Abstract
In the last decade, a sporadic tree health syndrome affecting high-density apple plantings in North America has become known as Rapid Apple Decline (RAD) or Sudden Apple Decline (SAD). The affected apple trees were typically grafted on small dwarfing rootstocks, often displayed necrosis at the graft union, and suffered from sudden mortality that occurred over 2–3 weeks amid the growing season or a gradual decline. In 2019 and 2020, we conducted a multi-site investigation in the south Okanagan, British Columbia, Canada, to assess the stem hydraulic characteristics, stomatal conductance, leaf δ13C‰, and fruit dry matter accumulation of the declining trees during disease progression. In trees that died, mortality appeared to be associated with severe disruption in xylem water transport at the damaged graft union, followed by abrupt hydraulic failure. In contrast, symptomatic trees that did not die exhibited the moderately declined plant water relations and a reduction in fruit dry matter accumulation followed by either further deterioration or eventual recovery. This pattern indicates the risk of carbohydrate depletion over gradual hydraulic decline and the importance of timely horticultural remedies. In the present study, we discuss potential horticultural practices to mitigate hydraulic dysfunctions and enhance crop tolerance.
1. Introduction
Since the 2010s, a sporadic tree health syndrome noted as Rapid Apple Decline (RAD) or Sudden Apple Decline (SAD) has been reported in high-density apple plantings in various locations in North America [1,2,3,4,5,6]. With the underlying casual factors yet to be identified, a damaged (typically in the form of a necrotic lesion) graft union was observed as a common symptom in the affected dwarfing apple trees, followed by sudden dieback within a few weeks, or a gradual decline throughout the growing season [5]. Despite the high mortality rate, some affected trees demonstrated a gradual recovery.
Water transport is essential to plant survival [7]. In dwarfing apple trees, the scion is grafted onto dwarfing rootstocks which possess limited root volume and water uptake capacity [8,9,10,11,12,13,14]; water transport is conducted through the narrow vascular passage in the graft union. This modified hydraulic structure is the foundation for the efficient restriction on tree vigor and the alteration of the balance between vegetative and reproductive growth. This system brings horticultural advantages such as higher planting density based on smaller size of mature trees, precocity, higher crop load, and higher yield efficiency [14]. However, it also makes the crop more vulnerable to adverse water regimes and extreme temperature events [8,9,10,11,12,15,16,17]. The graft union and small root volume are inherently weak spots in the hydraulic system [8,9,10,12,18], and could be less resilient to damages and stresses; their hydraulic recovery is challenged therefore the loss of hydraulic function is more likely to be irreversible in comparison to non-dwarfing rootstock systems. In addition, tree vigor influences photosynthetic assimilation at the whole tree level and photosynthate partitioning towards vegetative growth [19,20]; trees with less photosynthates in their perennial tissues would be more prone to carbohydrate depletion and have less capacity for hydraulic recovery and systemic defense [7,19,20,21]. Hydraulic failure to different temporal or spatial extents can be manifested as either rapid or gradual tree health decline, tree dieback, or sudden tree mortality [21].
Potential factors associated with sudden apple decline that may play a role in hydraulic dysfunctions in this growing region include: (1) girdling, massive vascular tissue necrosis and permanent loss of the entire water transport capacity due to stressors such as winter injury on a large area of xylem tissues [22], severe infestation of trunk-boring insects such as Synanthedon myopaeformis (apple clearwing moth) [5] and Scolytus rugulosus (shothole borers, also known as ambrosia beetles) [23], or canker-causing fungi (e.g., Diplodia) [24], and (2) factors that cause localized and reversible loss of hydraulic functions, such as xylem embolism due to drought or an abrupt increase in evapotranspiration demand, and partial root inactivity under waterlogging [9,25,26]. To date, the majority of these biotic and abiotic factors, and their confluent stress-induced legacy effects, remain under investigation or are speculative. Understanding the hydraulic dysfunctions is a critical step to trace back the legacy effects of environmental stresses underlying tree decline, potentially leading to effective horticultural mitigation measures to alleviate stresses and prevent decline. In order to investigate the hydraulic disruptions during the progression of apple tree decline, we conducted a multi-site survey (Table 1) in the southern Okanagan Valley, British Columbia, Canada, in 2019 and 2020, to assess the stem hydraulic characteristics prior to tree mortality, and to evaluate tree–water relations and fruit dry matter accumulation in the declining trees. These preliminary findings could inform future investigations on the roles of hydraulic dysfunction and carbohydrate depletion in apple tree vulnerability and decline, and on potential horticultural practices that could be deployed to enhance resilience under a changing climate and emerging biotic threats.

Table 1.
Tree and soil descriptions of five sampling sites.
