Isolation, Optimization and Characterization of Rhodotorula alborubescens for Dietary Pigment β-Carotene Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Screening
2.2. Morphological, Molecular and Biochemical Characterization
2.3. Blood Agar Testing
2.4. Selection of Media
2.5. Carotenoid Production Optimization
2.6. Extraction of Carotenoids
2.7. Analysis of Carotenoids
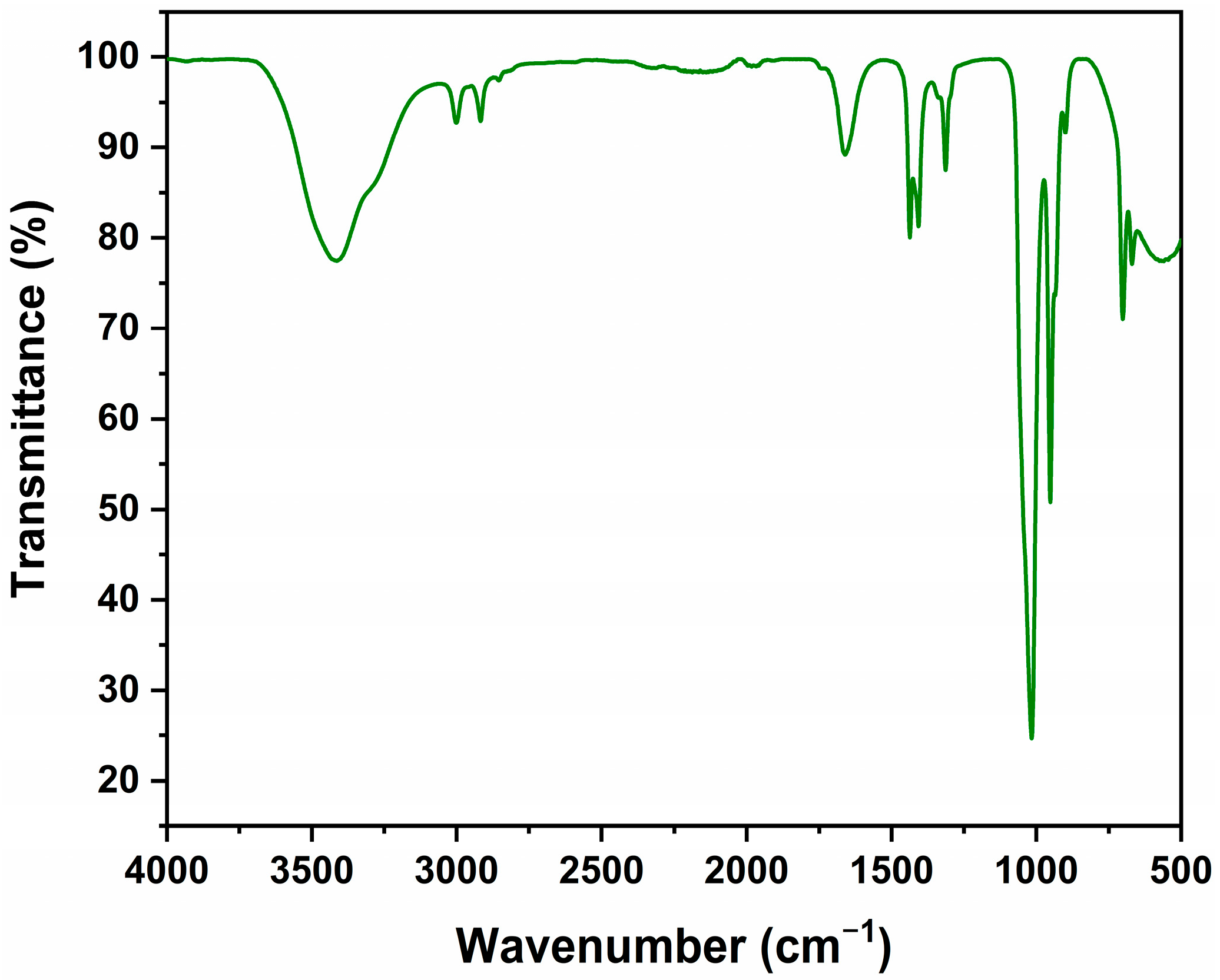
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. High-Performance Liquid Chromatography (HPLC)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Isolation, Identification and Biochemical Testing of the Isolate
3.2. Blood Agar Test
3.3. Optimization of Abiotic Parameters for Enhanced Total Carotenoid and Biomass Yield from R. alborubescens
3.3.1. Growth Medium and Incubation Time
3.3.2. Incubation Temperature
3.3.3. Growth Media pH
3.3.4. White/Dark Period Analysis
3.4. Pigment Quantification and Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashir, I.; Pandey, V.K.; Dar, A.H.; Dash, K.K.; Shams, R.; Mir, S.A.; Fayaz, U.; Khan, S.A.; Singh, R.; Zahoor, I. Exploring sources, extraction techniques and food applications: A review on biocolors as next-generation colorants. Phytochem. Rev. 2024, 23, 1–26. [Google Scholar] [CrossRef]
- Kushwaha, A.; Talukdar, S.; Mohanan, V.P.; Lata, S.; Gupta, M.; Goswami, L.; Kim, B.S. Bio-treatment of the swine wastewater and resource recovery: A sustainable approach towards circular bioeconomy. In Bio-Based Materials and Waste for Energy Generation and Resource Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 299–329. [Google Scholar]
- Dufossé, L. Current and potential natural pigments from microorganisms (bacteria, yeasts, fungi, and microalgae). In Handbook on Natural Pigments in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2024; pp. 419–436. [Google Scholar]
- Anshi Kapil, S.; Goswami, L.; Sharma, V. Unveiling the intricacies of microbial pigments as sustainable alternatives to synthetic colorants: Recent trends and advancements. Micro 2024, 4, 621–640. [Google Scholar] [CrossRef]
- Singh, A.; Kushwaha, A.; Goswami, S.; Tripathi, A.; Bhasney, S.M.; Goswami, L.; Hussain, C.M. Roadmap from microalgae to biorefinery: A circular bioeconomy approach. In Emerging Trends to Approaching Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 339–360. [Google Scholar]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2021, 62, 6932–6946. [Google Scholar] [CrossRef] [PubMed]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and identification of carotenoid-producing Rhodotorula sp. from Pinaceae forest ecosystems and optimization of in vitro carotenoid production. Biotechnol. Rep. 2021, 32, e00687. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.A.; El-Ewasy, S.M. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef]
