar to mammalian insulins are native to unicellular eukaryotes. Proc. Natl. Acad. Sci. USA 1980, 77, 6184–6188. [Google Scholar] [CrossRef]
- LeRoith, D.; Shiloach, J.; Roth, J.; Lesniak, M.A. Insulin or a closely related molecule is native to Escherichia coli. J. Biol. Chem. 1981, 256, 6533–6536. [Google Scholar] [CrossRef]
- LeRoith, D.; Shiloach, J.; Heffron, R.; Rubinovitz, C.; Tanenbaum, R.; Roth, J. Insulin-related material in microbes: Similarities and differences from mammalian insulins. Can. J. Biochem. Cell Biol. 1985, 63, 839–849. [Google Scholar] [CrossRef]
- Christensen, S. Insulin rescues the unicellular eucaryote Tetrahymena from dying in a complete, synthetic nutrient medium. Cell Biol. Intern. 1993, 17, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, B.J.; Viselli, S.M. Effect of insulin on microbial growth. Curr. Microbiol. 2000, 41, 60–64. [Google Scholar] [CrossRef]
- Plotkin, B.J.; Halkyard, S.; Spoolstra, E.; Micklo, A.; Kaminski, A.; Sigar, I.M.; Konaklieva, M.I. The Role of the Insulin/Glucose Ratio in the Regulation of Pathogen Biofilm Formation. Biology 2023, 12, 1432. [Google Scholar] [CrossRef] [PubMed]
- Adler, J.; Hazelbauer, G.L.; Dahl, M.M. Chemotaxis toward sugars in Escherichia coli. J. Bacteriol. 1973, 115, 824–847. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D. Basal insulin: Physiology, pharmacology, and clinical implications. Postgrad. Med. 2011, 123, 17–26. [Google Scholar] [CrossRef]
- Rasmussen, H.; Zawalich, K.C.; Ganesan, S.; Calle, R.; Zawalich, W.S. Physiology and pathophysiology of insulin secretion. Diabetes Care 1990, 13, 655–666. [Google Scholar] [CrossRef]
- Di Santo Meztler, P.; Fait, M.E.; Foresti, M.L.; Morcelle, S.R. Biocatalytic characterization of a naturally immobilized lipase found in Araujia sericifera Brot. (Apocynaceae) latex. Catal. Sci. Technol. 2014, 4, 1386–1394. [Google Scholar] [CrossRef]