2. Results
2.1. Hydraulic Characteristics Prior to Tree Mortality
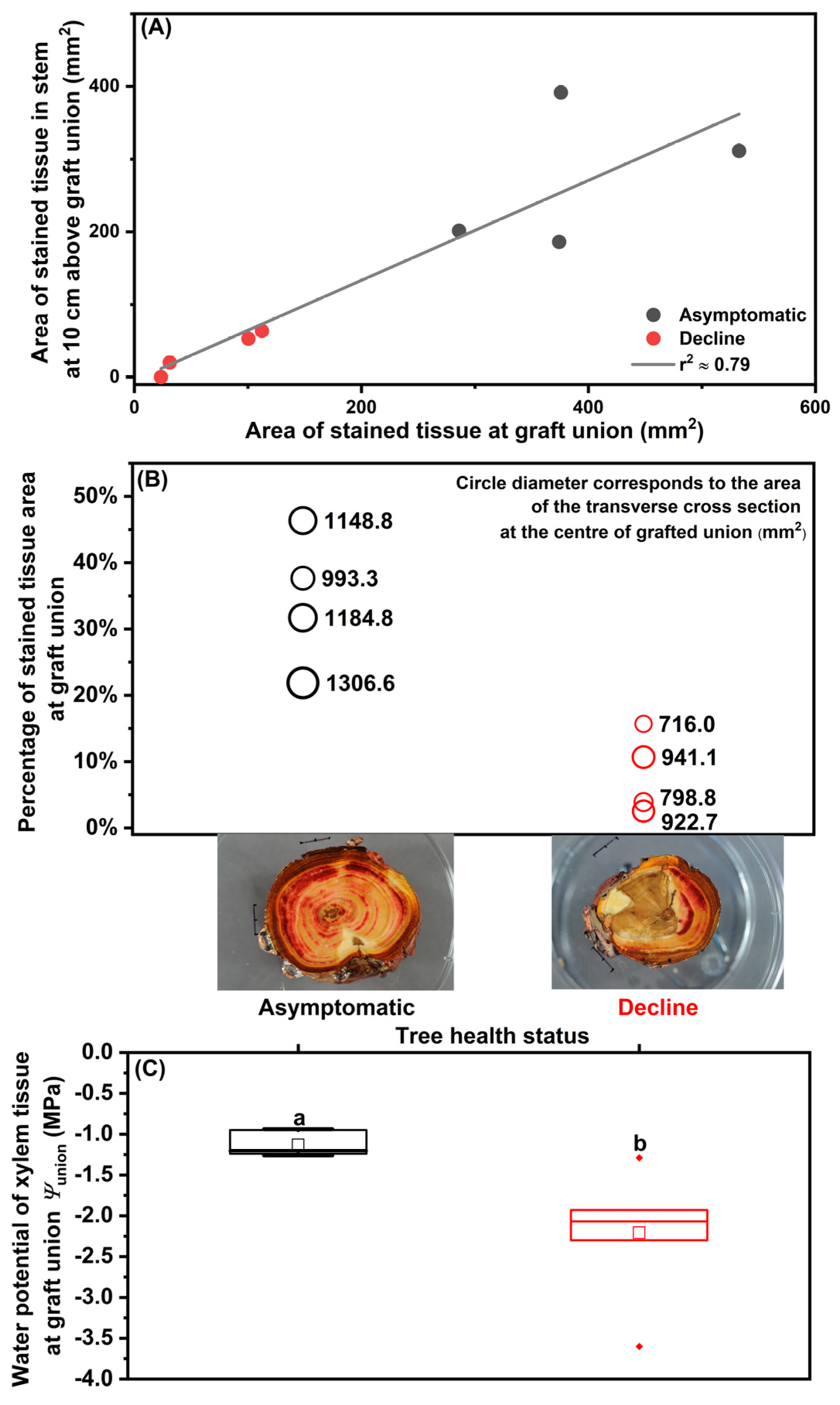
The ‘Salish’/M.9 trees in severe decline at Site A were analyzed to reveal the hydraulic characteristics across the graft union prior to mortality. The area of stained tissue in the scion stem at 10 cm above the graft union was positively correlated with the area of stained tissue in the center of the graft union (Figure 1A). Visual inspection showed damaged bark and necrotic tissues in the outer and inner sap wood that blocked dye transport in the declining trees. Compared to the asymptomatic trees, the declining trees had significantly smaller area of water transporting vascular tissues through the graft union (392.11 ± 51.34 mm2 versus 66.85 ± 23.05 mm2, mean ± standard error; F(1,7) = 33.40, p = 0.001) and in scion stem (272.76 ± 48.49 mm2 versus 34.45 ± 14.62 mm2; F(1,7) = 22.13, p = 0.003) (Figure 1A), and a smaller cross sectional area at the center of the graft union (1158.39 ± 64.54 mm2 versus 844.64 ± 53.28 mm2; F(1,7) = 14.1, p = 0.01) (Figure 1B). The percentage of active transporting xylem tissues to the total cross section area of the center of the graft union was 8% ± 3% (mean ± standard error) in the declining trees, significantly smaller than that in the asymptomatic trees (34% ± 5%) (Figure 1B; F(1,7) = 19.1, p = 0.005). Xylem tissue water potential of the graft union (Ψunion) in asymptomatic trees was -1.13 ± 0.06 MPa with little variation; in contrast, Ψunion in the declining trees was significantly lower and more variable among the trees (−2.21 ± 0.31) (Figure 1C; F(1,11) = 11.5, p = 0.007).