- Hassan, M.G.; El-Sayyad, G.S.; Abdel-Monem, M.O.; Malash, M.N.; Kishk, M.A.; El Awady, M.E.; El-Khonezy, M.I. Unravelling the outcome of L-glutaminase produced by Streptomyces sp. strain 5 M as an anti-neoplasm activity. Microb. Cell Factories 2025, 24, 4. [Google Scholar] [CrossRef]
- Bozhuyuk, F.M.; Ozdal, M. Characterization of melanin from the fungus Scolecobasidium Musae and its antioxidant and photoprotective properties. Arch. Microbiol. 2025, 207, 77. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Caicedo-Paz, A.V.; Farias, F.O.; de Souza Mesquita, L.M.; Giuffrida, D.; Dufossé, L. Microbial bacterioruberin: The new C50 carotenoid player in food industries. Food Microbiol. 2024, 124, 104623. [Google Scholar] [CrossRef]
- Zhao, D.; Li, C.; Zeng, N.; Wang, D.; Zhang, N.; Li, B. Current advances in the biosynthesis and sustainable production strategies of carotenoids and their multifaceted applications in the food industry: A comprehensive review. Food Biosci. 2025, 64, 105864. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, M.; Jiang, W.; Zhang, L.; Shen, L.; Bai, Y. Functional Role and Mutational Analysis of the Phytoene Synthase from the Halophilic Euryarchaeon Haloferax volcanii in Bacterioruberin Biosynthesis. J. Agric. Food Chem. 2025, 73, 2393–2403. [Google Scholar] [CrossRef]
- Yadav AP, S.; Dwivedi, V.; Kumar, S.; Kushwaha, A.; Goswami, L.; Reddy, B.S. Cyanobacterial extracellular polymeric substances for heavy metal removal: A mini review. J. Compos. Sci. 2020, 5, 1. [Google Scholar] [CrossRef]
- Fang, Y.; Feng, P.; Wang, L.; Qin, L.; Wang, Z.; Zhu, S.; Fan, Y. Evaluation of culture conditions of Trichosporon oleaginosus DSM11815 for enhancement of growth and lipid production in sugarcane bagasse hydrolysate as a substrate. Process Biochemistry. 2025. [Google Scholar] [CrossRef]
- Bisht, A.; Sahu, S.C.; Kumar, A.; Maqsood, S.; Barwant, M.M.; Jaiswal, S.G. Recent Advances in Conventional and Innovative Extraction Techniques for Recovery of High-Added Value Compounds for Food Additives and Nutraceuticals. Food Phys. 2025, 2, 100047. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Kamran, F.; Stankov, S.; Rathod, N.B.; Teixeira-Costa, B.E.; Fidan, H.; Bhat, M.I.; Sofi, S.A. Unveiling the Diversity of Non Conventional Proteins-From Sources, Extraction, Technofunctionality, Nutraceutical Potential to Advancement in Food Applications-A Systematic Review. Waste Biomass Valoriz. 2025, 16, 29–51. [Google Scholar] [CrossRef]
- Chatragadda, R.; Dufossé, L. Ecological and biotechnological aspects of pigmented microbes: A way forward in development of food and pharmaceutical grade pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef]
- Mannazzu, I.; Landolfo, S.; Da Silva, T.L.; Buzzini, P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef]
