Abstract
Immune checkpoint blockade targeting PDCD1 (PD-1) or CD274 (PD-L1) has demonstrated efficacy and interest across multiple cancers. However, the exact determinants of the response and cancer-specific molecular features remain unclear. A recent pan-cancer study identified a PDCD1/CD274-related immunotherapy network of 40 genes that had differential patient survival associations across multiple cancers. However, the survival relevance of this network in mesothelioma could not be assessed due to a lack of available survival data for the mesothelioma study included. Mesothelioma, a rare cancer that most commonly arises in the pleural membranes around the lung, does have immune checkpoint blockade as an approved treatment strategy, yet questions over its efficacy remain. RNA-seq data from 87 pleural mesothelioma patients were interrogated on cBioPortal to assess the role of the PDCD1/CD274 network identified in a previous study, in addition to identifying repurposed drugs that may have therapeutic efficacy. Extensive literature searches were conducted to identify known information from the literature around the genes shown to impact patient survival (CCR5, GATD3A/GATD3, CXCR6, GZMA, and TBC1D10C). The same literature validation was performed for putative repurposed drugs that were identified as potential immunotherapeutic adjuvants in the context of mesothelioma (disulfiram, terfenadine, maraviroc, clioquinol, chloroxine, and oxyphenbutazone). Only disulfiram returned a specifically focused research article based on the literature search. This article demonstrated cytotoxicity in a panel of five human MPM cell lines of mixed histology (epithelioid, biphasic, and sarcomatoid). There was little information on the remaining five drugs, yet the clear preclinical efficacy of disulfiram validates the methodology used herein and prompts further exploration of the remaining drugs in mesothelioma. This study ultimately sheds light on novel preclinical information of genes related to PDCD1/CD274 in mesothelioma, as well as identifying putative drugs that may have therapeutic efficacy either independently or as an immunotherapeutic adjuvant.
Keywords:
PD-1; PD-L1; mesothelioma; cBioPortal; drug repurposing; immunotherapy; Kaplan–Meier survival curves 1. Introduction
Mesothelioma is a rare form of cancer that arises from the mesothelial cells that line organs such as the heart (pericardial mesothelioma), abdomen (peritoneal mesothelioma), testes (testicular mesothelioma), and the lungs (pleural mesothelioma) [1]. Pleural mesothelioma is the most common of these, accounting for approximately 90% of mesothelioma cases [2]. Outcomes remain poor, with a median overall survival of 8 months based on pleural and peritoneal mesothelioma data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results–Medicare database [3]. The standard first-line therapy for mesothelioma was traditionally platinum-based therapy and anti-folates, though, as with many cancers, there have been significant efforts to utilize immunotherapy, particularly immune checkpoint blockade [4].
Immune checkpoint blockade relies on the premise that blocking the immune-downregulatory signals that cancer cells exhibit to evade the immune system leads to the immune system remaining active and targeting the cancer [5,6]. The most common targets for immune checkpoint blockade are CTLA-4, PD-1 (PDCD1), and PD-L1 (CD274) [7]. However, the response to immune checkpoint blockade in mesothelioma has been mixed at best and is often associated with side effects [8]. Although first-line combination nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) was approved in October 2020 for unresectable MPM [9], based on the CheckMate 743 trial [10], side-effects and adverse-related events persist. The efficacy of the therapy has also been questioned, with a comparative study finding no survival benefit in the CheckMate 743 trial relative to a trial studying cisplatin + pemetrexed + bevacizumab against cisplatin + pemetrexed [11]. As such, there is a need to delineate the molecular differences underpinning immune checkpoint blockade on a per-cancer type basis and, in the case of mesothelioma, develop further understanding of its biology, pathology, and drivers of response to therapy.
A recent study [12] utilized patient RNA-seq data from cBioPortal [13,14] to interrogate the association between expression of genes in a PDCD1/CD274 network and patient survival on a pan-cancer basis. The study, beginning with CD274 and PDCD1, identified 40 genes that were correlated with PDCD1 or CD274 (either positively or negatively) and undertook Kaplan–Meier analysis based on patient RNA-seq data to identify genes that were significantly associated with patient outcomes [12]. Different patterns of clinical relevance emerged in terms of which genes of the network were significant and how they influenced survival, with renal and esophageal cancers being notably different from the thirteen other cancer types. A limitation of the study, however, was that the mesothelioma study included (Mesothelioma (TCGA, Firehose Legacy)) lacked usable survival data and, therefore, the clinical relevance of the genes in this PDCD1/CD274 network could not be assessed [12].
However, a second study (Mesothelioma (TCGA, PanCancer Atlas) [15]) on the same patient cohort is available on cBioPortal, which contains the survival data alongside the “mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM)” data format analyzed in the original study [12]. Due to the inclusion criteria in the preceding study, the Mesothelioma (TCGA, PanCancer Atlas) study could not be included. Therefore, this present study assessed the clinical relevance of PDCD1, CD274, and the 40 pan-cancer correlated genes in the PDCD1/CD274 network for malignant pleural mesothelioma (MPM). Five genes (CCR5, GATD3A/GATD3, CXCR6, TBC1D10C, and GZMA) were shown to be significantly associated with survival in mesothelioma patients, with each demonstrating high expression to be significantly associated with patient survival and in silico drug repurposing identifying potentially repurposed prospective immunotherapeutic adjuvants for mesothelioma.
2. Materials and Methods
2.1. Study Selection
In order to assess the clinical relevance of CD274, PDCD1, and genes in the PDCD1/CD274 network, the cBioPortal database (https://www.cbioportal.org/, accessed on 12 January 2022) was used [13,14]. The database contains easily accessible patient-level multi-omics data for over 300 individual studies/analyses. The “Mesothelioma (TCGA, PanCancer Atlas)” study was the dataset of choice, as it contains the same patients from the “Mesothelioma (TCGA, Firehose Legacy)” study used in the preceding publication [12].
