Modeling the Double Peak Phenomenon in Drug Absorption Kinetics: The Case of Amisulpride
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Non-Linear Mixed Effect Modeling
2.2.1. General
2.2.2. Structural Model and Absorption Models
2.2.3. Model Evaluation
2.3. Simulations
3. Results
3.1. Developed Model
3.2. Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hadryś, T.; Rymaszewska, J. Amisulpride—Is it as all other medicines or is it different? An update. Psychiatr. Pol. 2020, 54, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, L.; Shang, D.W.; Wen, Y.G.; Ning, Y.P. A systematic review and combined meta-analysis of concentration of oral amisulpride. Br. J. Clin. Pharmacol. 2020, 86, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.C.; Paletta, S.; Di Pace, C.; Reggiori, A.; Cirnigliaro, G.; Valli, I.; Altamura, A.C. Clinical pharmacokinetics of atypical antipsychotics: An update. Clin. Pharmacokinet. 2018, 57, 1493–1528. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Örey, D.; Richter, F.; Davis, J.M. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Osser, D.N.; Roudsari, M.J.; Manschreck, T. The psychopharmacology algorithm project at the Harvard South Shore Program: An update on schizophrenia. Harv. Rev. Psychiatry 2013, 21, 18–40. [Google Scholar] [CrossRef]
- Leucht, S.; Crippa, A.; Siafis, S.; Patel, M.X.; Orsini, N.; Davis, J.M. Dose-Response Meta-Analysis of Antipsychotic Drugs for Acute Schizophrenia. Am. J. Psychiatry 2020, 177, 342–353. [Google Scholar] [CrossRef]
- Sabe, M.; Zhao, N.; Crippa, A.; Kaiser, S. Antipsychotics for negative and positive symptoms of schizophrenia: Dose-response meta-analysis of randomized controlled acute phase trials. NPJ Schizophr. 2021, 7, 43. [Google Scholar] [CrossRef]
- Hiemke, C.; Baumann, P.; Bergemann, N.; Conca, A.; Dietmaier, O.; Egberts, K.; Zernig, G. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: Update 2011. Pharmacopsych 2011, 21, 195–235. [Google Scholar] [CrossRef]
- Lako, I.M.; van den Heuvel, E.R.; Knegtering, H.; Bruggeman, R.; Taxis, K. Estimating dopamine D(2) receptor occupancy for doses of 8 antipsychotics: A meta-analysis. J. Clin. Psychopharmacol. 2013, 33, 675–681. [Google Scholar] [CrossRef]
- Sparshatt, A.; Taylor, D.; Patel, M.X.; Kapur, S. Amisulpride—Dose, plasma concentration, occupancy and response: Implications for therapeutic drug monitoring. Acta Psychiatr. Scand. 2009, 120, 416–428. [Google Scholar] [CrossRef]
- Urban, A.E.; Cubała, W.J. Therapeutic drug monitoring of atypical antipsychotics. Psychiatr Pol. 2017, 51, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Zhou, Q.; Tian, L.; Shen, Y.; Zhou, Z. Amisulpride steady-state plasma concentration and adverse reactions in patients with schizophrenia: A study based on therapeutic drug monitoring data. Int. Clin. Psychopharmacol. 2022, 37, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.K.; Spigset, O.; Reis, M. A Compilation of Serum Concentrations of 12 Antipsychotic Drugs in a Therapeutic Drug Monitoring Setting. Ther. Drug Monit. 2019, 41, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Huang, J.; Long, Y.; Wang, X.; Wang, Y.; Yang, Y.; Hei, G.; Sun, M.; Zhao, J.; Li, L.; et al. Optimizing and Individualizing the Pharmacological Treatment of First-Episode Schizophrenic Patients: Study Protocol for a Multicenter Clinical Trial. Front. Psychiatry 2021, 12, 611070. [Google Scholar] [CrossRef] [PubMed]