- Dufossé, L. Biotechnological approaches in the production of fungal pigments. In Fungal Biotechnology; Academic Press: Cambridge, MA, USA, 2025; pp. 449–466. [Google Scholar]
- Thilakan, M.L.J.; Amsaveni, S.; Dharani, G. Microwave pretreatment: A promising strategy to improve the clean extraction yield of microalgae-based products. In Algal Bioreactors; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2025; pp. 233–247. [Google Scholar]
- Tinoi, J.; Rakariyatham, N.; Deming, R.L. Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem. 2005, 40, 2551–2557. [Google Scholar] [CrossRef]
- de Lima, J.G.O.; Veríssimo, N.V.P.; de Azevedo Lima, C.; Picheli, F.P.; de Paula, A.V.; Santos-Ebinuma, V.D.C. Improvement of torularhodin production by Rhodotorula glutinis through the stimulation of physicochemical stress and application of the bioproduct as an additive in the food industry. Bioprocess Biosyst. Eng. 2025, 48, 543–563. [Google Scholar] [CrossRef]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. (Eds.) The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Kapil, S.; Bhattu, M.; Sharma, V.; Kumar, T. Racemization rate and biomolecular characterization of D-serine synthesizing bacteria Bacillus tequilensis A1C1. Lett. Appl. Microbiol. 2023, 76, ovac017. [Google Scholar] [CrossRef]
- Gedela, R.; Prabhu, A.; Veeranki, V.D.; Kannan, P. High yield production of lipid and carotenoids in a newly isolated Rhodotorula mucilaginosa by adapting process optimization approach. Biofuels 2023, 14, 509–520. [Google Scholar] [CrossRef]
- Lopes, N.A.; Remedi, R.D.; dos Santos Sá, C.; Burkert, C.A.V.; de Medeiros Burkert, J.F. Different cell disruption methods for obtaining carotenoids by Sporodiobolus pararoseus and Rhodothorula mucilaginosa. Food Sci. Biotechnol. 2017, 26, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Ravi, G.; Venkata Dasu, V.; Pakshirajan, K. Exploring the impact of sodium acetate on lipid and carotenoid production in Rhodotorula mucilaginosa. Prep. Biochem. Biotechnol. 2025, 55, 590–605. [Google Scholar] [CrossRef]
- Cutzu, R.; Coi, A.; Rosso, F.; Bardi, L.; Ciani, M.; Budroni, M.; Zara, G.; Zara, S.; Mannazzu, I. From crude glycerol to carotenoids by using a Rhodotorula glutinis mutant. World J. Microbiol. Biotechnol. 2013, 29, 1009–1017. [Google Scholar] [CrossRef]
- Goodwin, T.W. Biosynthesis of carotenoids: An overview. Methods Enzymol. 1993, 214, 330–340. [Google Scholar]
- Moise, A.R.; Al-Babili, S.; Wurtzel, E.T. Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 2014, 114, 164–193. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.V.D.; Amore, T.D.; Teixeira, E.C.; de Medeiros Burkert, J.F. Carotenoid production by Rhodotorula mucilaginosa in batch and fed-batch fermentation using agroindustrial byproducts. Food Technol. Biotechnol. 2019, 57, 388. [Google Scholar] [CrossRef]