2.2. Identification of Genes of Interest
A total of 42 genes (CD274, PDCD1, and the 40 genes in the PDCD1/CD274 network) were obtained from the preceding publication [12], as these were highlighted in the first study as being correlated pan-cancer either positively or negatively with either CD274 or PDCD1.
To validate the interconnectivity between these genes/proteins, which was not performed in the original study, STRING [16] was used. The “Multiple proteins” search function was used; at which point, the 42 network constituents were inputted, and “Homo sapiens” used as the species. After ensuring each input was mapped to the appropriate STRING entry, the resultant protein connectivity map was saved and exported, alongside the node connectivity assessment.
2.3. Assessing the Mesothelioma-Specific Importance and Survival Association of PDCD1, CD274, and the 40 PDCD1/CD274-Related Genes
To assess the survival relevance of the PDCD1/CD274 network, the “Mesothelioma (TCGA, PanCancer Atlas)” study on the cBioPortal homepage was selected and “Explore Selected Studies” was chosen. Patients with “mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM)” were selected from the “Genomic Profile Sample Counts” section and each gene was queried, in turn, using the “Gene Specific” tab under “Charts”. Patients were divided into high and low expression of each gene, in turn, based on the median value. “mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM)” was the “type” of mRNA expression analysis performed. A Kaplan–Meier plot was then computed based on the two groups (low and high expression of each gene in turn) to determine the putative association of each gene with patient survival. A raw p ≤ 0.05 (based on a survival logrank test as calculated by cBioPortal) was considered statistically significant for that gene, and the determination of low or high expression being beneficial for the patient was determined by examination of the overall pattern of the curve. Due to the exploratory nature of this and the preceding study, multiple comparison corrections on p-values were not strictly required [17,18] and the interpretation downstream in this article considers raw p-values. However, Benjamini–Hochberg corrections were performed for reference on the p-values for the Kaplan–Meier curves https://www.sdmproject.com/utilities/?show=FDR, accessed on 28 October 2022) and are available in Supplementary Table S1, as is the raw p value distribution (Supplementary Figure S1).
For genes that were statistically significantly associated with survival, the gene expression and patient survival data were downloaded from cBioPortal and input to KMplot using the upload function [19,20]. This allowed for the recapitulation of the cBioPortal groupings and analysis and enhanced it by also calculating hazard ratios.
To validate the importance of these genes in the context of mesothelioma, cBioPortal was again accessed and the “Mesothelioma (TCGA, PanCancer Atlas)” study on the cBioPortal homepage was selected and “Query by Gene” was chosen. “mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM)” was selected as the “type” of mRNA expression and the default Patient/Case set was used. PDCD1 and CD274 were then input separately in turn, and the Co-Expression tab under the “mRNA Expression, RSEM (Batch normalized from Illumina HiSeq_RNASeqV2) (82 Samples)” setting was used to identify correlations between PDCD1/CD274 and the significant genes (by Kaplan–Meier analysis) identified above.
2.4. Putative Drug Identification & Literature Searches for Gene & Drug Validation
A list of drugs relevant to the present study were obtained from the preceding study [12]. The drugs that were selected were those that targeted genes that were identified to have a statistically significant survival association in mesothelioma based on the results from the above methods section.
To assess the depth of the literature available for the drugs and their relationship to mesothelioma, the following search term was used on PubMed: “(mesothelioma[Title/Abstract]) AND ([DRUG])”, where “[DRUG]” was replaced with each drug in turn. The date of the search was 24 June 2022. The resultant literature was scanned at the abstract level in the first instance, and then full papers were accessed if the paper was deemed relevant (based on the simple criteria of assessing to what extent the drug has been tested in mesothelioma). A second search was performed to identify non-published links such as current clinical trials.
The depth of the literature available for any genes linked to patient survival in mesothelioma was also assessed. The following search term was used on PubMed: “(mesothelioma [Title/Abstract]) AND ([OFFICIAL GENE SYMBOL])”, where “[OFFICIAL GENE SYMBOL]” was replaced with each official gene symbol in turn. The date of the search was 24 June 2022. The resultant literature was scanned at the abstract level in the first instance, and then the full papers were accessed if the paper was deemed relevant.
3. Results
3.1. Validation of the Connectivity of the PDCD1/CD274 Network
To validate the network identified in the previous study and extend the analysis, STRING v11.5 was accessed in order to determine the connectivity between the 42 network constituents, as shown below in Figure 1:
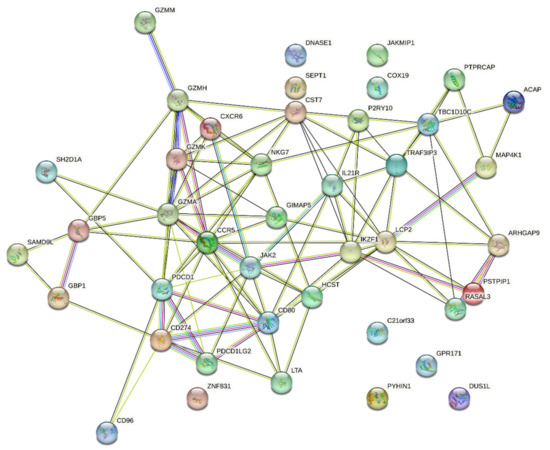
Figure 1.
STRING connectivity map of the PDCD1/CD274 network identified in the preceding study. As identified by STRING, the protein–protein interaction (PPI) enrichment p-value was less than 1.0 × 10−16.
As demonstrated above in Figure 1, there is a significant degree of interconnectivity between the 42 network elements in the PDCD1/CD274 network from the previous study [12], which is further supported by the node connectivity assessment (Supplementary Table S2). As this STRING analysis is conducted indepenently of the cBioPortal platform, it provides a confirmation of the validity of the PDCD1/CD274 network, warranting further investigation.