Figure 1.
The hydraulic characteristics of asymptomatic and severely declining ‘Salish’/M.9 apple trees sampled from Site A in September 2019: (A) The area of Safranin-O stained tissue in scion cross section at 10 cm above graft union and at the center of graft union (n = 4); (B) The cross section area and the percentage of stained tissue area to the total cross section area at the center of graft union (n = 4); (C) Xylem water potential of graft union (Ψunion, n = 6; one-way ANOVA, Tukey’s test, p ≤ 0.05). In (A), linear regression is shown as solid line; r2 refers to the coefficient of determination for correlation. In (C), horizontal lines of boxplots show median and interquartile ranges, dots show outliners and squares stand for mean.
2.2. Water Relations and Fruit Carbohydrate Accumulation during Tree Decline
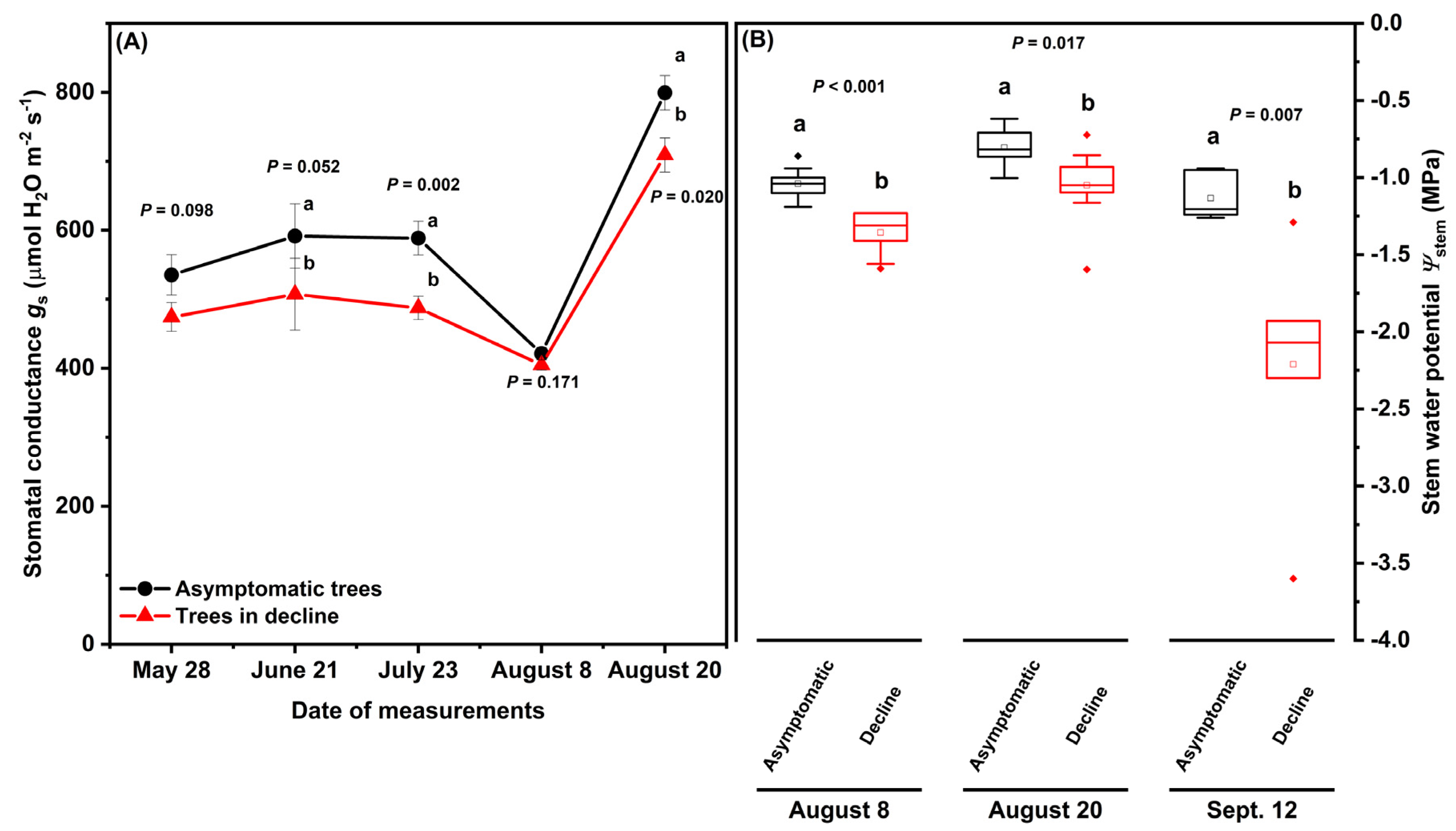
At Site S, stomatal conductance (gs) and stem water potential (Ψstem) were monitored in asymptomatic and declining ‘Ambrosia’/M.9 trees during the growing season of 2019, to demonstrate tree water relations during moderate tree decline. Tree health status had significant effects on gs (Figure 2A; F(1,122) = 10.65, p = 0.001) and on Ψstem (Figure 2B; F(1,47) = 13.65, p < 0.001). The gs of asymptomatic and declining trees showed similar trends across the growing season: relatively stable on May 28, June 21, and July 23, decreasing by 15–25% on August 8 