- Faghihnejad, A.; Zeng, H. Hydrophobic interactions between polymer surfaces: Using polystyrene as a model system. Soft Matter. 2012, 8, 2746–2759. [Google Scholar] [CrossRef]
- Christensen, G.; Simpson, W.; Younger, J.; Baddour, L.; Barrett, F.; Melton, D.; Beachy, E. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 966–1006. [Google Scholar] [CrossRef]
- Hu, J.; Lv, X.; Niu, X.; Yu, F.; Zuo, J.; Bao, Y.; Yin, H.; Huang, C.; Nawaz, S.; Zhou, W. Effect of nutritional and environmental conditions on biofilm formation of avian pathogenic Escherichia coli. J. Appl. Microbiol. 2022, 132, 4236–4251. [Google Scholar] [CrossRef]
- Song, S.; Wood, T.K. The Primary Physiological Roles of Autoinducer 2 in Escherichia coli Are Chemotaxis and Biofilm Formation. Microorganisms 2021, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Gomes, L.C.; Monteiro, G.A.; Mergulhão, F.J. The influence of nutrient medium composition on Escherichia coli biofilm development and heterologous protein expression. Appl. Sci. 2021, 11, 8667. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Kim, J.A.; Neupane, G.P.; Cho, M.H.; Lee, C.S.; Lee, J. Low concentrations of honey reduce biofilm formation, quorum sensing, and virulence in Escherichia coli O157:H7. Biofouling 2011, 27, 1095–1104. [Google Scholar] [CrossRef]
- Sutrina, S.L.; Callender, S.; Grazette, T.; Scantlebury, P.; O’Neal, S.; Neal, S.; Thomas, K.; Harris, D.C.; Mota-Meira, M. The quantity and distribution of biofilm growth of Escherichia coli strain ATCC 9723 depends on the carbon/energy source. Microbiology 2019, 165, 47–64. [Google Scholar] [CrossRef]
- Ha, J.-H.; Hauk, P.; Cho, K.; Eo, Y.; Ma, X.; Stephens, K.; Cha, S.; Jeong, M.; Suh, J.-Y.; Sintim, H.O.; et al. Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr. Sci. Adv. 2018, 4, eaar7063. [Google Scholar] [CrossRef]
- Domka, J.; Lee, J.; Bansal, T.; Wood, T.K. Temporal gene-expression in Escherichia coli K-12 biofilms. Environ. Microbiol. 2007, 9, 332–346. [Google Scholar] [CrossRef]