3.2. Identifying the Clinical Relevance of PDCD1, CD274, and 40 PDCD1/CD274-Related Genes
To identify the clinical relevance of PDCD1, CD274, and the 40 genes in the PDCD1/CD274 network [12], the cBioPortal was accessed, as described in Materials and Methods [13,14]. Of the 42 genes, only five had a statistically significant p-value (p < 0.05) in pleural mesothelioma based on the logrank test calculated by cBioPortal (Supplemental Figure S1). To calculate hazard ratios, the cBioPortal analysis was recapitulated in KMplot [19,20]. The survival curves for the five genes are shown below in Figure 2.
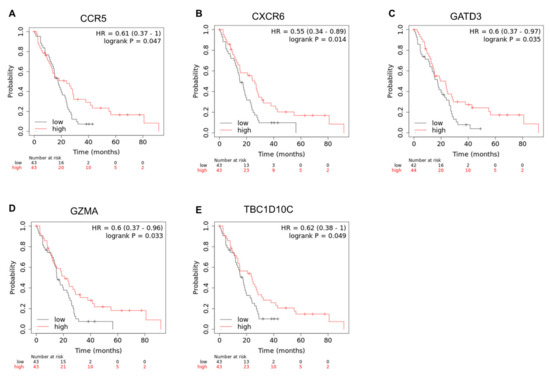
Figure 2.
Statistically significant Kaplan−Meier plots for CD274, PDCD1, and 40 PDCD1/CD274-related genes. Only statistically significant survival curves are shown. For each graph above (A–E), the overall survival in months can be seen on the x-axis, whereas the probability of survival is shown on the y-axis. Hazard ratio (HR) and logrank p value for the analysis is indicated. High expression of CCR5 (A), CXCR6 (B), GATD3A/GATD3 (C), GZMA (D), and TBC1D10C (E) is shown in red, whereas low expression for each gene is shown in black. Data were generated using the mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM) for the Mesothelioma (TCGA, PanCancer Atlas) study [15] available on cBioPortal, and Kaplan–Meier analysis was performed via Kaplan–Meier Plotter.
The mesothelioma-specific correlations between these genes and PDCD1/CD274 can be seen in the co-expression analysis results in Supplementary Figures S2–S6, which shows that all the genes here are, in a mesothelioma context, significantly correlated either positively or negatively.
In terms of the genes correlated with CD274 (CCR5 and GATD3A/GATD3), the previous study [12] found that a high expression of CCR5 (a gene positively correlated with CD274) was beneficial for patients with liver, head and neck, and melanoma cancers, which was also the case for mesothelioma (Figure 2A). However, the survival profile for mesothelioma shown above is unique, as a high expression of GATD3A/GATD3 (a gene negatively correlated with CD274) was shown to be beneficial for patients (Figure 2C), something which was only identified in pancreatic cancer in the previous study [12].
In terms of the genes correlated with PDCD1 (CXCR6, GZMA, TBC1D10C, all positively correlated), a high expression of all of these were shown to be beneficial for patients in mesothelioma (Figure 2B,D,E). This is consistent with multiple cancers in each case. A high expression of CXCR6 was shown to be beneficial for patients in cervical, liver, head and neck, breast, melanoma, lung, and bladder cancers [12]. A high expression of GZMA was shown to be beneficial in breast, melanoma, and bladder cancers [12]. Finally, a high expression of TBC1D10C was shown to be beneficial in cervical, liver, head and neck, breast, melanoma, and lung cancers [12].
3.3. Drug Repositioning
The preceding study [12] was accessed to retrieve drugs targeting the genes identified above (initial analysis was via the DRUGSURV database [21,22]). Six drugs (disulfiram, terfenadine, maraviroc, clioquinol, chloroxine, and oxyphenbutazone) targeting five genes (CCR5, CXCR6, TBC1D10C, GZMA, GATD3A/GATD3) were relevant. A literature search was then conducted on PubMed to investigate the degree to which each gene and drug had been investigated in mesothelioma, summarized in Table 1.

Table 1.
Assessment of the literature linking mesothelioma and the genes identified in Figure 2 and literature validation of the putative repurposed drugs identified. Approval information was taken from [12] Bolded entries indicate search terms used.
Of the five genes identified to influence patient survival, only two were shown to be linked to mesothelioma in the literature. These were CCR5 [23] and GZMA [24,25]. However, the literature linking CCR5 was limited [23]. Davidson et al. (2007) analyzed chemokine receptor expression in malignant mesothelioma (11 samples), reactive mesothelium (16 samples), and lymphocytes in effusions, and found that chemokine receptors were infrequently expressed in malignant mesothelioma, whereas it was uniformly negative in reactive mesothelium [23].
Literature validation also highlighted the potential cytotoxic effect of disulfiram in mesothelioma [26], discussed in more depth below.
4. Discussion
This study builds on previous work which identified a 42-gene PDCD1/CD274 network [12]. The original study used only cBioPortal analysis, though on a pan-cancer basis, to identify this network. To validate the network through an independent platform, STRING [16] was used to assess the degree of interconnectivity between the network constituents. As shown in Figure 1, there is a significant degree of interconnectivity, and this is supported by the PPI enrichment p-value of <1.0 × 10−16. Per STRING, this highlights that the input list—the PDCD1/CD274 network—has significantly more interactions between the network elements than would be expected for a random list of proteins. Thus, this PPI enrichment indicates that the proteins are, at least partially, biologically connected [16].
In Figure 1, there is evidence that certain “hubs” are identifiable. For example, CCR5, which was identified in this study as high expression being significantly associated with mesothelioma patient survival, is one of the most interconnected nodes, with a node degree of twelve (Supplementary Table S2). Similarly, GZMA, identified again in this study as beneficial for patient survival, was also significantly connected to other genes/proteins in the network, with a node degree of fourteen. This independent STRING analysis confirms the potential importance and composition of the PDCD1/CD274 network.