but increasing by more than 75% on August 20, when Ψstem and gs was higher than on any other measurement date (Figure 2A). Compared to asymptomatic trees, the declining trees had significantly lower gs on June 21, July 23, and August 20 at p = 0.05 (Figure 2A), and lower Ψstem on all three measurements dates (August 8: −1.04 ± 0.04 MPa versus −1.36 ± 0.05 MPa, F(1,17) = 29.4; August 20: −0.8 ± 0.04 MPa versus −1.05 ± 0.08 MPa, F(1,17) = 7.07; September 12: −1.13 ± 0.06 MPa versus −2.21 ± 0.31 MPa, F(1,11) = 11.48) (Figure 2B). Ψstem in the declining trees dropped below −1.5 MPa on September 12.
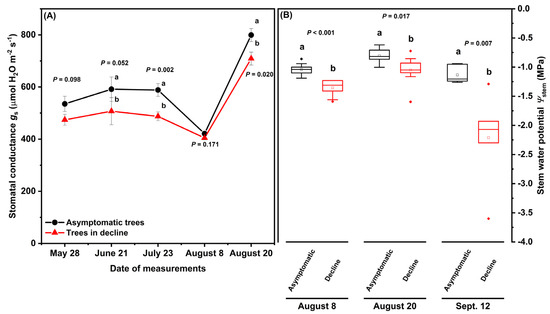
Figure 2.
Stomatal conductance gs (A) and mid-day stem water potential Ψstem (B) of asymptomatic and declining ‘Ambrosia’/M.9 apple trees at Site S in the growing season of 2019. Different letters stand for significant difference at p = 0.05 between asymptomatic and declining trees on each date (n = 6; p values from one-way ANOVA with tree heath status as the fixed effect are noted for each pair of comparison, Tukey’s test). In (A), dots with error bars are means ± standard errors. In (B), horizontal lines show median and interquartile ranges, dots show outliners and squares stand for mean.
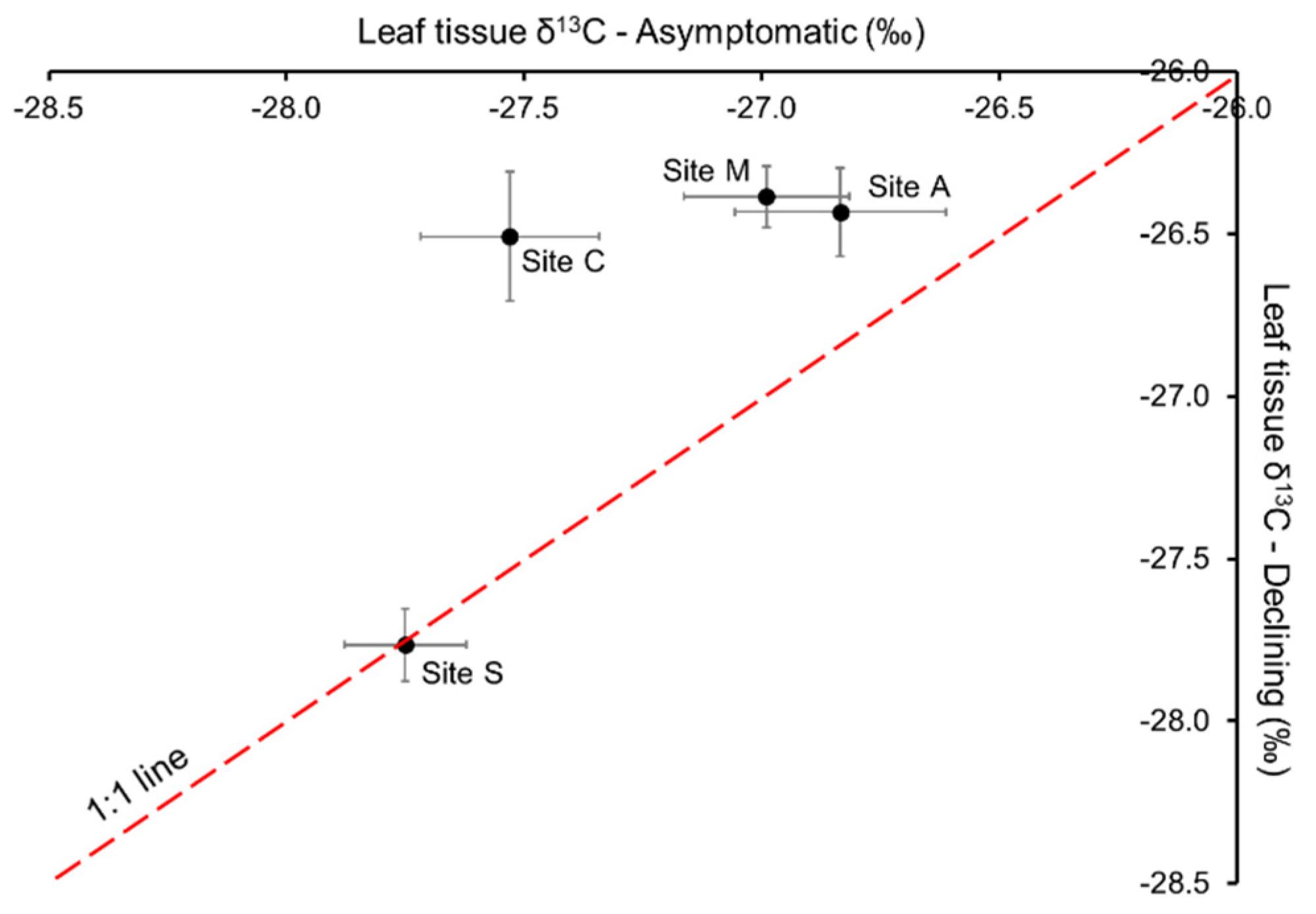
Asymptomatic and declining trees at Site S had similar leaf δ13C‰. In contrast, leaf δ13C‰ in declining trees was higher (less negative) than in asymptomatic trees at Site A, C and M (Figure 3).