- Mosqueda-Martínez, E.; Chiquete-Félix, N.; Castañeda-Tamez, P.; Ricardez-García, C.; Gutiérrez-Aguilar, M.; Uribe-Carvajal, S.; Mendez-Romero, O. In Rhodotorula mucilaginosa, active oxidative metabolism increases carotenoids to inactivate excess reactive oxygen species. Front. Fungal Biol. 2024, 5, 1378590. [Google Scholar] [CrossRef]
- Ghilardi, C.; Sanmartin Negrete, P.; Carelli, A.A.; Borroni, V. Evaluation of olive mill waste as substrate for carotenoid production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020, 7, 52. [Google Scholar] [CrossRef]
- Maldonade, I.R.; Rodriguez-Amaya, D.B.; Scamparini, A.R. Carotenoids of yeasts isolated from the Brazilian ecosystem. Food Chem. 2008, 107, 145–150. [Google Scholar] [CrossRef]
- Libkind, D.; Sommaruga, R.; Zagarese, H.; van Broock, M. Mycosporines in carotenogenic yeasts. Syst. Appl. Microbiol. 2005, 28, 749–754. [Google Scholar] [CrossRef]
- El-Banna, A.A.E.R.; Abd El-Razek, A.M.; El-Mahdy, A.R. Isolation, identification and screening of carotenoid-producing strains of Rhodotorula glutinis. Food Nutr. Sci. 2012, 3, 627–633. [Google Scholar]
- Hu, P.; Mao, J.; Zeng, Y.; Sun, Z.; Deng, H.; Chen, C.; Sun, W.; Tang, Z. Isolation, identification, and function of Rhodotorula mucilaginosa TZR2014 and its effects on the growth and health of weaned piglets. Front. Microbiol. 2022, 13, 922136. [Google Scholar] [CrossRef] [PubMed]
- Hof, H. Rhodotorula spp. in the gut–foe or friend? GMS Infect. Dis. 2019, 7, Doc02. [Google Scholar] [PubMed]
- Akinjogunla, O.J.; Adefiranye, O.O.; Edem, E.N.; Adenugba, I.T.; Ogboona, F.C.; Oshosanya, G.O. Antibiotic resistance profile, multidrug-, extensively drug-, and pandrug-resistant bacterial isolates: Hemagglutination and hemolytic activities against human erythrocytes. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2025, 95, 207–222. [Google Scholar] [CrossRef]
- Russell, F.M.; Biribo, S.S.N.; Selvaraj, G.; Oppedisano, F.; Warren, S.; Seduadua, A.; Mulholland, E.K.; Carapetis, J.R. As a bacterial culture medium, citrated sheep blood agar is a practical alternative to citrated human blood agar in laboratories of developing countries. J. Clin. Microbiol. 2006, 44, 3346–3351. [Google Scholar] [CrossRef]
- Hewedy, M.A.; Ashour, S.M. Production of a melanin like pigment by Kluyveromyces marxianus and Streptomyces chibaensis. Aust. J. Basic Appl. Sci. 2009, 3, 920–927. [Google Scholar]
- Keneni, A.; Gupta, V.K. Characterization of a Red bacterium strain isolated from root nodule of a Faba Bean (Vicia faba L.) for Growth Pigment Production. J. Adv. Lab. Res. Biol. 2011, 2, 138–146. [Google Scholar]
- Ramírez, J.; Obledo, N.; Arellano, M.; Herrera, E. Astaxanthin production by Phaffia rhodozyma in a fedbatch culture using a low cost medium feeding. e-Gnosis 2006, 4, 5. [Google Scholar]