A demonstrated in Figure 2, five genes (CCR5, CXCR6, GZMA, TBC1D10C, and GATD3A/GATD3) from the PDCD1/CD274 network demonstrated that high expression levels were associated with improved outcomes in pleural mesothelioma. Furthermore, the co-expression analysis (Supplementary Figures S2–S6) supports the importance of these five genes in relation to PDCD1/CD274 specifically in the context of mesothelioma, as all five were statistically significantly correlated with either PDCD1, CD274, or both. Apart from GATD3A/GATD3 (encoding glutamine amidotransferase class 1 domain containing 3 protein), of which little is known for its role in cancer, the other genes are all involved in the immune response.
Two of these genes (CCR5 and CXCR6) encode for proteins that act as chemokine receptors, primarily involved in the recruitment and migration of immune cells, and they can play complex roles in cancer. The role of CCR5 and other chemokine receptors in cancer is multifactorial and has previously been reviewed in depth [27]. Both pro- and anti-tumor activities have been identified. The recruitment of immune cells into the tumor microenvironment is believed to underlie many of the impacts of chemokines. A range of different immune cells can be found within the tumor in pleural mesothelioma, with significant patient heterogeneity [28] and a bias towards immunosuppressive cells. The number and nature of immune cells present in pleural mesothelioma is believed to have prognostic value [29] and may impact the response to immunotherapy. Tumor-associated macrophages (TAMs) comprise a large proportion of the immune milieu and are associated with poor outcomes. These cells are likely derived from peripheral blood monocytes recruited via mesothelioma cell-derived chemokines. While the CCL5-CCR5 axis is capable of such recruitment, evidence suggests that CCR2 (via interactions with CCL2), rather than CCR5, is the dominant driver in MPM [30].
CXCR6 and its ligand CXCL16 are responsible for the recruitment of lymphocytes, including both CD4+ (specifically Th1) and CD8+ T cells, into areas of inflammation [31]. Hypermethylation of this gene has been identified as a potential biomarker for the identification of mesothelioma in those exposed to asbestos [32]. Although a high expression of CXCR6 and its ligand have been proposed to have a role in cancer progression and metastasis in several cancers, including prostate and breast cancer [33], that is not consistent with the protective role seen in the mesothelioma data from this study.
In addition to the expression on immune cells, CCR5 can also be expressed by a range of cancer cells, including hematological (acute lymphoblastic leukemia [34], Hodgkin’s lymphoma [35], and multiple myeloma [36]) and solid tumors (liver [37], head and neck [38], melanoma [39], and breast [40]). Along with other chemokine receptors and leukocyte markers, CCR5 can be occasionally detected on a minority of malignant mesothelioma cells [23], with 1/11 samples showing CCR5 staining. Although there may be a role for tumor-intrinsic chemokine receptor expression, the correlation of high CCR5 (and potentially CXCR6) and improved outcomes in this study is likely to reflect chemokine receptor expression levels on T cell subsets within the tumor. Both CD8+ and CD4+ T cells can be detected in most mesothelioma patients, and the beneficial role of tumor-infiltrating lymphocytes has been established in a range of different cancers [41].
The gene TBC1D10C [42] encodes the immune protein carabin which is capable of negatively regulating both Ras signaling and calcineurin, inhibiting both T cells [43] and B cells [44]. Despite its inhibitory role in immune cells, this gene has been found to have a strong positive correlation with infiltrating immune cells, including CD8+ T cells and activated B cells. In a study of breast cancer patients, this gene was identified to correlate with both overall survival and progression-free survival [45]. The potential for a beneficial effect of recruited immune cells is echoed by this study’s finding that high levels of the gene encoding the protease granzyme A (GZMA) are also associated with improved survival. Expressed in cytotoxic lymphocytes (primarily CD8+ T cells and NK cells), granzyme A is a member of the serine protease family of granzymes and promotes anti-tumor activity via the induction of programmed cell death. The evaluation of the expression level of this gene has been used, together with perforin (PRF1), as a measure of the cytolytic activity (CYT) present within a tumor [46]. Although a recent meta-analysis found that there was large amounts of tumor heterogeneity in the level of CYT and that the prognostic value of this parameter is complicated by other factors in the tumor microenvironment [47], this study supports a protective, rather than tumor-permissive, role for increased immune activity within the mesothelioma tumor environment.
Drug repurposing against the five genes identified to influence patient survival led to a total of six putative drugs being identified that may have therapeutic benefit in mesothelioma. As highlighted in the Results, only one drug—disulfiram—returned a specifically-focused research article in the context of mesothelioma [26]. In this study, copper-complexed dithiocarbamate compound disulfiram (DSF-Cu) was shown to be cytotoxic in a panel of five human MPM cell lines. This panel included mesothelioma cell lines that were epithelioid, biphasic, and sarcomatoid in nature, indicating a potential wider use of the drug. This is key, as the histology of mesothelioma is associated with prognosis, with sarcomatoid being linked to a poorer prognosis [1]. The drug also showed efficacy on a mouse MPM allograft [26].
A 2017 conference paper from the National Cancer Research Institute Cancer Conference highlighted that disulfiram in combination with copper was cytotoxic in mesothelioma cells and curative at the in vivo level [48]. The same conference paper highlighted that disulfiram in combination with copper abolished PD-L1 expression in mesothelioma cells, which provides further rationale for its use in combination with PD-1/PD-L1 immune checkpoint blockade, as posited herein.
A recent review on drug repurposing in mesothelioma [49] did discuss disulfiram, though in the context of mesothelioma, only the article by Cheriyan et al. (2014) was discussed. This further highlights the relative infancy of investigation of disulfiram in mesothelioma, signposting a need for further studies to confirm its effects.
As shown in Table 1, disulfiram was the only drug to return specific results for having been tested in mesothelioma. The remaining five drugs (terfenadine, maraviroc, clioquinol, chloroxine, and oxyphenbutazone) did not return search results and are, therefore, under-investigated in the context of mesothelioma. They have, however, been tested in a variety of other cancers, as discussed below, and an expanded literature search identified one drug screening article that investigated both disulfiram and terfenadine [50].