- Coukell, A.; Benfield, P. Amisulpride: A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of schizophrenia. CNS Drugs 1996, 6, 237–256. [Google Scholar] [CrossRef]
- Hamon-Vilcot, B.; Chaufour, S.; Deschamps, C.; Canal, M.; Zieleniuk, I.; Ahtoy, P.; Chretien, P.; Rosenzweig, P.; Nasr, A.; Piette, F. Safety and pharmacokinetics of a single oral dose of amisulpride in healthy elderly volunteers. Eur. J. Clin. Pharmacol. 1998, 54, 405–409. [Google Scholar] [CrossRef]
- Rosenzweig, P.; Canal, M.; Patat, A.; Bergougnan, L.; Zieleniuk, I.; Bianchetti, G. A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers. Hum. Psychopharmacol. 2002, 17, 1–13. [Google Scholar] [CrossRef]
- Musenga, A.; Mandrioli, R.; Morganti, E.; Fanali, S.; Raggi, M.A. Enantioselective analysis of amisulpride in pharmaceutical formulations by means of capillary electrophoresis. J. Pharm. Biomed. Anal. 2008, 46, 966–970. [Google Scholar] [CrossRef]
- Wang, R.; Sun, X.; Deng, Y.S.; Qiu, X.W. ABCB1 1199G>A Polymorphism Impacts Transport Ability of P-gp-Mediated Antipsychotics. DNA Cell Biol. 2018, 37, 325–329. [Google Scholar] [CrossRef]
- Sutar, R.; Atlani, M.K.; Chaudhary, P. Antipsychotics and hemodialysis: A systematic review. Asian J. Psychiatr. 2021, 55, 102484. [Google Scholar] [CrossRef]
- Dos Santos Pereira, J.N.; Tadjerpisheh, S.; Abu Abed, M.; Saadatmand, A.R.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Brockmöller, J.; Tzvetkov, M.V. The poorly membrane permeable antipsychotic drugs amisulpride and sulpiride are substrates of the organic cation transporters from the SLC22 family. AAPS J. 2014, 16, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.W.; Schinkel, A.H. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2 and 3 (SLC22A1-3). J. Pharmacol. Exp. Ther. 2004, 308, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.; Bertrand, J.; D’Antonio, F.; McLachlan, E.; Nair, A.; Brownings, S.; Greaves, S.; Smith, A.; Taylor, D.; Howard, R. A population approach to characterise amisulpride pharmacokinetics in older people and Alzheimer’s disease. Psychopharmacology 2016, 233, 3371–3381. [Google Scholar] [CrossRef]
- Reeves, S.; Bertrand, J.; McLachlan, E.; D’Antonio, F.; Brownings, S.; Nair, A.; Greaves, S.; Smith, A.; Dunn, J.T.; Marsden, P.; et al. A population approach to guide amisulpride dose adjustments in older patients with Alzheimer’s disease. J. Clin. Psych. 2017, 78, e844–e851. [Google Scholar] [CrossRef]
- Glatard, A.; Guidi, M.; Delacrétaz, A.; Dubath, C.; Grosu, C.; Laaboub, N.; von Gunten, A.; Conus, P.; Csajka, C.; Eap, C.B. Amisulpride: Real-World Evidence of Dose Adaptation and Effect on Prolactin Concentrations and Body Weight Gain by Pharmacokinetic/Pharmacodynamic Analyses. Clin. Pharmacokinet. 2020, 59, 371–382. [Google Scholar] [CrossRef]
- Huang, S.; Li, L.; Wang, Z.; Xiao, T.; Li, X.; Liu, S.; Zhang, M.; Lu, H.; Wen, Y.; Shang, D. Modeling and Simulation for Individualized Therapy of Amisulpride in Chinese Patients with Schizophrenia: Focus on Interindividual Variability, Therapeutic Reference Range and the Laboratory Alert Level. Drug Des. Devel. Ther. 2021, 15, 3903–3913. [Google Scholar] [CrossRef] [PubMed]
- Alagga, A.A.; Gupta, V. Drug absorption. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557405/ (accessed on 12 January 2023).