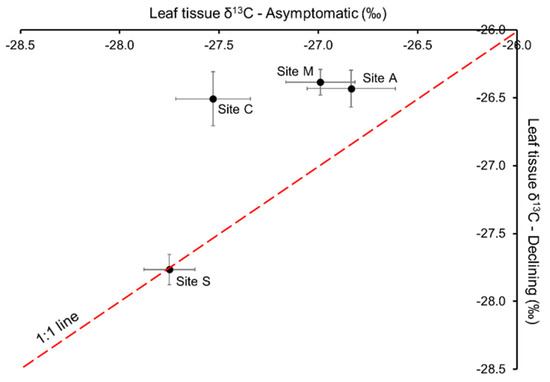
Figure 3.
The δ‰13C composition of leaf tissue collected from asymptomatic and declining trees at Sites A (n = 4), S (n = 8), C (n = 4) and M (n = 4), in 2019. Values are means ± standard error of the mean.
Fruit quality was analyzed on ‘Ambrosia’ apples collected from three sites in 2019 and four sites in 2020. Except for Site S in 2020, the declining trees had lower average fruit mass and lower estimated fruit dry matter weight (Table 2). Fruit firmness was higher on declining trees at Site S in 2019 and at Site C, Site M, and Site P in 2020. In both years, site, tree health status, and their interactions had significant effects on water potential of fruit hypanthium (Ψfruit) (2019: Site[F(2,85) = 74.9], Tree health[F(1,86) = 16.2], Interaction[F(2,85) = 23.6, Model[F(5,82) = 44.8], p < 0.001) (2020: Site[F(3,96) = 27.2], Tree health[F(1,98) = 23.1], Model[F(7,92) = 16.9], p < 0.001; Interaction[F(3,96) = 4.4, p = 0.006). The fruits on declining trees had higher soluble solids content (SSC) and lower Ψfruit at Site C in both years and at Site M in 2020 (Table 2).

Table 2.
Fruit mass, dry matter content (DMC), estimated dry matter weight, soluble solids content (SSC), firmness, and flesh water potential of ‘Ambrosia’ apple on asymptomatic and declining trees, from three sites in 2019 and four sites in 2020.