Terfenadine, an antihistamine, has been tested in several cancers and the use of antihistamines in cancer in general has been previously reviewed [51]. Terfenadine has shown efficacy in human melanoma [52,53,54], human mast cell leukemic cells [55], breast cancer [56], prostate cancer [57], and non-small cell lung cancer [58]. The drug screening study that assessed terfenadine in mesothelioma [50] highlighted it as having defined cytotoxic potential against a panel of mesothelioma cell lines. Though disulfiram was also assayed, it demonstrated less potential than a significant number of other drugs assessed [50].
Maraviroc, an antiretroviral CCR5 antagonist [59], has been tested in cancers, including breast [60], gastric [61], and colorectal cancers [62]. Maraviroc has recently been tested in 2022 in a phase one clinical trial, where it was combined with pembrolizumab (anti-PD-1) in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer [63]. In this study, the combination therapy was feasible and demonstrated a beneficial toxicity pattern [63]. Clioquinol, an antifungal and antiprotozoal drug [64], has been tested in a variety of cancers and shown efficacy at the preclinical level, though low efficacy was demonstrated in a clinical trial [65]. This prompted studies into alternative applications and combinations of clioquinol-based therapy [65].
The anticancer efficacies of chloroxine and oxyphenbutazone have also been investigated, with chloroxine restoring platinum sensitivity in high-grade serous ovarian cancer cells [66] and oxyphenbutazone promoting cytotoxicity in hepatocellular carcinoma at the in vitro and in vivo level [67].
It is important to note that there are several limitations to this study. Most principally, the analyses have only been conducted on a single patient cohort of mesothelioma patients. This limitation is one that arises quite naturally in mesothelioma as a result of its rarity and understudied nature. Additionally, as highlighted in a previous study [12], patient-level omics analyses are increasingly moving towards the integration of multi-omics datasets [6] to allow for the capture of information flow along several levels of biology. However, as an initial exploratory study, such analysis was considered beyond the scope of this article. Further studies are needed to independently validate the findings presented in this article in another patient cohort and through the integration of multi-omics data.
Building on the patient survival-associated discoveries at the mRNA level, this study identified numerous putative repurposed drugs for use in mesothelioma. Although no drugs other than disulfiram and terfenadine have been studied in the context of mesothelioma, the fact remains that the consistent methodology used to identify these drugs validates the panel as potential treatments for mesothelioma. However, further studies and building a solid preclinical rationale for their use are, of course, required, as is the delineation of their solo efficacy and their prospective use as adjuvants to immune checkpoint blockade. A rational starting point to investigate these drugs would be basic cell viability assays, such as an SRB or MTS approach, which would identify any standalone cytotoxic effect in mesothelioma cells [68]. One way to then take this forward would be animal studies where the putative immunotherapeutic adjuvants are used in conjunction with anti-PD-1/anti-PD-L1 therapy. One example of a model that could be used for this would be the AB1-HA murine mesothelioma model [69,70]. Should these experiments yield promising results, clinical trials could be designed which, provided the use of effective endpoints and design, could provide novel treatment combinations for this underserved patient population.
5. Conclusions
This study has validated a previously identified CD274/PDCD1-related network in the context of mesothelioma. Of the 42 genes screened, only 5 genes demonstrated a significant survival association, whereas drug repurposing against the 5 genes led to 6 drugs being identified as potential immune checkpoint adjuvants for mesothelioma. Though evidence is lacking for the use of most of these drugs in mesothelioma (either alone or as immunotherapeutic adjuvants), the demonstrated efficacy of disulfiram and terfenadine validates the method used to identify the drugs. Future in vitro studies are required to determine cytotoxicity in mesothelioma, as well as the molecular mechanisms of any identified efficacy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedinformatics2040037/s1, Figure S1: Distribution of p-values from Kaplan–Meier plots for CD274, PDCD1, and 40 PDCD1/CD274-related genes; Figure S2: Co-expression analysis of PDCD1 and CD274 with CCR5; Figure S3: Co-expression analysis of PDCD1 and CD274 with GATD3; Figure S4: Co-expression analysis of PDCD1 and CD274 with CXCR6; Figure S5: Co-expression analysis of PDCD1 and CD274 with GZMA; Figure S6: Co-expression analysis of PDCD1 and CD274 with TBC1D10C; Table S1: Kaplan-Meier curve p-values obtained from cBioPortal and Benjamini-Hochberg adjusted p-values; Table S2: Node degree distribution for the PDCD1/CD274 network as identified by STRING analysis.