- Falcoz, C.; Guzy, S.; Kovač, J.; Meister, I.; Coulibaly, J.; Sayasone, S.; Wesche, D.; Lin, Y.W.; Keiser, J. R-praziquantel integrated population pharmacokinetics in preschool-and school-aged African children infected with Schistosoma mansoni and S. haematobium and Lao adults infected with Opisthorchis viverrini. J. Pharmacokinet. Pharmacodyn. 2022, 49, 293–310. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; Tan, W.; Li, J.; Haughey, M.; Masters, J.; Hibma, J.; Lin, S. Pharmacokinetic models to characterize the absorption phase and the influence of a proton pump inhibitor on the overall exposure of dacomitinib. Pharmaceutics 2020, 12, 330. [Google Scholar] [CrossRef]
- Gasthuys, E.; Vermeulen, A.; Croubels, S.; Millecam, J.; Schauvliege, S.; van Bergen, T.; De Bruyne, P.; Vande Walle, J.; Devreese, M. Population Pharmacokinetic Modeling of a Desmopressin Oral Lyophilisate in Growing Piglets as a Model for the Pediatric Population. Front. Pharmacol. 2018, 31, 41. [Google Scholar] [CrossRef]
- Savic, R.M.; Jonker, D.M.; Kerbusch, T.; Karlsson, M.O. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J. Pharmacokinet. Pharmacodyn. 2007, 34, 711–726. [Google Scholar] [CrossRef]
- Holford, N.H.; Ambros, R.J.; Stoeckel, K. Models for describing absorption rate and estimating extent of bioavailability: Application to cefetamet pivoxil. J. Pharmacokinet. Biopharm. 1992, 20, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Kousovista, R.; Karali, G.; Vlasopoulou, K.; Karalis, V. Validation of population pharmacokinetic models: A comparison of internal and external validation approaches for hydrochlorothiazide. Xenobiotica 2021, 51, 1372–1388. [Google Scholar] [CrossRef]