- Sharma, R.; Ghoshal, G. Optimization of carotenoids production by Rhodotorula mucilaginosa (MTCC-1403) using agro-industrial waste in bioreactor: A statistical approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Malisorn, C.; Suntornsuk, W. Optimization of β-carotene production by Rhodotorula glutinis DM28 in fermented radish brine. Bioresour. Technol. 2008, 99, 2281–2287. [Google Scholar] [CrossRef]
- Hayman, E.P.; Yokoyama, H.; Chichester, C.O.; Simpson, K.L. Carotenoid biosynthesis in Rhodotorula glutinis. J. Bacteriol. 1974, 120, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Maldonade, I.R.; Scamparini, A.R.; Rodriguez-Amaya, D.B. Selection and characterization of carotenoid-producing yeasts from Campinas region, Brazil. Braz. J. Microbiol. 2007, 38, 65–70. [Google Scholar] [CrossRef]
- Ochoa-Viñals, N.; Alonso-Estrada, D.; Pacios-Michelena, S.; García-Cruz, A.; Ramos-González, R.; Faife-Pérez, E.; Iliná, A. Current Advances in Carotenoid Production by Rhodotorula sp. Fermentation 2024, 10, 190. [Google Scholar] [CrossRef]
- Latha, B.V.; Jeevaratnam, K.; Murali, H.S.; Manja, K.S. Influence of growth factors on carotenoid pigmentation of Rhodotorula glutinis DFR-PDY from natural source. IJBT 2005, 4, 353–357. [Google Scholar]
- Nasrabadi, M.R.N.; Razavi, S.H. Optimization of β-carotene production by a mutant of the lactose-positive yeast Rhodotorula acheniorum from whey ultrafiltrate. Food Sci. Biotechnol. 2011, 20, 445–454. [Google Scholar] [CrossRef]
- Serra-Cardona, A.; Canadell, D.; Ariño, J. Coordinate responses to alkaline pH stress in budding yeast. Microb. Cell 2015, 2, 182. [Google Scholar] [CrossRef]
- Casado, C.; González, A.; Platara, M.; Ruiz, A.; Ariño, J. The role of the protein kinase A pathway in the response to alkaline pH stress in yeast. Biochem. J. 2011, 438, 523–533. [Google Scholar] [CrossRef]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef]
- Weeks, O.B.; Saleh, F.K.; Wirahadiku-Sumah, M.; Berry, R.A. Photoregulated carotenoid biosynthesis in non-photosynthetic microorganisms. Pure Appl. Chem. 1973, 35, 63–80. [Google Scholar] [CrossRef][Green Version]
- Yen, H.W.; Zhang, Z. Enhancement of cell growth rate by light irradiation in the cultivation of Rhodotorula glutinis. Bioresour. Technol. 2011, 102, 9279–9281. [Google Scholar] [CrossRef]
- Moliné, M.; Flores, M.R.; Libkind, D.; del Carmen Dieguez, M.; Farías, M.E.; van Broock, M. Photoprotection by carotenoid pigments in the yeast Rhodotorula mucilaginosa: The role of torularhodin. Photochem. Photobiol. Sci. 2010, 9, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Balraj, J.; Pannerselvam, K.; Jayaraman, A. Isolation of pigmented marine bacteria Exiguobacterium sp. from peninsular region of India and a study on biological activity of purified pigment. Int. J. Sci. Technol. Res. 2014, 3, 375–384. [Google Scholar]
- Goswami, G.; Chaudhuri, S.; Dutta, D. Effect of pH and temperature on pigment production from an isolated bacterium. Chem. Eng. Trans. 2010, 20, 127–132. [Google Scholar]