Author Contributions
Conceptualization, G.M.O. and E.Y.B.; methodology, G.M.O. and E.Y.B.; software, G.M.O. and E.Y.B.; validation G.M.O. and E.Y.B.; formal analysis, G.M.O. and E.Y.B.; investigation, G.M.O. and E.Y.B.; resources, G.M.O. and E.Y.B.; data curation, G.M.O. and E.Y.B.; writing—original draft preparation, G.M.O. and E.Y.B.; writing—review and editing, G.M.O. and E.Y.B.; visualization, G.M.O. and E.Y.B.; project administration, G.M.O. and E.Y.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data accessed in this study are available on multiple publicly available databases, as described in Materials and Methods.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bakker, E.; Guazzelli, A.; Krstic-Demonacos, M.; Lisanti, M.; Sotgia, F.; Mutti, L. Current and prospective pharmacotherapies for the treatment of pleural mesothelioma. Expert Opin. Orphan Drugs 2017, 5, 455–465. [Google Scholar] [CrossRef][Green Version]
- Mezei, G.; Chang, E.T.; Mowat, F.S.; Moolgavkar, S.H. Epidemiology of mesothelioma of the pericardium and tunica vaginalis testis. Ann. Epidemiol. 2017, 27, 348–359.e311. [Google Scholar] [CrossRef] [PubMed]
- Beebe-Dimmer, J.L.; Fryzek, J.P.; Yee, C.L.; Dalvi, T.B.; Garabrant, D.H.; Schwartz, A.G.; Gadgeel, S. Mesothelioma in the United States: A Surveillance, Epidemiology, and End Results (SEER)-Medicare investigation of treatment patterns and overall survival. Clin. Epidemiol. 2016, 8, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Popat, S. Pleural mesothelioma (PM)—The status of systemic therapy. Cancer Treat. Rev. 2021, 100, 102265. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Park, B.B.; Uhm, J. Understanding Immune Evasion and Therapeutic Targeting Associated with PD-1/PD-L1 Pathway in Diffuse Large B-cell Lymphoma. Int. J. Mol. Sci. 2019, 20, 1326. [Google Scholar] [CrossRef]
- Leung, K.L.; Verma, D.; Azam, Y.J.; Bakker, E. The use of multi-omics data and approaches in breast cancer immunotherapy: A review. Future Oncol. 2020, 16, 2101–2119. [Google Scholar] [CrossRef]
- Thapa, B.; Walkiewicz, M.; Rivalland, G.; Murone, C.; Asadi, K.; Barnett, S.; Knight, S.; Watkins, N.; Russell, P.A.; John, T. Immune microenvironment in mesothelioma: Looking beyond PD-L1. J. Clin. Oncol. 2017, 35, 8515. [Google Scholar] [CrossRef]
- Thapa, B.; Watkins, D.N.; John, T. Immunotherapy for malignant mesothelioma: Reality check. Expert Rev. Anticancer Ther. 2016, 16, 1167–1176. [Google Scholar] [CrossRef]
- Tantibanchachai, C. FDA Approves Drug Combination for Treating Mesothelioma. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-drug-combination-treating-mesothelioma (accessed on 20 August 2022).
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Meirson, T.; Pentimalli, F.; Cerza, F.; Baglio, G.; Gray, S.G.; Correale, P.; Krstic-Demonacos, M.; Markel, G.; Giordano, A.; Bomze, D.; et al. Comparison of 3 Randomized Clinical Trials of Frontline Therapies for Malignant Pleural Mesothelioma. JAMA Netw Open 2022, 5, e221490. [Google Scholar] [CrossRef]
- Kannan, S.; O’Connor, G.M.; Bakker, E.Y. Molecular Mechanisms of PD-1 and PD-L1 Activity on a Pan-Cancer Basis: A Bioinformatic Exploratory Study. Int. J. Mol. Sci. 2021, 22, 5478. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e411. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Althouse, A.D. Adjust for Multiple Comparisons? It’s Not That Simple. Ann. Thorac. Surg. 2016, 101, 1644–1645. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Györffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat 2010, 123, 725–731. [Google Scholar] [CrossRef]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Antonov, A.V. BioProfiling.de: Analytical web portal for high-throughput cell biology. Nucleic Acids Res. 2011, 39, W323–W327. [Google Scholar] [CrossRef]
- Antonov, A.V.; Krestyaninova, M.; Knight, R.A.; Rodchenkov, I.; Melino, G.; Barlev, N.A. PPISURV: A novel bioinformatics tool for uncovering the hidden role of specific genes in cancer survival outcome. Oncogene 2014, 33, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Dong, H.P.; Holth, A.; Berner, A.; Risberg, B. Chemokine receptors are infrequently expressed in malignant and benign mesothelial cells. Am. J. Clin. Pathol. 2007, 127, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Bacolod, M.D.; Barany, F.; Fisher, P.B. Can CpG methylation serve as surrogate markers for immune infiltration in cancer? Adv. Cancer Res. 2019, 143, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Miura, Y.; Maeda, M.; Kumagai, N.; Murakami, S.; Hayashi, H.; Fukuoka, K.; Nakano, T.; Otsuki, T. Impairment in cytotoxicity and expression of NK cell- activating receptors on human NK cells following exposure to asbestos fibers. Int. J. Immunopathol. Pharmacol. 2009, 22, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Cheriyan, V.T.; Wang, Y.; Muthu, M.; Jamal, S.; Chen, D.; Yang, H.; Polin, L.A.; Tarca, A.L.; Pass, H.I.; Dou, Q.P.; et al. Disulfiram suppresses growth of the malignant pleural mesothelioma cells in part by inducing apoptosis. PLoS ONE 2014, 9, e93711. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Minnema-Luiting, J.; Vroman, H.; Aerts, J.; Cornelissen, R. Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2018, 19, 1041. [Google Scholar] [CrossRef]