- Godfrey, K.R.; Arundel, P.A.; Dong, Z.; Bryant, R. Modelling the double peak phenomenon in pharmacokinetics. IFAC Proc. Vol. 2009, 42, 127–132. [Google Scholar] [CrossRef]
- Jaber, M.M.; Al-Kofahi, M.; Sarafoglou, K.; Brundage, R.C. Individualized Absorption Models in Population Pharmacokinetic Analyses. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Cirincione, B.; Edwards, J.; Mager, D.E. Population pharmacokinetics of an extended-release formulation of exenatide following single-and multiple-dose administration. AAPS J. 2017, 19, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, M.S.; Seong, S.J.; Park, S.M.; Gwon, M.R.; Han, S.; Lee, S.M.; Kin, W.; Yoon, Y.R.; Yoo, H.D. Population pharmacokinetic analysis of the multiple peaks phenomenon in sumatriptan. Transl. Clin. Pharmacol. 2015, 23, 66–74. [Google Scholar] [CrossRef]
- Godfrey, K.R.; Arundel, P.A.; Dong, Z.; Bryant, R. Modelling the double peak phenomenon in pharmacokinetics. Comput. Methods. Programs Biomed. 2011, 104, 62–69. [Google Scholar] [CrossRef]
- Shen, J.; Boeckmann, A.; Vick, A. Implementation of dose superimposition to introduce multiple doses for a mathematical absorption model (transit compartment model). J. Pharmacokinet. Pharmacodyn. 2012, 39, 251–262. [Google Scholar] [CrossRef]
- Moffat, A.C.; Osselton, M.D.; Widdop, B.O. Clarke’s Analysis of Drugs and Poisons; Pharmaceutical Press: London, UK, 2011. [Google Scholar]
- Oberle, R.L.; Amidon, G.L. The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine; an explanation for the double peak phenomenon. J. Pharmacokinet. Biopharm. 1987, 15, 529–544. [Google Scholar] [CrossRef]
- Bowskill, S.V.; Patel, M.X.; Handley, S.A.; Flanagan, R.J. Plasma amisulpride in relation to prescribed dose, clozapine augmentation, and other factors: Data from a therapeutic drug monitoring service, 2002–2010. Hum. Psychopharmacol. 2012, 27, 507–513. [Google Scholar] [CrossRef]