- Szotkowski, M.; Plhalová, Ž.; Sniegoňová, P.; Holub, J.; Chujanov, O.; Špačková, D.; Blažková, J.; Márová, I. Conversion of mixed waste food substrates by carotenogenic yeasts of Rhodotorula sp. genus. Microorganisms 2023, 11, 1013. [Google Scholar] [CrossRef]
- Salih, A.I. Effect of some types of oils and Surfactant factors on the production of carotenoids from the yeast of Rhodotorula glutinis. Al-Kitab J. Pure Sci. 2022, 2, 292–304. [Google Scholar] [CrossRef]
- Moosavi Nasab Marzieh Abedi, E.; Moosavi-Nasab, S. Utilization of date syrup as a substrate for carotenoid production by Rhodotorula glutinis. Iran Agric. Res. 2015, 34, 8–13. [Google Scholar]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of lipids and carotenoids by Rhodotorula gracilis ATCC 10788 yeast in a bioreactor using low-cost wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Banzatto, D.; Freita, L.A.D.; Mutton, M.J.R. Carotenoid production by Rhodotorula rubra cultivated in sugarcane juice, molasses, and syrup. Food Sci. Technol. 2013, 33, 14–18. [Google Scholar] [CrossRef]
- Keskin, A.; Fırat, M.; Büyüktopcu, A.E.Ü. Valorization of shalgam juice plant waste for the production of carotenoids by Rhodotorula glutinis. Int. J. Agric. Environ. Food Sci. 2023, 7, 79–87. [Google Scholar] [CrossRef]
- Ibrahim, G.S.; El-Shall, F.N.; Arafa, A.A.; Shalabi, A.; El Awady, M.E. Bio-production and characterization of carotenoid yellow pigment from Kocuria sp. GMA and exploring its sustainable antioxidant, antimicrobial and antibiofilm properties. Egypt. J. Chem. 2024, 67, 57–68. [Google Scholar] [CrossRef]
- Reksamunandar, R.P.; Edikresnha, D.; Munir, M.M.; Damayanti, S. Encapsulation of β-carotene in poly (vinylpyrrolidone)(PVP) by electrospinning Technique. Procedia Eng. 2017, 170, 19–23. [Google Scholar] [CrossRef]
- Hagos, M.; Redi-Abshiro, M.; Chandravanshi, B.S.; Yaya, E.E. Development of Analytical Methods for Determination of β-Carotene in Pumpkin (Cucurbita maxima) Flesh, Peel, and Seed Powder Samples. Int. J. Anal. Chem. 2022, 2022, 9363692. [Google Scholar] [CrossRef] [PubMed]
- Moh, M.H.; Man, Y.C.; Badlishah, B.S.; Jinap, S.; Saad, M.S.; Abdullah, W.J.W. Quantitative analysis of palm carotene using Fourier transform infrared and near infrared spectroscopy. J. Am. Oil Chem. Soc. 1999, 76, 249–254. [Google Scholar] [CrossRef]
- Martin, D.; Amado, A.M.; Gonzalvez, A.G.; Marques, M.P.M.; Batista de Carvalho, L.A.; Ureña, Á.G. FTIR Spectroscopy and DFT calculations to probe the kinetics of β-carotene thermal degradation. J. Phys. Chem. A 2019, 123, 5266–5273. [Google Scholar] [CrossRef]
- Iqbal, I.; Sarwar, W.; Ali, Q.; Ahmed, S. Characterization of Pigment Production by Endophytic Rhodotorula mucilaginosa MGI from Tagetes erecta. Microbiol. Biotechnol. Lett. 2024, 52, 314–324. [Google Scholar] [CrossRef]