3. Discussion
3.1. Hydraulic Dysfunctions under Environmental Stresses
Despite well-known horticultural advantages, hydraulic restriction rendered by the dwarfing rootstock and the graft union can make apple trees susceptible to environmental adversities. Mild-to-moderate structural damage on trunk vascular tissues can restrict water transport in apple trees [3,27]. Soil water deficit, waterlogging, and/or sudden increase in transpirational demand can tilt the balance between root water supply and scion water demand [9,15,26], which could further cause xylem embolism and localized loss of hydraulic function. Under these circumstances, a decrease in gs and Ψstem is often observed, manifesting the compromised plant–water relations [15,26]. On one hand, this can lead to a reduction in transpirational cooling and the aggravation of subsequent water and heat stresses. On the other hand, it could cause the reduction in photosynthesis and photosynthate accumulation; over the growing season, it would change carbohydrate reserves in the affected trees [19,20]. This likely reduced the carbohydrate partitioning to reproductive growth, shown as a reduction in fruit mass and dry matter weight at harvest in the declining but surviving trees (Table 2). In the recent events of apple decline, hydraulic dysfunctions appeared to be linked to the deteriorating tree health according to the investigators’ visual observations; based on the extent of deterioration, we suggested the dysfunctions be categorized as either severe hydraulic disruption or gradual hydraulic decline.
Enrichment in leaf tissue 13C is indicative of moisture deficits [28]. Natural discrimination against 13C occurs as CO2 diffuses into the leaf tissue via stomates; the enzyme Rubisco also discriminates against 13C. As a result, photosynthates produced by trees that are not water stressed tend to be depleted in 13C relative to the atmosphere. When the internal concentration of CO2 within the leaf tissue decreases due to reduced stomatal conductance, more 13CO2 is incorporated into photosynthate and the 13C of leaf tissue increases [28]. Previous studies have demonstrated the relationship between lower Ψstem (more negative) or drought stress and higher leaf δ13C‰ (less negative) [27,29,30,31]. Leaf δ13C‰ in both asymptomatic and declining trees was particularly high at Site A (Figure 3), suggesting water was less available to the trees at this site, as 13C-enriched leaf tissue is consistent with a reduction in available intracellular CO2 due to partial stomatal closure caused by water deficit [28]. This was associated with a higher moisture deficit during the growing season recorded in the weather station adjacent to Site A, compared to the stations in the proximity of other sites (Table S1). Furthermore, δ13C‰ in declining trees was about 0.4‰ higher than in asymptomatic trees, suggesting declining trees were even more stressed (with marginal statistical significance p = 0.087).
At Site A, the significantly reduced area of active transporting xylem tissues (Figure 1A,B) and the fatally low tissue water potential (Figure 1C) at the wounded graft union showed the severe loss of hydraulic function in this structural bottleneck, which led to abrupt hydraulic failure and tree mortality. As the planting was situated on a south-facing slope with a shallow and sandy topsoil layer, several legacy effects may have induced the stress. First, soils in this region tend to have particularly poor soil water retention capacity [32,33]. This lack of ability to retain irrigation water may predispose trees in this region to periodical water deficits, particularly during hot periods, when rates of evapotranspiration are high. Indeed, summers in the Okanagan Valley are becoming hotter. Between 1970 and 1989, the average number of days with a maximum air temperature >35 °C was 1.8 (mode = 0) at the Summerland Research and Development Centre; this increased to 4.1 (mode = 1) between 1990 and 2009, and to 5.1 (mode = 3) between 2010 and 2021 [34]. Second, during the winter months, the sudden temperature fluctuations might cause rapid freeze–thaw cycles on the surface of the southwest-facing side of the lower trunk section; furthermore, the inconsistent composition of the scion–rootstock fusion could lead to uneven swelling and shrinking of the graft union. This symptom, commonly described as ‘Southwest Injury’ in North American apple orchards, can cause massive bark cracking, which directly exposes vascular tissues to secondary stressors [1] (Brownlee R, pers. comm.; Quamme H, pers. comm.). Third, freezing under prolonged extreme minimum temperature in the winter could cause the necrosis of vascular tissues; it was reported that xylem, cambium, and phloem tissues in the roots of dwarfing apple rootstocks were susceptible to such injury, which was speculated to be a main cause for apple decline in New York State (USA) following the cold winters of 2014 and 2015 [22]. Last, due to the compromised water relations, the systemic defense in the predisposed trees was weakened; bark cracking and damaged vascular tissues served as the entries for pathogens and insect pests. These conditions could accelerate and expand the infestation of biotic stressors, such as the apple clearwing moth (S. myopaeformis), shothole borer (S. rugulosus), Phytophthora and Diaporthe pathogens [5,23,24]. Infestation can further damage vascular tissues and deplete carbohydrates, leading to irreversible tree decline.