- Schembri, M.A.; Kjærgaard, K.; Klemm, P. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 2003, 48, 253–267. [Google Scholar] [CrossRef]
- Tao, H.; Bausch, C.; Richmond, C.; Blattner, F.R.; Conway, T. Functional genomics: Expression analysis of Escherichia coli growing on minimal and rich media. J. Bacteriol. 1999, 181, 6425–6440. [Google Scholar] [CrossRef]
- Miyakoshi, M. Multilayered regulation of amino acid metabolism in Escherichia coli. Curr. Opin. Microbiol. 2024, 77, 102406. [Google Scholar] [CrossRef]
- Huang, J.-F.; Shen, Z.-Y.; Mao, Q.-L.; Zhang, X.-M.; Zhang, B.; Wu, J.-S.; Liu, Z.-Q.; Zheng, Y.-G. Systematic Analysis of Bottlenecks in a Multibranched and Multilevel Regulated Pathway: The Molecular Fundamentals of l-Methionine Biosynthesis in Escherichia coli. ACS Synth. Biol. 2018, 7, 2577–2589. [Google Scholar] [CrossRef]
- Charlier, D.; Bervoets, I. Regulation of arginine biosynthesis, catabolism and transport in Escherichia coli. Amino Acids 2019, 51, 1103–1127. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Dingemans, J.; Gowett, M.; Sauer, K. Glucose-6-Phosphate acts as an extracellular signal of SagS to modulate Pseudomonas aeruginosa c-di-GMP levels, attachment, and biofilm formation. mSphere 2021, 6, e01231-1220. [Google Scholar] [CrossRef]
- Jeckelmann, J.-M.; Erni, B. Transporters of glucose and other carbohydrates in bacteria. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 1129–1153. [Google Scholar] [CrossRef]
- Romling, U.; Gomelsky, M.; Galperin, M. C-di-GMP: The dawning of a novel bacterial signalling system. Mol. Microbiol. 2005, 53, 629–639. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Z.; Luo, J.; Kong, J.; Ding, Y.; Chen, Y.; Wang, K. Insulin treatment enhances Pseudomonas aeruginosa biofilm formation by increasing intracellular cyclic di-GMP levels, leading to chronic wound infection and delayed wound healing. Am. J. Transl. Res. 2019, 11, 3261–3279. [Google Scholar]