- Wang, Z.Z. Research on therapeutic drug monitoring and clinical application of amisulpride tablets. Chin. J. Clin. Pharmacol. 2018, 34, 2704–2706. [Google Scholar]
- Wang, S.T.; Li, Y. Development of a UPLC-MS/MS method for routine therapeutic drug monitoring of aripiprazole, amisulpride, olanzapine, paliperidone and ziprasidone with a discussion of their therapeutic reference ranges for Chinese patients. Biomed. Chromatogr. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
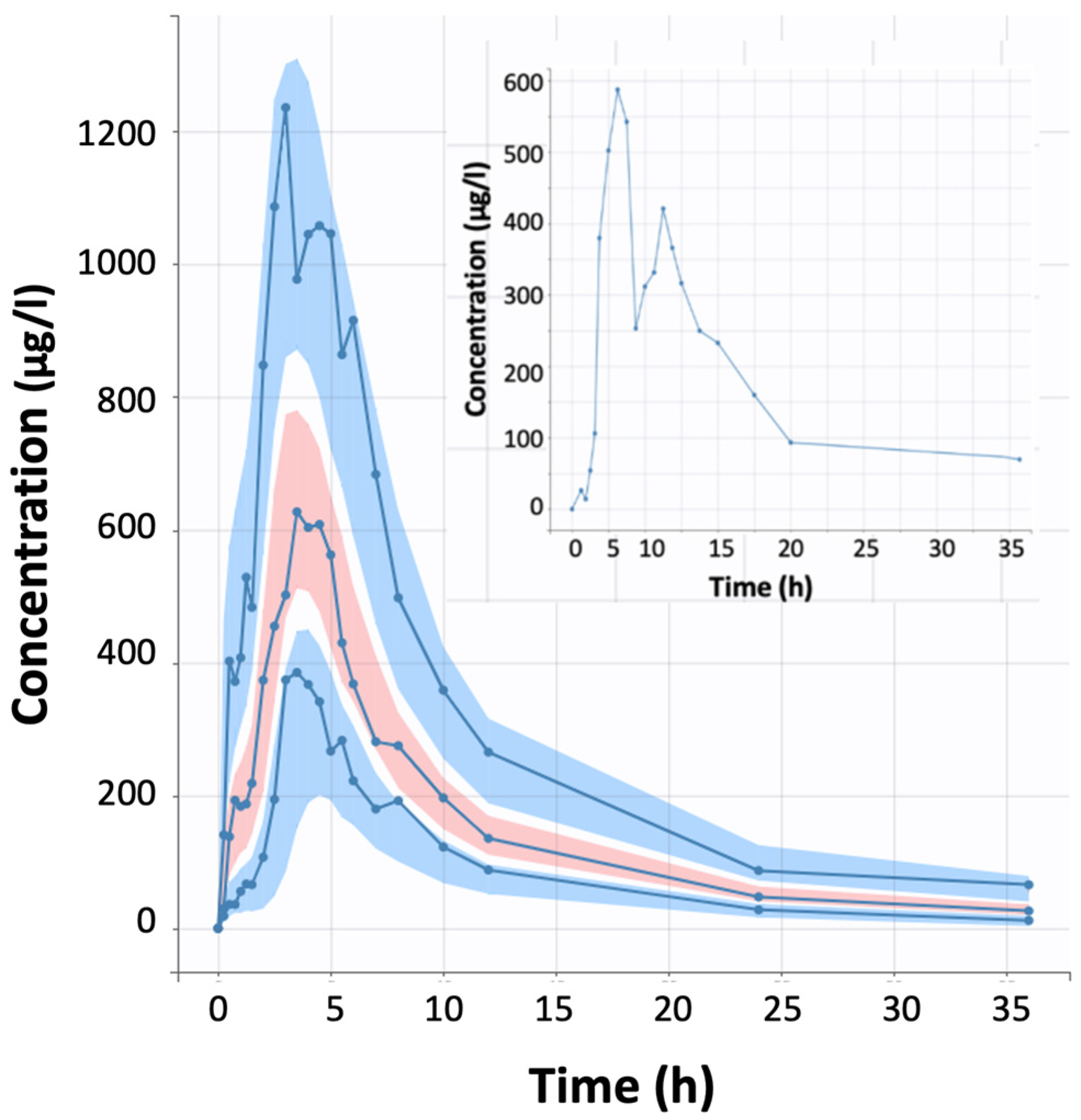
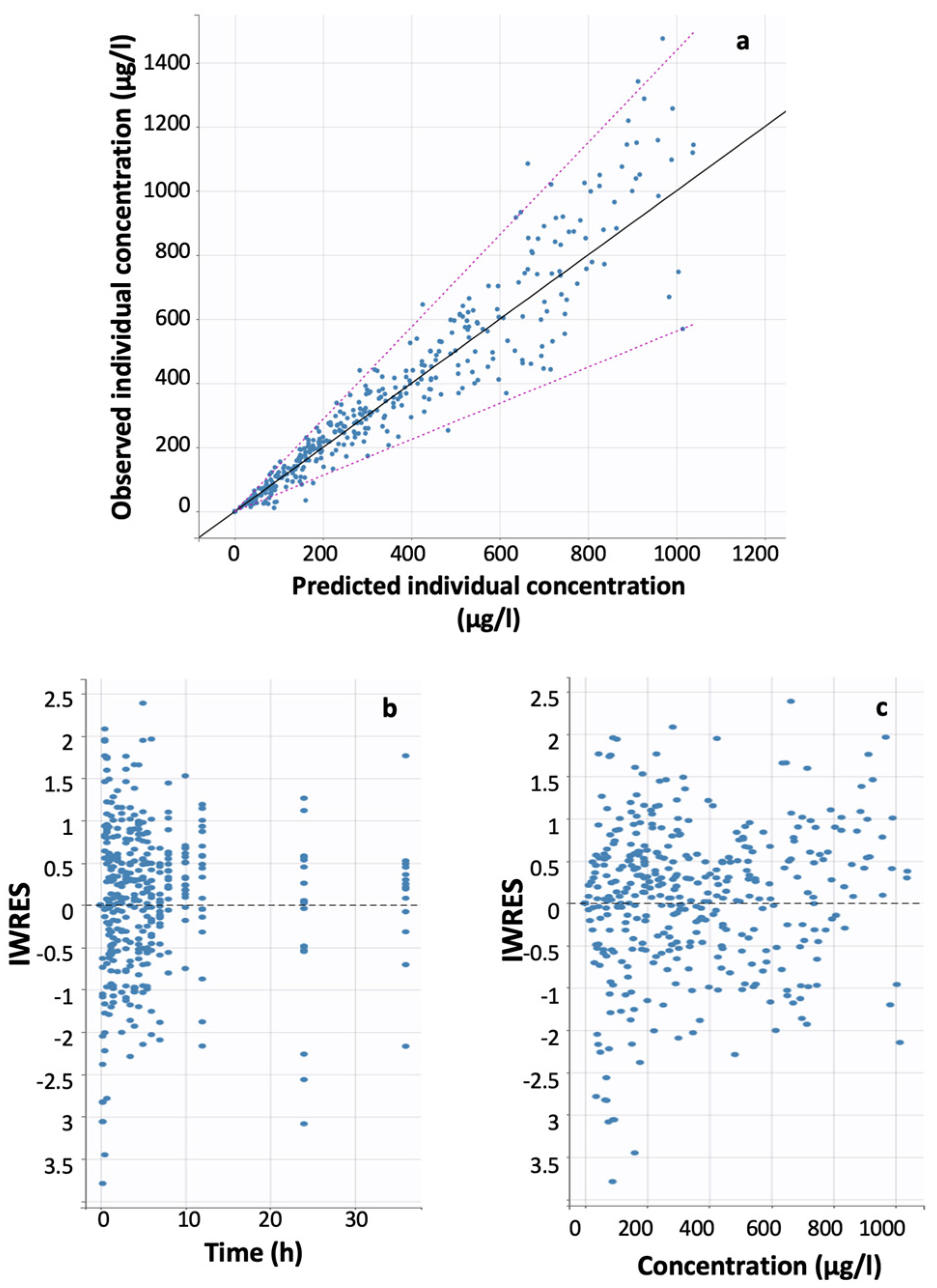
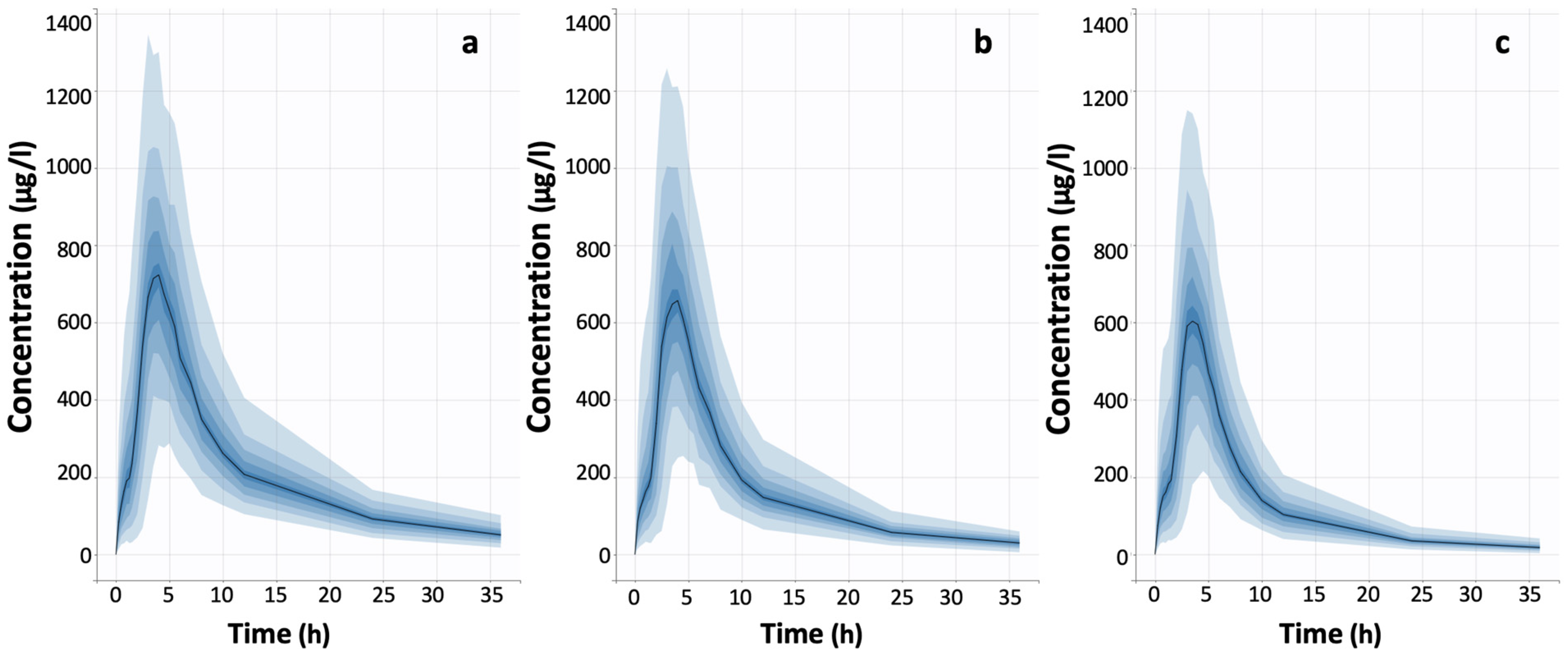

| Model ID | Model Short Description | Statistical Criterion a | ||
|---|---|---|---|---|
| −2LL | AIC | BIC | ||
| 1 | 1-compartment model, first-order absorption | 3642.7 | 3656.7 | 3663.31 |
| 2 | 1-compartment model, first-order absorption with lag time | 3615.59 | 3633.59 | 3642.09 |
| 3 | 1-compartment model, first-order absorption, and transit compartment | 3539.82 | 3561.82 | 3572.21 |
| 4 | 2-compartment model, first-order absorption | 3572.16 | 3594.16 | 3604.55 |
| 5 | 2-compartment model, first-order absorption with lag time | 3533.54 | 3559.54 | 3571.81 |
| 6 | 2-compartment model, first-order absorption, and transit compartment | 3477.93 | 3507.93 | 3522.09 |
| 7 | 2-compartment, first-order absorption followed by first-order absorption | 3574.6 | 3604.6 | 3618.76 |
| 8 | 2-compartment, first-order absorption followed by first-order absorption with lag time | 3287.38 | 3321.38 | 3337.44 |
| 9 | 2-compartment, first-order absorption followed by zero-order absorption with lag time | 3297.2 | 3331.2 | 3347.25 |