- Noguchi, T.; Mitsuka, T.; Inoue, Y. Fourier transform infrared spectrum of the radical cation of β-carotene photoinduced in photosystem II. FEBS Lett. 1994, 356, 179–182. [Google Scholar] [CrossRef]
- Goswami, L.; Kayalvizhi, R.; Dikshit, P.K.; Sherpa, K.C.; Roy, S.; Kushwaha, A.; Kim, B.S.; Banerjee, R.; Jacob, S.; Rajak, R.C. A critical review on prospects of bio-refinery products from second and third generation biomasses. Chem. Eng. J. 2022, 448, 137677. [Google Scholar] [CrossRef]
- Saha, P.; Goswami, L.; Kim, B.S. Novel Biobased Non-isocyanate polyurethanes from Microbially Produced 7,10-Dihydroxy-8(E)-Octadecenoic acid for potential packaging and coating applications. ACS Sustain. Chem. Eng. 2022, 10, 4623–4633. [Google Scholar] [CrossRef]
- Kushwaha, A.; Yadav, A.N.; Singh, B.; Dwivedi, V.; Kumar, S.; Goswami, L.; Hussain, C.M. Life cycle assessment and techno-economic analysis of algae-derived biodiesel: Current challenges and future prospects. In Waste-to-Energy Approaches Towards Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–372. [Google Scholar]
- Goswami, L.; Kushwaha, A.; Napathorn, S.C.; Kim, B.S. Valorization of organic wastes using bioreactors for polyhydroxyalkanoate production: Recent advancement, sustainable approaches, challenges, and future perspectives. Int. J. Biol. Macromol. 2023, 247, 125743. [Google Scholar] [CrossRef]
- Kafle, S.R.; Kushwaha, A.; Goswami, L.; Maharjan, A.; Kim, B.S. A holistic approach for process intensification of nicotinamide mononucleotide production via high cell density cultivation under exponential feeding strategy. Bioresour. Technol. 2023, 390, 129911. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S. Advancement in innovative strategies for poly (ethylene terephthalate) biodegradation. Curr. Opin. Chem. Eng. 2025, 48, 101121. [Google Scholar] [CrossRef]
- Padigala, C.T.; Satpati, G.G.; Singhvi, M.; Goswami, L.; Kushwaha, A.; Oraon, S.; Aleksanyan, K.; Smykovskaya, R.S.; Rawindran, H.; Wei, L.J.; et al. Nanotechnological advancement in green hydrogen production from organic waste: Recent developments, techno–economic, and life cycle analyses. Int. J. Hydrogen Energy 2024, 92, 672–693. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Kim, B.S. An Integrated Chemical and Biological Approach for Poly (ethylene terephthalate) Depolymerization and Biopolyol Production. ACS Sustain. Chem. Eng. 2024, 12, 11866–11878. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Saha, P.; Kim, B.S. A novel integrated membrane bioreactor-stirred tank bioreactor system for simultaneous biodegradation of bisphenol A contaminated wastewater and biopolyol production: An approach towards circular bioeconomy. Chem. Eng. J. 2024, 490, 151740. [Google Scholar] [CrossRef]
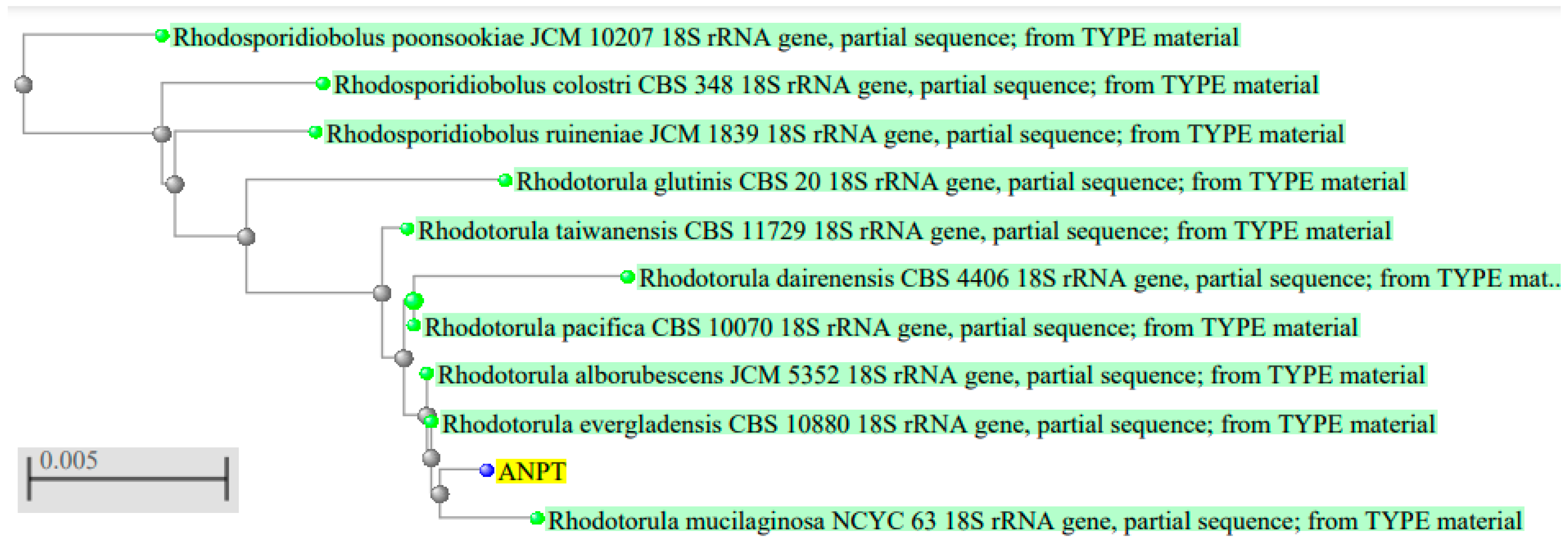
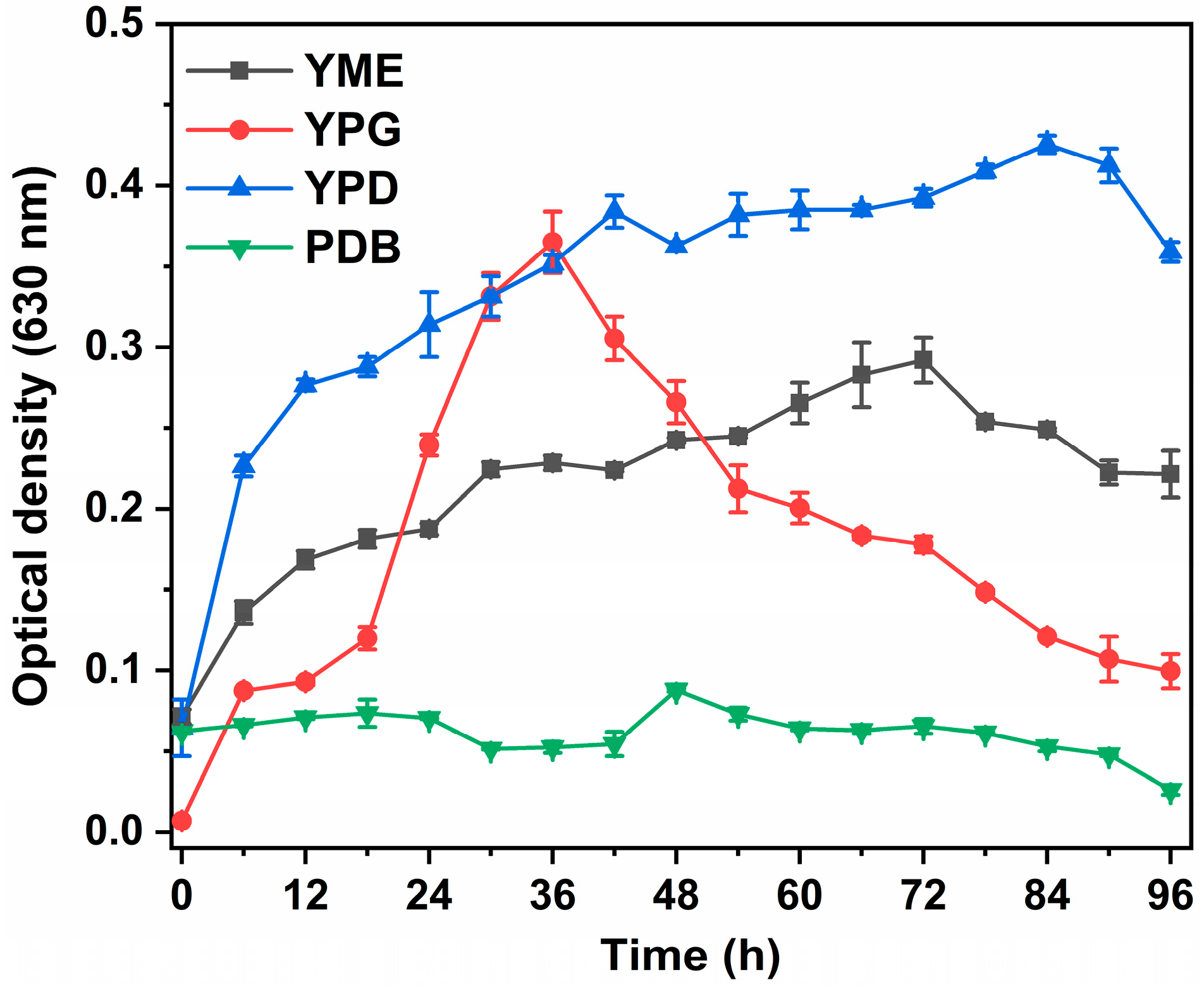

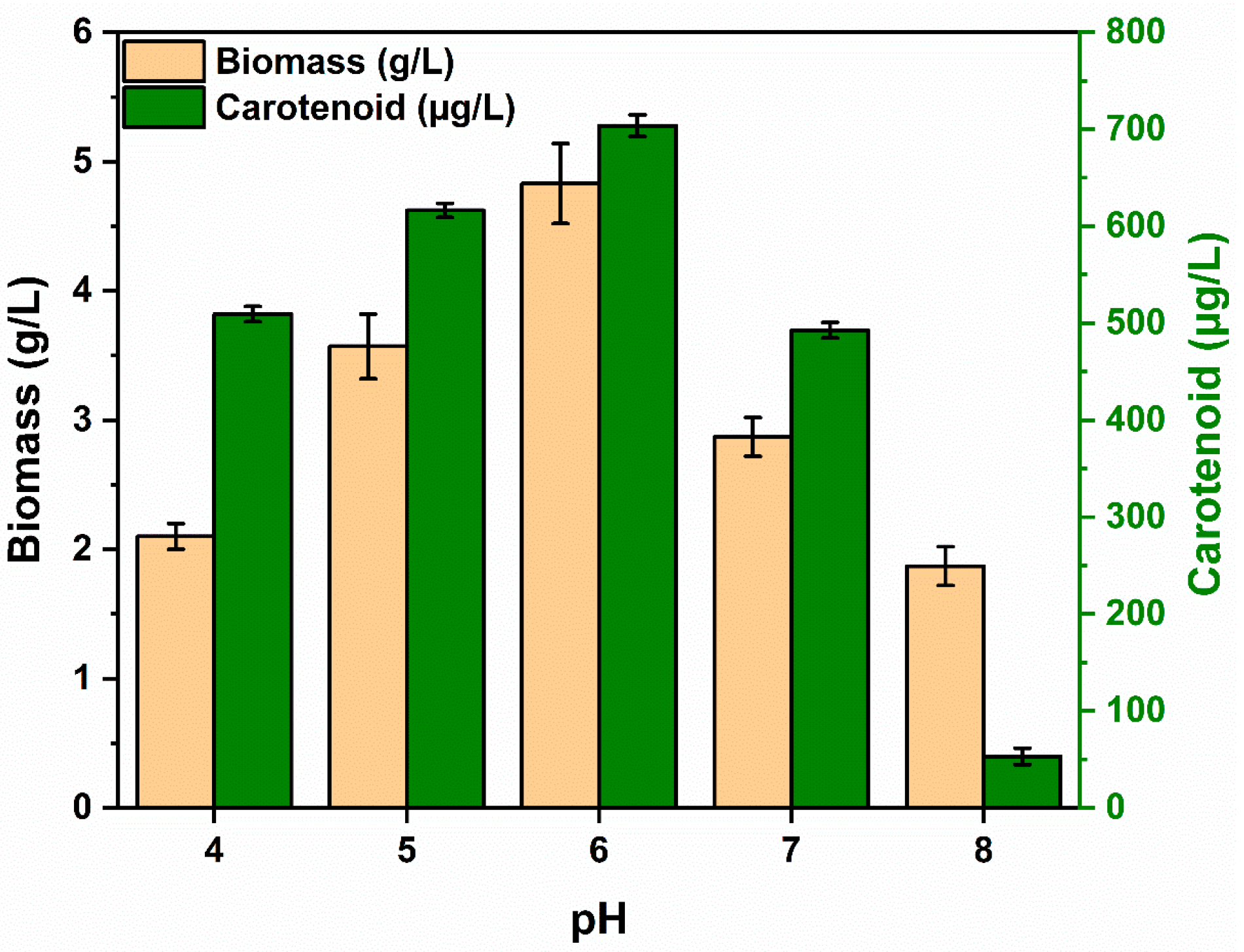