- Park, S.; Sauer, K. Controlling biofilm development through cyclic di-GMP signaling. Adv. Exp. Med. Biol. 2022, 1386, 69–94. [Google Scholar] [CrossRef]
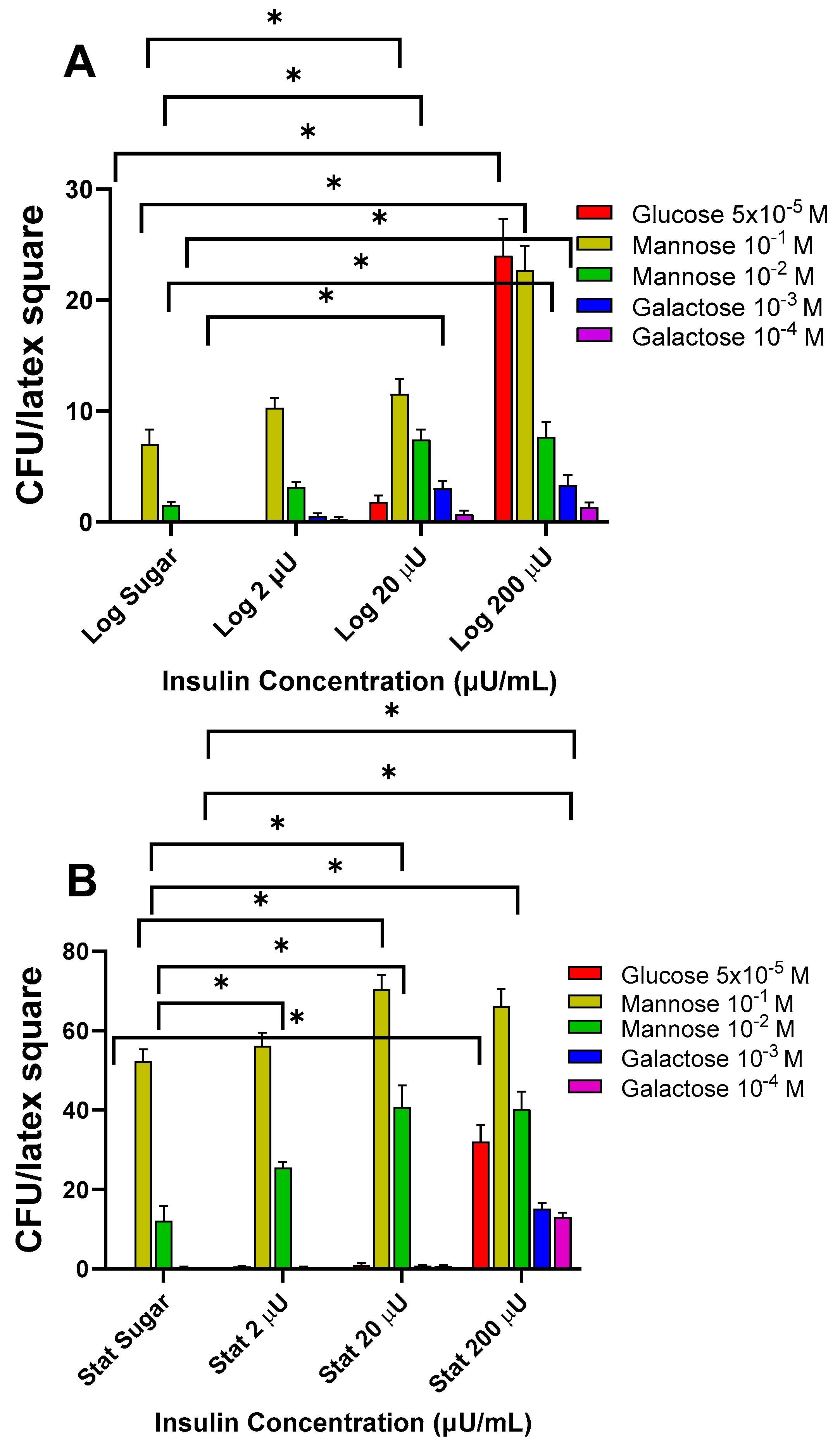

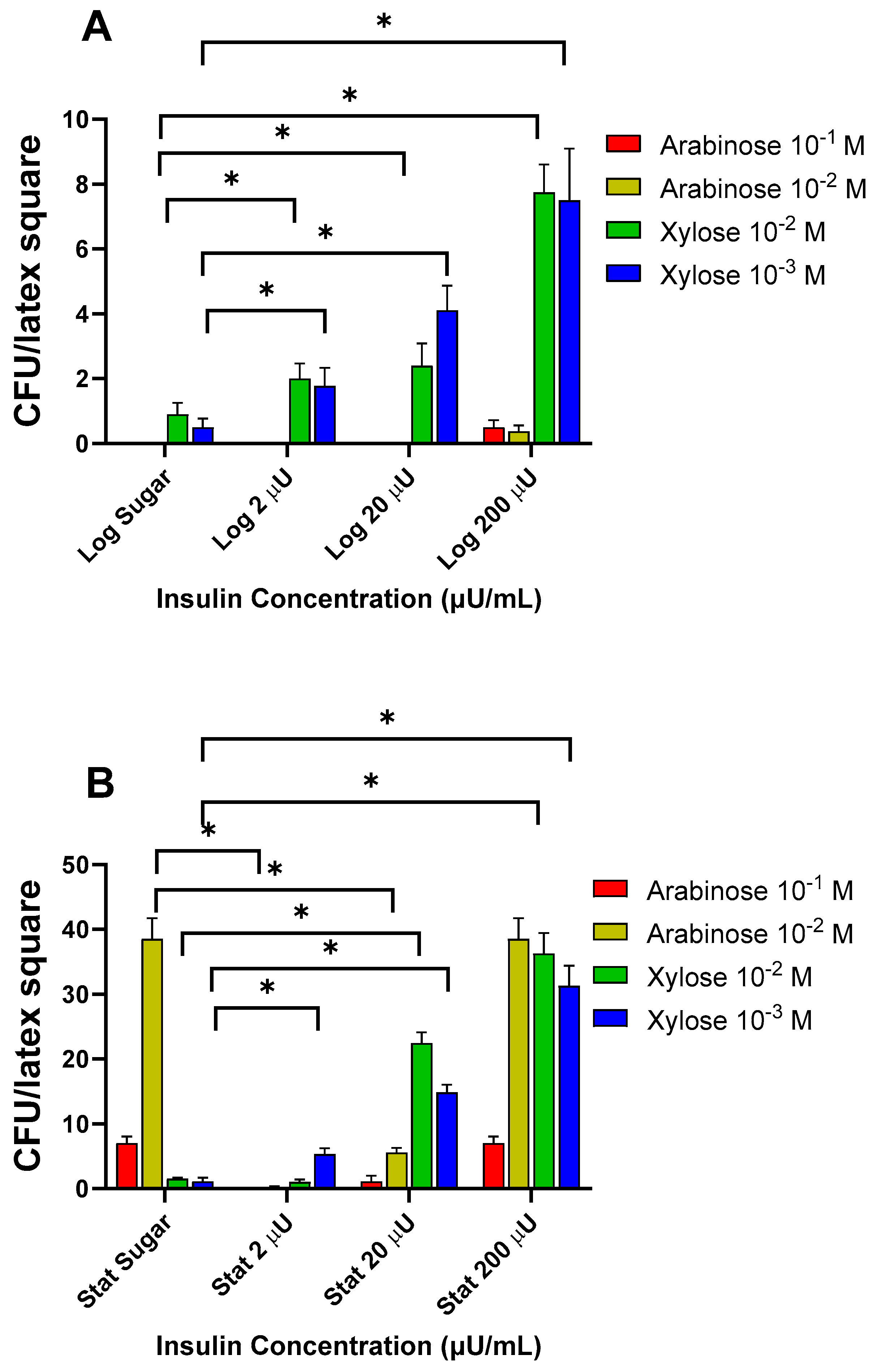
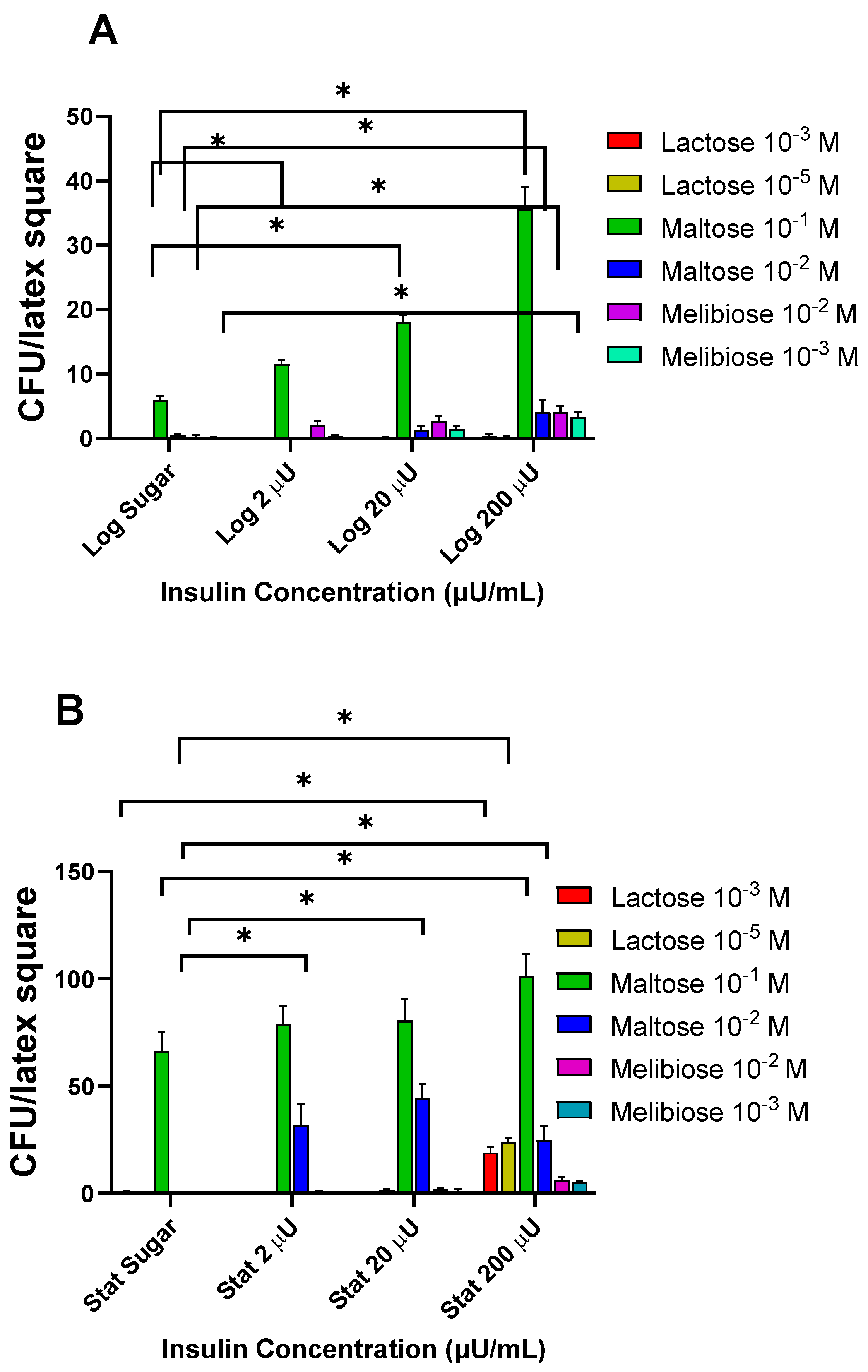
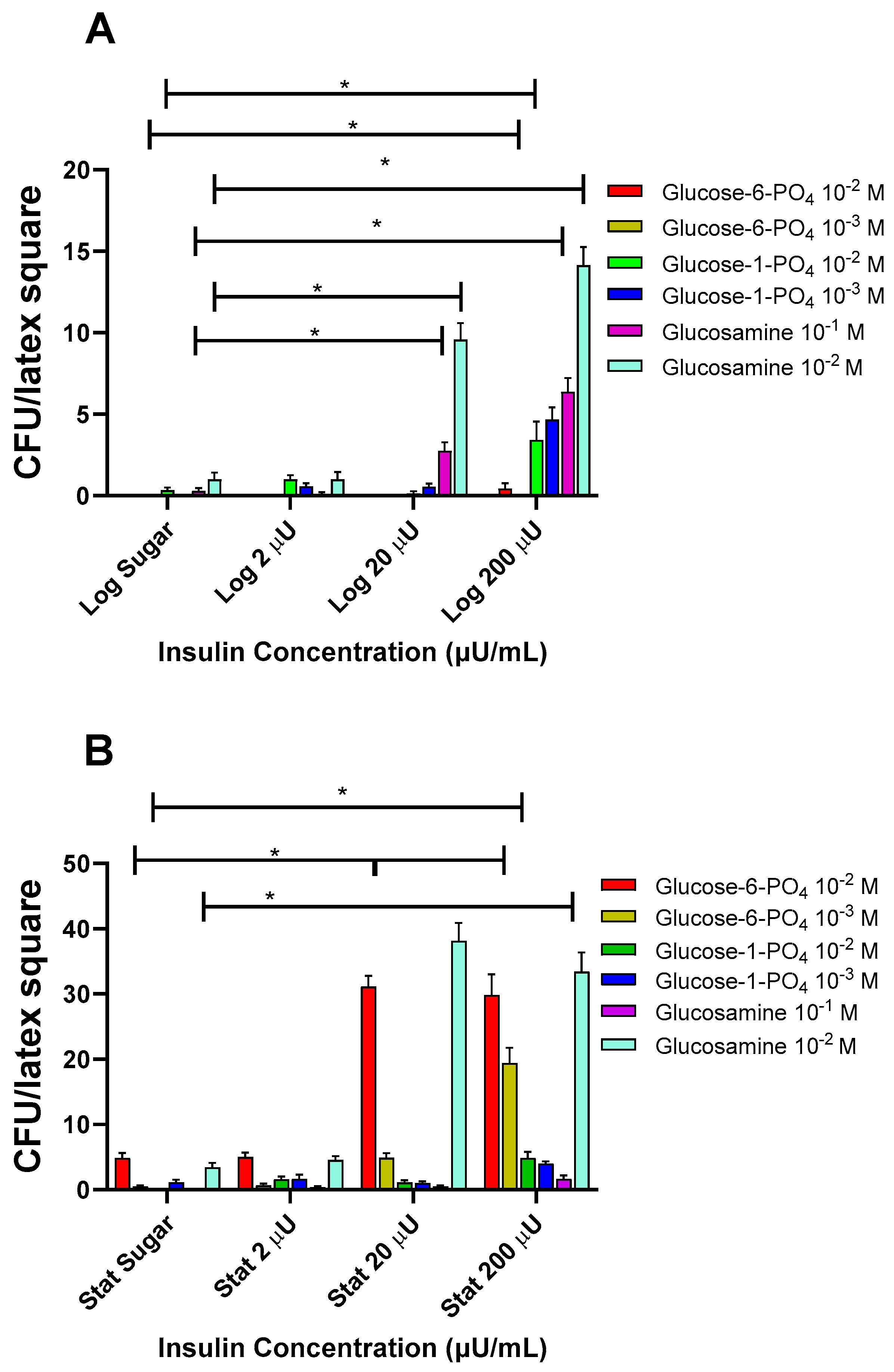

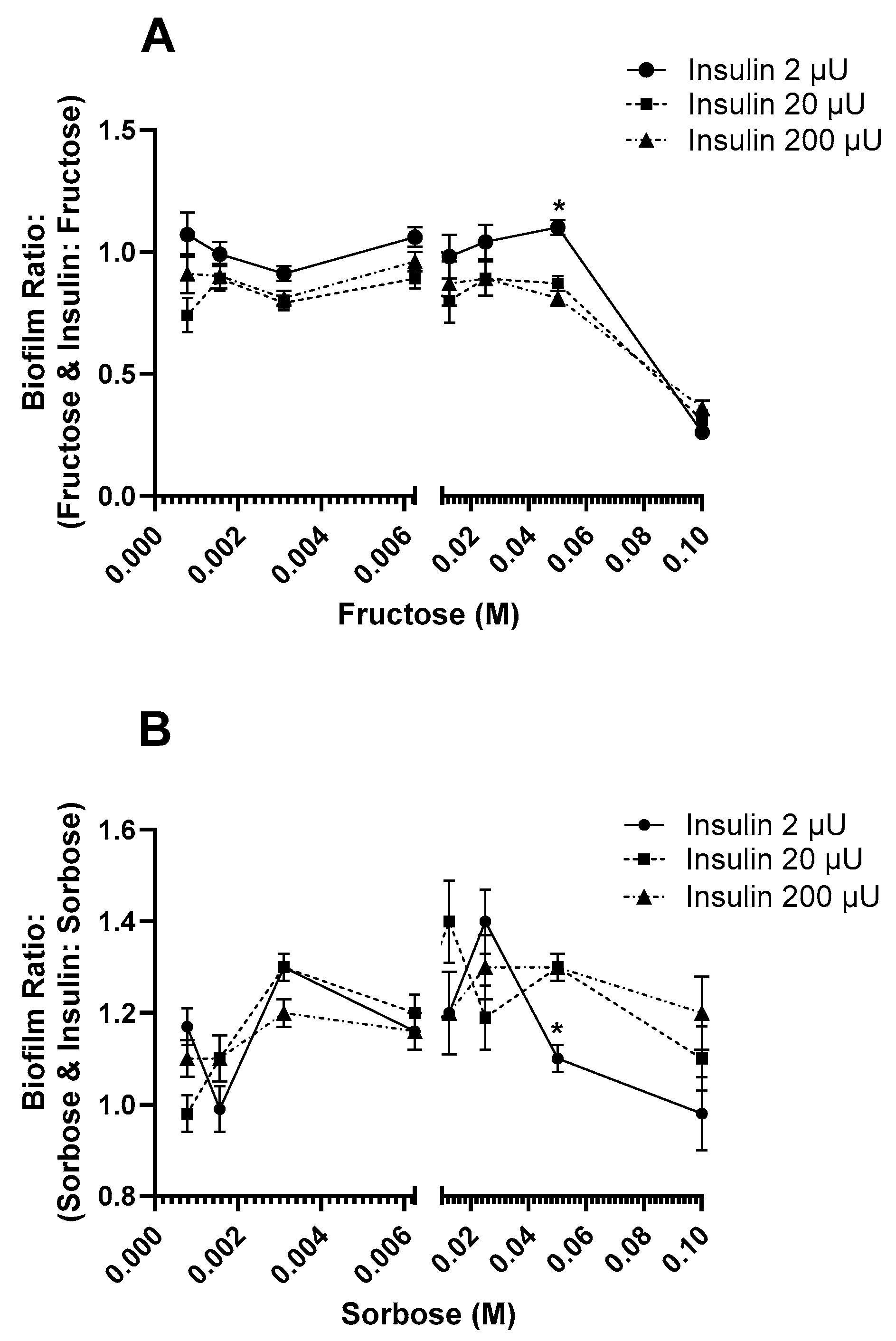
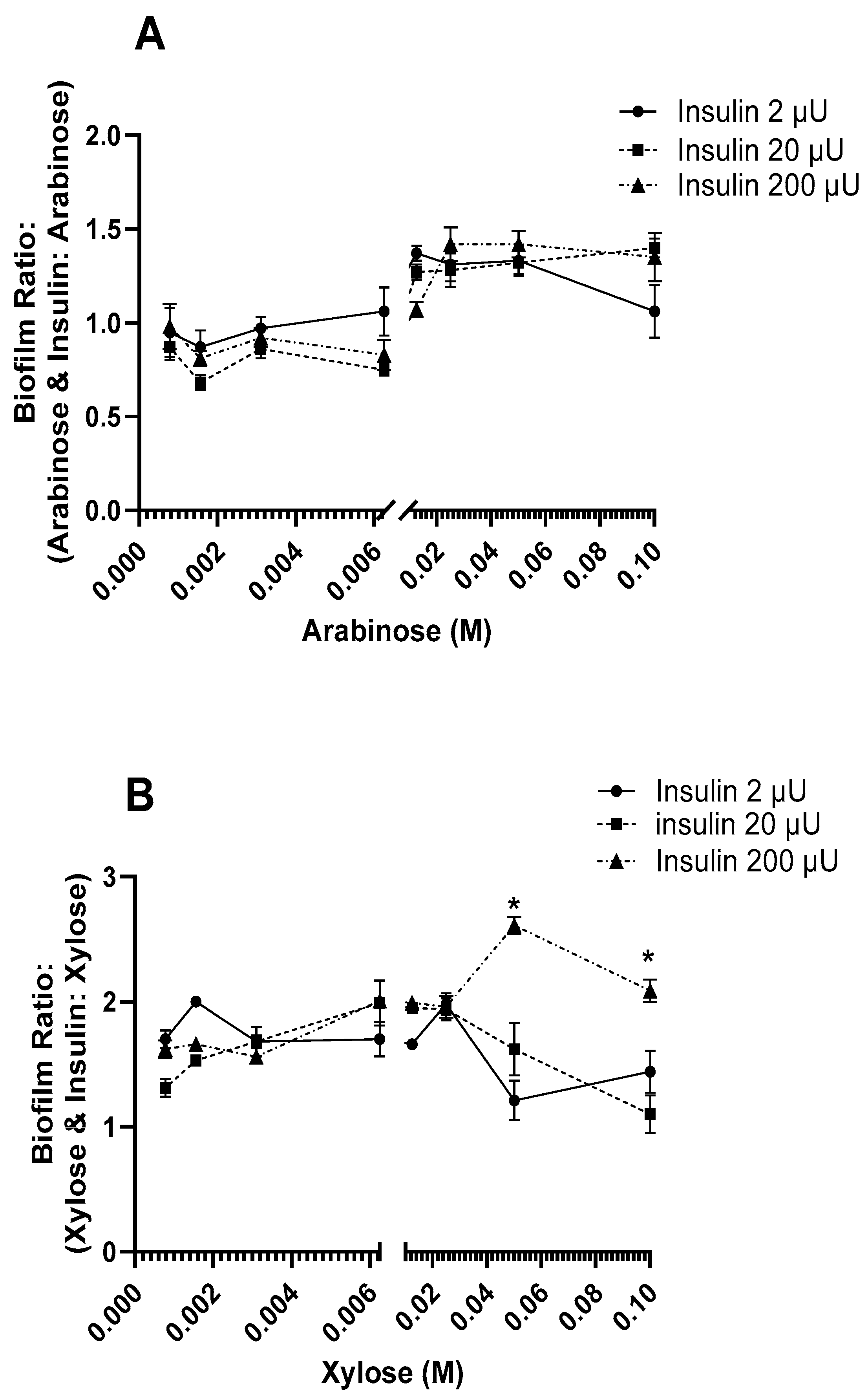
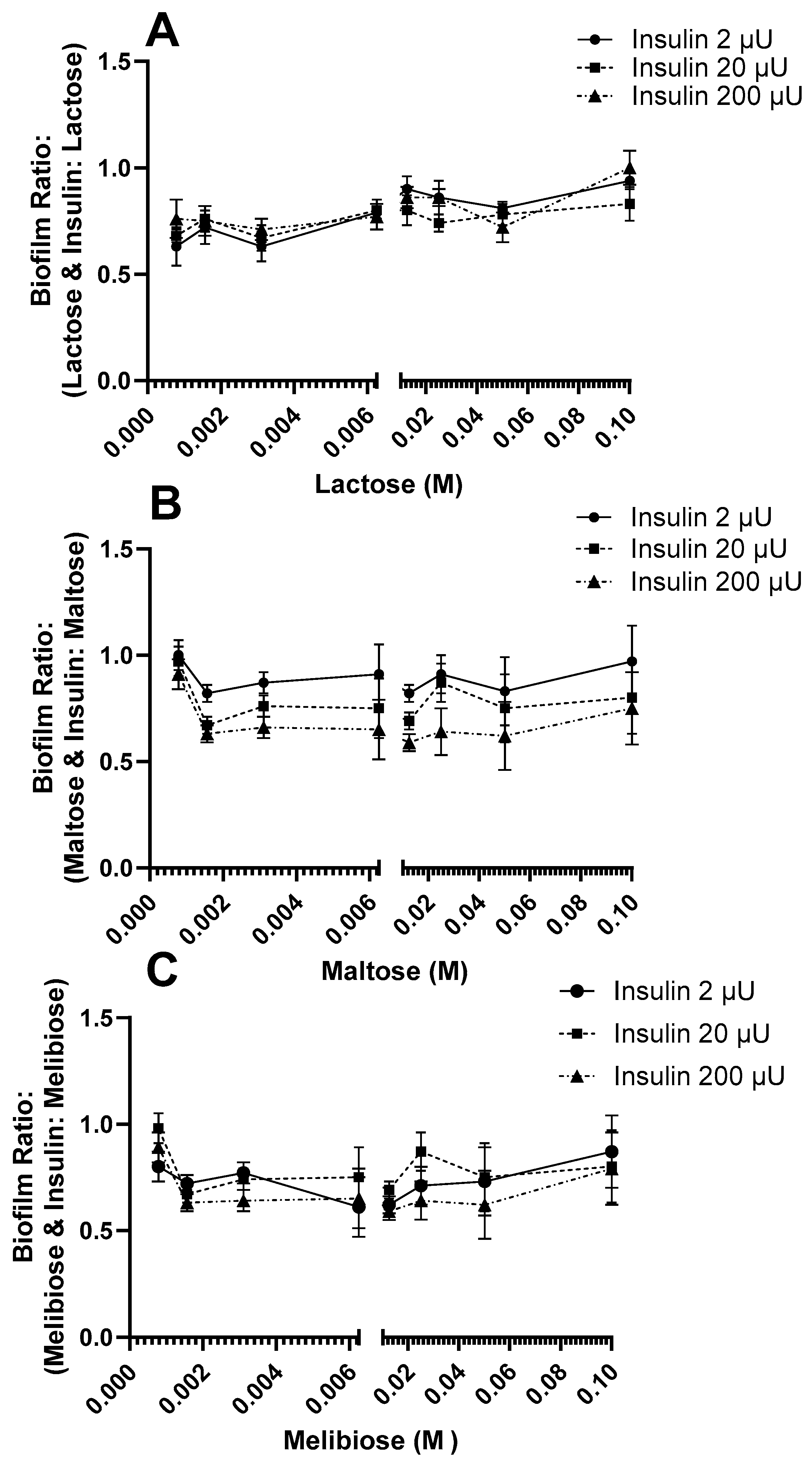
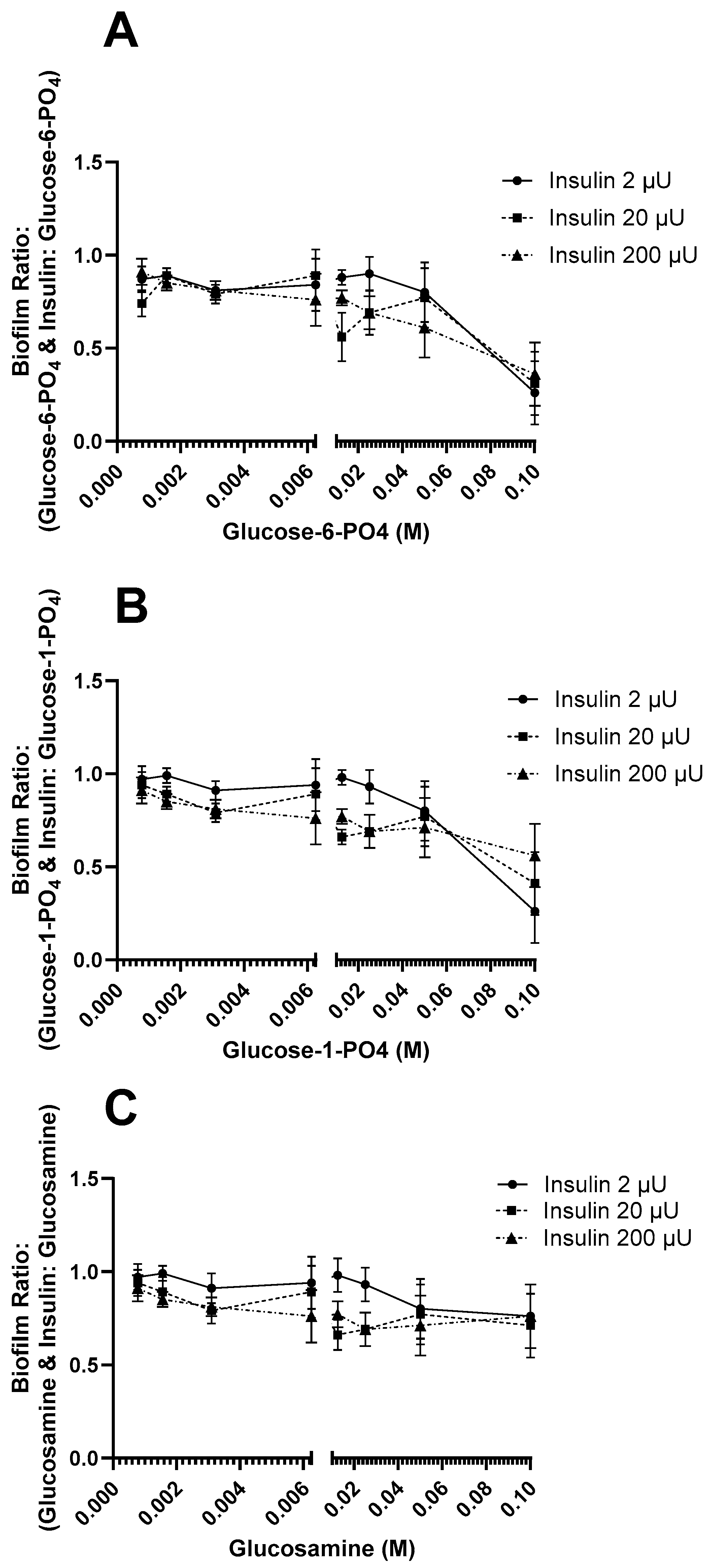
| 6-Carbon Aldo Sugars | 6-Carbon Keto Sugars | 5-Carbon Sugars | Disaccharides | Altered Glucose Compounds |
|---|---|---|---|---|
| Glucose (5 × 10−5 M) | Fructose (10−3, 10−4 M) | Arabinose (10−1, 10−2 M) | Lactose (10−3, 10−5 M) | Glucose-6-Phosphate (10−2, 10−3 M) |
| Mannose (10−1, 10−2 M) | Sorbose (10−2, 10−3 M) | Xylose (10−2, 10−3 M) | Maltose (10−1, 10−2 M) | Glucosamine (10−1, 10−2 M) |