| 10 | 2-compartment model, zero-order absorption followed by first-order absorption with lag time | 3348.72 | 3382.72 | 3398.78 |
| 11 | 3-compartment model, first-order absorption | 3572.91 | 3602.91 | 3617.08 |
| 12 | 3-compartment model, first-order absorption with lag time | 3533.13 | 3567.13 | 3583.18 |
| 13 | 3-compartment model, first-order absorption, and transit compartment | 3486.41 | 3524.41 | 3542.35 |
| 14 | 2-compartment, first-order absorption followed by first-order absorption with lag time Covariates: body weight on V1/F (p = 0.28) | 3286.95 | 3322.95 | 3339.95 |
| 15 | 2-compartment, first-order absorption followed by first-order absorption with lag time Covariates: body weight on V2/F (p = 0.68) | 3286.11 | 3322.11 | 3339.11 |
| 16 | 2-compartment, first-order absorption followed by first-order absorption with lag time Covariates: age on CL/F (p = 0.53) | 3285.61 | 3321.61 | 3338.61 |
| 17 | 2-compartment, first-order absorption followed by first-order absorption with lag time Covariates: gender on CL/F (p = 0.40) and body weight on CL/F (p = 0.00) | 3279.23 | 3317.23 | 3335.18 |
| 18 | 2-compartment, first-order absorption followed by first-order absorption with lag time Covariates: age on CL/F (p = 0.48) and body weight on CL/F (p = 0.00) | 3278.46 | 3316.46 | 3334.41 |
| 19 | 2-compartment, first-order absorption followed by first-order absorption with lag time Covariates: body weight on CL/F (p = 0.012) | 3278.89 | 3314.89 | 3331.89 |
| 20 | 2-compartment, first-order absorption followed by first-order absorption with lag time Covariates: body weight on CL/F (p = 0.01) Correlations: Q/F and CL/F, ka1 and F | 3254.61 | 3294.61 | 3313.5 |
| Parameters (Units) | Value | Standard Error | Relative Standard Error (%) | p-Value |