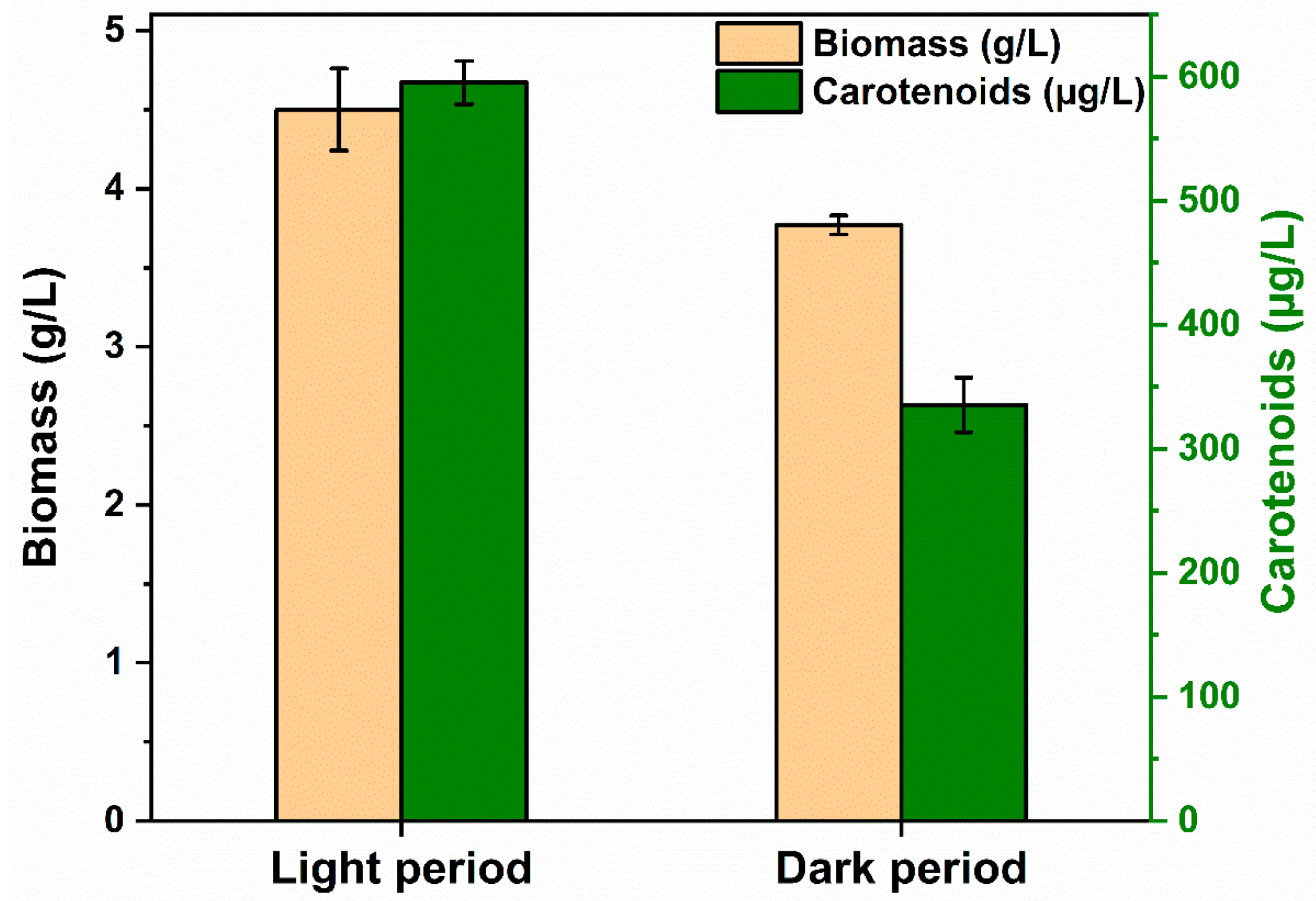
| Characteristics | Results | Characteristics | Results |
|---|---|---|---|
| color | Pink | Glycerol | + |
| Appearance | Glossy and Smooth | D-Mannitol | - |
| Colony shape | Oval | D-Glucitol (10%) | - |
| Cell Shape | Ovoid | Citrate | - |
| Texture | Mucoid | Succinate (10%) | + |
| Elevation | Raised | Vitamin-free | - |
| Mycelium | - | Nitrate | - |
| Conjugation | - | Starch formation | - |
| Ascospore | - | Insulin | - |
| Sucrose (10%) | + | Urease | + |
| Galactose (10%) | + | Gelatin Liquification | - |
| Maltose (10%) | + | Growth—25 °C | + |
| Glucose (10%) | + | Growth—37 °C | + |
| Lactose | - | Growth—42 °C | - |
| Methanol | - | Sedimentation | + |
| Ethanol | - | Soluble starch | - |
| Source of Variation | SS | df | MS | F | p-Value | F Crit |
|---|---|---|---|---|---|---|
| Between Groups | 33,021.69 | 4 | 8255.423 | 50.5839 | 1.43 × 10−20 | 2.493696 |
| Within Groups | 12,240.19 | 75 | 163.2026 | |||
| Total | 45,261.89 | 79 |
| S. No. | Strain | Substrate Utilized | Carotenoid Yield | Reference |
|---|---|---|---|---|
| 1. | Rhodotorula mucilaginosa | Alpeerujo water | 0.78 mg/g | [34] |
| 2. | Rhodotorula mucilaginosa | Sugarcane molasses and corn steep liquor | 1.248 mg/L | [32] |
| 3. | Rhodotorula kratochvilovae | Waste animal fat | 4.930 mg/g | [60] |
| 4. | Rhodotorula toruloides | Waste glycerol and coffee oil | 10.302 mg/g | [60] |
| 5. | Rhodotorula glutinis | Black seed oil | 1.057 mg/L | [61] |
| 6. | Rhodotorula glutinis | Date syrup | 7.94 mg/L | [62] |
| 7. | Rhodotorula gracilis | Potato wastewater and glycerol | 6.24 mg/L | [63] |
| 8. | Rhodotorula rubra | Molasses | 2.74 mg/L | [64] |
| 9. | Rhodotorula glutinis | Shalgam juice | 1.221 mg/L | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anshi; Kaur, H.; Goswami, L.; Kapil, S.; Sharma, V. Isolation, Optimization and Characterization of Rhodotorula alborubescens for Dietary Pigment β-Carotene Production. Appl. Microbiol. 2025, 5, 54. https://doi.org/10.3390/applmicrobiol5020054
Anshi, Kaur H, Goswami L, Kapil S, Sharma V. Isolation, Optimization and Characterization of Rhodotorula alborubescens for Dietary Pigment β-Carotene Production. Applied Microbiology. 2025; 5(2):54. https://doi.org/10.3390/applmicrobiol5020054
Chicago/Turabian StyleAnshi, Hardeep Kaur, Lalit Goswami, Shikha Kapil, and Vipasha Sharma. 2025. "Isolation, Optimization and Characterization of Rhodotorula alborubescens for Dietary Pigment β-Carotene Production" Applied Microbiology 5, no. 2: 54. https://doi.org/10.3390/applmicrobiol5020054
APA StyleAnshi, Kaur, H., Goswami, L., Kapil, S., & Sharma, V. (2025). Isolation, Optimization and Characterization of Rhodotorula alborubescens for Dietary Pigment β-Carotene Production. Applied Microbiology, 5(2), 54. https://doi.org/10.3390/applmicrobiol5020054