- Marcq, E.; Siozopoulou, V.; De Waele, J.; van Audenaerde, J.; Zwaenepoel, K.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology 2017, 6, e1261241. [Google Scholar] [CrossRef]
- Chéné, A.L.; d’Almeida, S.; Blondy, T.; Tabiasco, J.; Deshayes, S.; Fonteneau, J.F.; Cellerin, L.; Delneste, Y.; Grégoire, M.; Blanquart, C. Pleural Effusions from Patients with Mesothelioma Induce Recruitment of Monocytes and Their Differentiation into M2 Macrophages. J. Thorac. Oncol. 2016, 11, 1765–1773. [Google Scholar] [CrossRef]
- Kim, C.H.; Kunkel, E.J.; Boisvert, J.; Johnston, B.; Campbell, J.J.; Genovese, M.C.; Greenberg, H.B.; Butcher, E.C. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J. Clin. Investig. 2001, 107, 595–601. [Google Scholar] [CrossRef]
- Guarrera, S.; Viberti, C.; Cugliari, G.; Allione, A.; Casalone, E.; Betti, M.; Ferrante, D.; Aspesi, A.; Casadio, C.; Grosso, F.; et al. Peripheral Blood DNA Methylation as Potential Biomarker of Malignant Pleural Mesothelioma in Asbestos-Exposed Subjects. J. Thorac. Oncol. 2019, 14, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chen, N.; Li, Y.; Zheng, H.; Lei, Q. CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochim. Biophys. Acta 2010, 1806, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zi, J.; Yuan, S.; Qiao, J.; Zhao, K.; Xu, L.; Qi, K.; Xu, K.; Zeng, L. Treatment with the C-C chemokine receptor type 5 (CCR5)-inhibitor maraviroc suppresses growth and induces apoptosis of acute lymphoblastic leukemia cells. Am. J. Cancer Res. 2017, 7, 869–880. [Google Scholar] [PubMed]
- Casagrande, N.; Borghese, C.; Visser, L.; Mongiat, M.; Colombatti, A.; Aldinucci, D. CCR5 antagonism by maraviroc inhibits Hodgkin lymphoma microenvironment interactions and xenograft growth. Haematologica 2019, 104, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Menu, E.; De Leenheer, E.; De Raeve, H.; Coulton, L.; Imanishi, T.; Miyashita, K.; Van Valckenborgh, E.; Van Riet, I.; Van Camp, B.; Horuk, R.; et al. Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: A study in the 5TMM model. Clin. Exp. Metastasis 2006, 23, 291–300. [Google Scholar] [CrossRef]
- Singh, S.K.; Mishra, M.K.; Rivers, B.M.; Gordetsky, J.B.; Bae, S.; Singh, R. Biological and Clinical Significance of the CCR5/CCL5 Axis in Hepatocellular Carcinoma. Cancers 2020, 12, 883. [Google Scholar] [CrossRef]
- González-Arriagada, W.A.; Lozano-Burgos, C.; Zúñiga-Moreta, R.; González-Díaz, P.; Coletta, R.D. Clinicopathological significance of chemokine receptor (CCR1, CCR3, CCR4, CCR5, CCR7 and CXCR4) expression in head and neck squamous cell carcinomas. J. Oral Pathol. Med. 2018, 47, 755–763. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Ma, X.; Tian, Y.; Wang, C.; Fu, Y.; Luo, Y. High expression of CCR5 in melanoma enhances epithelial-mesenchymal transition and metastasis via TGFβ1. J. Pathol. 2019, 247, 481–493. [Google Scholar] [CrossRef]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Villagomez, F.R.; Diaz-Valencia, J.D.; Ovalle-García, E.; Antillón, A.; Ortega-Blake, I.; Romero-Ramírez, H.; Cerna-Cortes, J.F.; Rosales-Reyes, R.; Santos-Argumedo, L.; Patiño-López, G. TBC1D10C is a cytoskeletal functional linker that modulates cell spreading and phagocytosis in macrophages. Sci. Rep. 2021, 11, 20946. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Sun, L.; Kardian, D.B.; Whartenby, K.A.; Pardoll, D.M.; Liu, J.O. Feedback inhibition of calcineurin and Ras by a dual inhibitory protein Carabin. Nature 2007, 445, 433–436. [Google Scholar] [CrossRef]
- Schickel, J.N.; Pasquali, J.L.; Soley, A.; Knapp, A.M.; Decossas, M.; Kern, A.; Fauny, J.D.; Marcellin, L.; Korganow, A.S.; Martin, T.; et al. Carabin deficiency in B cells increases BCR-TLR9 costimulation-induced autoimmunity. EMBO Mol. Med. 2012, 4, 1261–1275. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Lv, R.; Pang, Y.; Yao, Z.; Zhou, X.; Zhu, W.; Zhou, W. Weighted Gene Coexpression Network Analysis Identifies TBC1D10C as a New Prognostic Biomarker for Breast Cancer. Anal. Cell Pathol. (Amst.) 2022, 2022, 5259187. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Roufas, C.; Chasiotis, D.; Makris, A.; Efstathiades, C.; Dimopoulos, C.; Zaravinos, A. The Expression and Prognostic Impact of Immune Cytolytic Activity-Related Markers in Human Malignancies: A Comprehensive Meta-analysis. Front. Oncol. 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, V.; Zhang, Z.; Liu, P.; Wang, Z.; Butcher, K.; Wang, W. Disulfiram: A possible panacea for malignant mesothelioma. In Proceedings of the NCRI Cancer Conference, Liverpool, UK, 5–8 November 2017. [Google Scholar]
- Boyer, A.; Pasquier, E.; Tomasini, P.; Ciccolini, J.; Greillier, L.; Andre, N.; Barlesi, F.; Mascaux, C. Drug repurposing in malignant pleural mesothelioma: A breath of fresh air? Eur. Respir. Rev. 2018, 27, 147. [Google Scholar] [CrossRef]
- Dell’Anno, I.; Melani, A.; Martin, S.A.; Barbarino, M.; Silvestri, R.; Cipollini, M.; Giordano, A.; Mutti, L.; Nicolini, A.; Luzzi, L.; et al. A Drug Screening Revealed Novel Potential Agents against Malignant Pleural Mesothelioma. Cancers 2022, 14, 2527. [Google Scholar] [CrossRef]
- Faustino-Rocha, A.I.; Ferreira, R.; Gama, A.; Oliveira, P.A.; Ginja, M. Antihistamines as promising drugs in cancer therapy. Life Sci. 2017, 172, 27–41. [Google Scholar] [CrossRef]