|---|---|---|---|---|
| Fixed effects | ||||
| ka1 (h−1) | 0.76 | 0.16 | 21.1 | |
| ka2 (h−1) | 0.91 | 0.17 | 18.6 | |
| z | 0.30 | 0.08 | 29.0 | |
| Tlag2 (h) | 1.95 | 0.16 | 8.38 | |
| CL/F (ml/h) | 57.87 | 4.34 | 7.49 | |
| V1/F (ml) | 202.12 | 22.62 | 39.0 | |
| Q/F (ml/h) | 46.74 | 9.45 | 11.2 | |
| V2/F (ml) | 467.11 | 120.07 | 20.2 | |
| Beta weight on CL | 0.82 | 0.32 | 25.2 | 0.01 |
| Random effects | ||||
| ω_ka1 | 1.32 | 0.32 | 23.8 | |
| ω_ka2 | 0.43 | 0.14 | 31.8 | |
| ω_z | 1.46 | 0.35 | 23.9 | |
| ω_Tlag2 | 0.32 | 0.06 | 19.4 | |
| ω_CL | 0.22 | 0.04 | 20.3 | |
| ω_V1 | 0.076 | 0.007 | 10.1 | |
| ω_Q | 0.65 | 0.14 | 21.3 | |
| ω_V2 | 0.5 | 0.16 | 32.5 | |
| Correlations | ||||
| p(Q, CL) | 0.8 | 0.15 | 18.8 | |
| p(ka1, z) | −0.68 | 0.19 | 28.5 | |
| Error model parameters | ||||
| b | 0.27 | 0.01 | 4.79 | |
| Estimated log-likelihood and information criteria | ||||
| −2LL | 3254.61 | |||
| AIC | 3294.61 | |||
| BIC | 3313.5 | |||
| BICc | 3340.97 | |||
| Clinical Study | Reeves et al., 2016 [23] | Reeves et al., 2017 [24] | Glatard et al., 2019 [25] | Huang et al., 2021 [26] |
|---|---|---|---|---|
| Patient population | Healthy elderly participants with Alzheimer’s disease | Healthy older people (n = 20) Alzheimer’s disease and very late onset schizophrenia-like psychosis (n = 31) | Patients with schizophrenia and schizotypal disorders | A retrospective study of data from psychiatric inpatients (Chinese patients with schizophrenia) (mean age = 32 years) |
| Sample size | 45 | 51 | 242 | 121 |
| Route of administration | oral 50 mg/day | oral 50 mg/day | oral | oral amisulpride with serum drug concentration monitoring |
| Software | Monolix | Monolix | NONMEM | ΝOΝΜΕΜ |