| Galactose (10−3, 10−4 M) | Melibiose (10−2, 10−3 M) | Glucose-1-Phosphate (10−2, 10−3 M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotkin, B.J.; Sigar, I.; Konaklieva, M. Effect of r-Human Insulin (Humulin®) and Sugars on Escherichia coli K-12 Biofilm Formation. Appl. Microbiol. 2025, 5, 58. https://doi.org/10.3390/applmicrobiol5030058
Plotkin BJ, Sigar I, Konaklieva M. Effect of r-Human Insulin (Humulin®) and Sugars on Escherichia coli K-12 Biofilm Formation. Applied Microbiology. 2025; 5(3):58. https://doi.org/10.3390/applmicrobiol5030058
Chicago/Turabian StylePlotkin, Balbina J., Ira Sigar, and Monika Konaklieva. 2025. "Effect of r-Human Insulin (Humulin®) and Sugars on Escherichia coli K-12 Biofilm Formation" Applied Microbiology 5, no. 3: 58. https://doi.org/10.3390/applmicrobiol5030058
APA StylePlotkin, B. J., Sigar, I., & Konaklieva, M. (2025). Effect of r-Human Insulin (Humulin®) and Sugars on Escherichia coli K-12 Biofilm Formation. Applied Microbiology, 5(3), 58. https://doi.org/10.3390/applmicrobiol5030058