- Jangi, S.-M.; Díaz-Pérez, J.L.; Ochoa-Lizarralde, B.; Martín-Ruiz, I.; Asumendi, A.; Pérez-Yarza, G.; Gardeazabal, J.; Díaz-Ramón, J.L.; Boyano, M.D. H1 histamine receptor antagonists induce genotoxic and caspase-2-dependent apoptosis in human melanoma cells. Carcinogenesis 2006, 27, 1787–1796. [Google Scholar] [CrossRef]
- Nicolau-Galmés, F.; Asumendi, A.; Alonso-Tejerina, E.; Pérez-Yarza, G.; Jangi, S.M.; Gardeazabal, J.; Arroyo-Berdugo, Y.; Careaga, J.M.; Díaz-Ramón, J.L.; Apraiz, A.; et al. Terfenadine induces apoptosis and autophagy in melanoma cells through ROS-dependent and -independent mechanisms. Apoptosis 2011, 16, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.M.; Ruiz-Larrea, M.B.; Nicolau-Galmés, F.; Andollo, N.; Arroyo-Berdugo, Y.; Ortega-Martínez, I.; Díaz-Pérez, J.L.; Boyano, M.D. Terfenadine-induced apoptosis in human melanoma cells is mediated through Ca2+ homeostasis modulation and tyrosine kinase activity, independently of H1 histamine receptors. Carcinogenesis 2008, 29, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Hadzijusufovic, E.; Peter, B.; Gleixner, K.V.; Schuch, K.; Pickl, W.F.; Thaiwong, T.; Yuzbasiyan-Gurkan, V.; Mirkina, I.; Willmann, M.; Valent, P. H1-receptor antagonists terfenadine and loratadine inhibit spontaneous growth of neoplastic mast cells. Exp. Hematol. 2010, 38, 896–907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Nogueira, P.; Noguera-Castells, A.; Fuster, G.; Recalde-Percaz, L.; Moragas, N.; López-Plana, A.; Enreig, E.; Jauregui, P.; Carbó, N.; Almendro, V.; et al. Histamine receptor 1 inhibition enhances antitumor therapeutic responses through extracellular signal-regulated kinase (ERK) activation in breast cancer. Cancer Lett. 2018, 424, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Chen, Y.H.; Hsu, J.L.; Leu, W.J.; Yu, C.C.; Chan, S.H.; Ho, Y.F.; Hsu, L.C.; Guh, J.H. Terfenadine induces anti-proliferative and apoptotic activities in human hormone-refractory prostate cancer through histamine receptor-independent Mcl-1 cleavage and Bak up-regulation. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 33–45. [Google Scholar] [CrossRef]
- An, L.; Li, D.D.; Chu, H.X.; Zhang, Q.; Wang, C.L.; Fan, Y.H.; Song, Q.; Ma, H.D.; Feng, F.; Zhao, Q.C. Terfenadine combined with epirubicin impedes the chemo-resistant human non-small cell lung cancer both in vitro and in vivo through EMT and Notch reversal. Pharmacol. Res. 2017, 124, 105–115. [Google Scholar] [CrossRef]
- Cooper, D.A.; Heera, J.; Goodrich, J.; Tawadrous, M.; Saag, M.; Dejesus, E.; Clumeck, N.; Walmsley, S.; Ting, N.; Coakley, E.; et al. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J. Infect. Dis. 2010, 201, 803–813. [Google Scholar] [CrossRef]
- Pervaiz, A.; Zepp, M.; Mahmood, S.; Ali, D.M.; Berger, M.R.; Adwan, H. CCR5 blockage by maraviroc: A potential therapeutic option for metastatic breast cancer. Cell Oncol. (Dordr.) 2019, 42, 93–106. [Google Scholar] [CrossRef]
- Mencarelli, A.; Graziosi, L.; Renga, B.; Cipriani, S.; D’Amore, C.; Francisci, D.; Bruno, A.; Baldelli, F.; Donini, A.; Fiorucci, S. CCR5 Antagonism by Maraviroc Reduces the Potential for Gastric Cancer Cell Dissemination. Transl. Oncol. 2013, 6, 784–793. [Google Scholar] [CrossRef]
- Pervaiz, A.; Ansari, S.; Berger, M.R.; Adwan, H. CCR5 blockage by maraviroc induces cytotoxic and apoptotic effects in colorectal cancer cells. Med. Oncol. 2015, 32, 158. [Google Scholar] [CrossRef]
- Haag, G.M.; Springfeld, C.; Grün, B.; Apostolidis, L.; Zschäbitz, S.; Dietrich, M.; Berger, A.-K.; Weber, T.F.; Zoernig, I.; Schaaf, M.; et al. Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer—The PICCASSO phase I trial. Eur. J. Cancer 2022, 167, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.R.; Sklar, L.A.; Chigaev, A. Clioquinol: To harm or heal. Pharmacol. Ther. 2019, 199, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, H.; Abdullah, Y.; Dou, Q.P. Feasibility of Repurposing Clioquinol for Cancer Therapy. Recent Pat. Anticancer Drug Discov. 2020, 15, 14–31. [Google Scholar] [CrossRef]
- Silva, V.L.; Saxena, J.; Nicolini, F.; Hoare, J.I.; Metcalf, S.; Martin, S.A.; Lockley, M. Chloroxine overrides DNA damage tolerance to restore platinum sensitivity in high-grade serous ovarian cancer. Cell Death Dis. 2021, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Khan, R.; Afzal, M.; Kazmi, I. Oxyphenbutazone promotes cytotoxicity in rats and Hep3B cellsvia suppression of PGE(2) and deactivation of Wnt/β-catenin signaling pathway. Mol. Cell Biochem. 2018, 444, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Guazzelli, A.; Meysami, P.; Bakker, E.; Demonacos, C.; Giordano, A.; Krstic-Demonacos, M.; Mutti, L. BAP1 Status Determines the Sensitivity of Malignant Mesothelioma Cells to Gemcitabine Treatment. Int. J. Mol. Sci. 2019, 20, 429. [Google Scholar] [CrossRef]
- Fear, V.S.; Tilsed, C.; Chee, J.; Forbes, C.A.; Casey, T.; Solin, J.N.; Lansley, S.M.; Lesterhuis, W.J.; Dick, I.M.; Nowak, A.K.; et al. Combination immune checkpoint blockade as an effective therapy for mesothelioma. Oncoimmunology 2018, 7, e1494111. [Google Scholar] [CrossRef]
- Principe, N.; Kidman, J.; Goh, S.; Tilsed, C.M.; Fisher, S.A.; Fear, V.S.; Forbes, C.A.; Zemek, R.M.; Chopra, A.; Watson, M.; et al. Tumor Infiltrating Effector Memory Antigen-Specific CD8(+) T Cells Predict Response to Immune Checkpoint Therapy. Front. Immunol. 2020, 11, 584423. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).