| Structural model | Two-compartment model with first-order elimination | Two-compartment with first-order absorption and elimination | One-compartment model with first-order absorption and elimination | One-compartment model with first-order absorption and elimination |
| Residual error model | proportional | proportional | proportional | proportional |
| Parameter estimates | ka = 0.87 h−1 Cl/F = 84 l/h V1/F = 668 l (men) V1/F = 399 (women) Q/F = 117 l/h V2/F = 808 l | ka = 0.83 h−1 Cl/F = 51.5 l/h V1/F = 440 l Q/F = 111 l/h V2/F = 741 l | ka = 0.9 h−1 Cl/F = 44 l/h V/F = 956 l | ka = 0.18 h−1 Cl/F = 61.1 l/h V1/F = 1720 l |
| Correlations | Between CL, Q, V1 and V2 | NA | NA | NA |
| Covariates | V1/F: Gender (β_gender = −0.52) V1/F: weight V2/F: weight Cl/F: weight Q/F: weight | NA | CL/F: Age, Weight | CL/F: Age |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kousovista, R.; Karali, G.; Karalis, V. Modeling the Double Peak Phenomenon in Drug Absorption Kinetics: The Case of Amisulpride. BioMedInformatics 2023, 3, 177-192. https://doi.org/10.3390/biomedinformatics3010013
Kousovista R, Karali G, Karalis V. Modeling the Double Peak Phenomenon in Drug Absorption Kinetics: The Case of Amisulpride. BioMedInformatics. 2023; 3(1):177-192. https://doi.org/10.3390/biomedinformatics3010013
Chicago/Turabian StyleKousovista, Rania, Georgia Karali, and Vangelis Karalis. 2023. "Modeling the Double Peak Phenomenon in Drug Absorption Kinetics: The Case of Amisulpride" BioMedInformatics 3, no. 1: 177-192. https://doi.org/10.3390/biomedinformatics3010013
APA StyleKousovista, R., Karali, G., & Karalis, V. (2023). Modeling the Double Peak Phenomenon in Drug Absorption Kinetics: The Case of Amisulpride. BioMedInformatics, 3(1), 177-192. https://doi.org/10.3390/biomedinformatics3010013