Hydraulic dysfunction can be gradual and sometimes reversible. Similar to what was reported in several woody perennial species [21], we speculated a gradual decline in hydraulic performance coupled with carbon reserve depletion during tree decline at Sites C and M. Delta 13C‰ of leaf tissue can be used as a proxy measurement of plant water status and leaf gas exchange over the growing season [28,35] and Ψfruit [15] represent the average plant water status over the period of fruit maturation. Drought stress in the declining trees was shown as higher leaf δ13C‰ (less negative) (Figure 3; p = 0.0041 at Site C, p = 0.0127 at Site M) and lower Ψfruit (more negative) (Table 2) than asymptomatic trees at both sites. Carbohydrates comprise 90% of dry matter content (DMC) in apple fruit [36]. Therefore, less fruit mass and dry matter accumulation at harvest could suggest a reduction in carbohydrate allocation to fruits in the affected trees in 2019, followed by further decrease in fruit mass in 2020 (Table 2). At Site S, the decrease in gs and Ψstem showed a transient water deficit in declining trees at the times of measurements (Figure 2A,B). However, neither leaf δ13C‰ (measured in July; Figure 3) nor Ψfruit (measured at harvest in October; Table 2) differed significantly between declining and asymptomatic trees, which suggests that water deficits in symptomatic trees measured periodically over the growing season at Site S (Figure 2) were transient and reversible. This could be attributed to sufficient access to water; over the growing season, water deficits at this site were not sufficiently severe to alter leaf δ13C‰ or Ψfruit in symptomatic trees. Indeed the lower leaf δ13C‰ at Site S relative to those measured at Sites A, M, and C suggests that trees at Site S may have had better moisture status over the growing season than those at the other three sites. Drought stress alleviation and hydraulic recovery at Site S could be attributed to more frequent and adequate water replenishment, as irrigation at this site was scheduled to replace 100% of the water lost to evapotranspiration (Et) every second day.
In addition to the deteriorated fruit quality, carbohydrate depletion at the whole tree level could weaken the tree crop resilience against secondary stresses such as winter injury or trunk borer attack. If the environmental challenges persist, the hydraulic decline can worsen over several growing seasons and eventually lead to tree mortality. Under more favorable conditions, however, the cavitation in xylem vessels can be refilled by sap inflow from adjacent vascular and parenchyma cells, rendering hydraulic recovery and tree water relations improvement [25]. Therefore, improving water and carbohydrate replenishment in hydraulic systems after prolonged stresses is important for remedying crop decline and enhancing orchard resilience.
3.2. Horticultural Mitigation
Effective horticultural approaches can mitigate environmental adversities and create favorable growing conditions. To prevent abrupt hydraulic failure and subsequent tree mortality, it is important to ensure the continuous function of vascular tissues in the graft union. Good grafting techniques are essential, including selecting good quality bud wood and healthy, non-juvenile rootstocks, conducting bench grafting and micropropagated plant grafting during dormancy, carefully aligning cambium tissues of the two parts, providing wound protection to prevent water loss and infection, and allowing ample time for callus development and new vascular tissue differentiation before acclimation. These practices help to prevent abnormal distribution of endogenous hormones between scion and rootstock as well as the formation of hyperplasia tissues around graft union [37]. Sufficient pruning and adequate water and nutrient supply is critical for successful establishment of new plantings, particularly when the orchard conditions are much different from where the grafting and early acclimation is conducted. The graft union of established trees can be protected using white latex paint to reflect radiation and reduce the fluctuation of trunk surface temperatures. Alternative weed control approaches should be tested to avoid damage caused by conventional herbicides application, weed tilling, and burning. Given that one of the most prevailing symptoms was a wounded graft union compromised by the infestation of fungal pathogens or trunk boring insects, early detection and treatment of these infestations is critical for keeping vascular tissue damage low and hydraulic disruption at non-fatal levels.
Horticultural practices that improve tree water relations can help trees regain a balanced C supply-demand, recover from gradual hydraulic decline, and enhance crop tolerance. Better moisture replenishment in the rhizosphere can be achieved by precision irrigation scheduling based on soil type and crop water status. Excessive evapotranspiration demand can be alleviated by the use of shade nets above the canopy [38,39,40,41]. A negative correlation was reported between wood starch concentrations and crop level in apple [42]; given the importance of nonstructural carbohydrates in hydraulic regulation and embolism recovery [43], maintaining a lower crop load can help the trees in early decline to allocate more resources for hydraulic recovery in woody tissues. At Site S, subsequent to the decline progression in 2019, fruit mass and DMC improved significantly in 2020 (Table 2; fruit mass increase by ~65%, F(1,213) = 352.8, p < 0.001; DMC increase by ~30%, F(1,213) = 1441.7, p < 0.001), which implied the recovered tree–water relations under a lighter cropping (average yield per tree: 21.77 Kg in 2019, 13.49 Kg in 2020; F(1,35) = 19.457, p < 0.001). In this line, maintaining a moderate crop load is particularly important for new plantings and small dwarfing rootstocks of limited trunk diameter to regain vigor and enhance resilience. Trees on larger dwarfing rootstocks possess higher water transporting capacity and more carbohydrate reserves in vegetative tissues. Recent studies suggested that, under sufficient water supply, larger dwarfing rootstocks could be more resilient to heat stress because of their higher Ψstem and denser canopy [16,17]. The feasibility of using larger dwarfing rootstocks to mitigate effects of a warming climate requires testing in more locations.
3.3. Limitations and Future Perspectives
This investigation on RAD has been different from a typical controlled experiment. The researchers attempted to understand the legacy effects of multiple putative factors on tree–water relations. However, without knowing the exact causal factor(s), stress treatments could not be implemented to reproduce the exact scenario(s) that could have induced tree decline. Under these restrictions, a two-year survey was conducted on multiple sites, with Site A to represent the symptoms of abrupt hydraulic failure, and other sites to represent gradual hydraulic loss; relevant assays were conducted to investigate hydraulic dysfunction at the graft union at Site A and plant–water relations at other sites, respectively. More assays were carried out at Site S—the main study site—than Site C, M, and P, due to resource constraints. Future studies are required to systematically monitor plant–water relations and environmental conditions across multiple sites that differ in the severity of hydraulic loss, during tree decline progression over years. In addition, controlled experiments are required to elucidate the effects of sequential abiotic and biotic stresses on vascular tissue damage and hydraulic dysfunction, particularly at the graft union.
In this study, the staining technique and potentiometer measurement helped to reveal the consequences of hydraulic failure in the samples from Site A. However, these destructive methods cannot be used to track the in situ hydraulic dynamics during tree decline. The advance in real-time imaging methods [44] can facilitate the non-destructive analysis of the flow dynamics and hydraulic functions in symptomatic trees in future studies. Furthermore, fruit DMC was used to estimate carbohydrate allocation to fruits in this study; carbohydrate contents in other plant tissues were not investigated. The quantification of structural and non-structural carbohydrate contents in fruits and vegetative tissues [20,25,42,43] is required to demonstrate the roles of carbohydrates during apple tree decline and recovery.
4. Materials and Methods
4.1. Site Selection and Symptom Description
The study sites were located in the apple production zone in the Okanagan-Similkameen Valley, interior British Columbia, Canada (Table 1). The climate in this region is semi-arid. Soils tend to be coarse-textured and poorly aggregated, with a limited capacity to retain soil water; irrigation is required to maintain commercial apple production.
Site A was a ‘Salish’/M.9 apple orchard located in Summerland, British Columbia, Canada (49.62° E, 119.67° W). Soil texture varied across this site (sand to loamy sand to sandy loam); soil organic matter (SOM) tended to be relatively high (4.3%), and pH relatively low (6.0). However, the part of the orchard in decline was situated on a south-facing slope with a particularly shallow and sandy topsoil layer. In September 2019, the apple trees in severe decline were at the brink of mortality; half to entire canopies showed leaf senescence and defoliation; bark and xylem from 2/3 to the entire graft union were damaged, with fungal canker and wooly aphid infestation found in the wounds. Due to tree mortality, the affected planting was terminated by the end of the growing season in 2019.
Site S was an ‘Ambrosia’/M.9 experimental trial located at Summerland Research and Development Center, Agriculture and Agri-Food Canada (49.57° E, 119.64° W). Soil texture was sand to loamy sand, with low SOM (1.6%) and moderate pH (6.8) (Table 1). In early summer of 2019, the declining trees were sparsely distributed across the block with no apparent pattern; the leaves on extension shoots appeared wilting and chlorotic; bark and xylem of about 1/4 to 1/3 of graft union was damaged, with frass and exit holes caused by apple clearwing moth larvae often found in the wounds. Many of the declining trees survived and produced fruits in 2019 and 2020.
Site C was an organic apple orchard in Cawston, BC (49.19° E, 119.75° W). Site M (49.57° E, 119.69° W) and Site P (49.57° E, 119.65° W) were two conventional apple orchards in Summerland, BC. These sites ranged in texture (loamy sand to sandy loam), soil organic matter (2.0% to 2.6%) and pH (6.2 to 7.8) (Table 1). The symptomatic ‘Ambrosia’/M9 trees on these sites manifested moderate decline symptoms similar to Site S. Most of the declining trees at these sites also survived the 2019/2020 growing seasons and produced fruits.
At all five of these sampling sites, apple trees were planted in high density of 3′ × 10′ spacing (0.91 m × 3.05 m). The canopy was trained into trellised Tall Spindle Axe system. Water was supplied by drip irrigation. Irrigation was scheduled based on 100% Et replacement at 2-day intervals at Site S. Irrigation at other sites was empirically scheduled in 3–7 day intervals, based on growers’ observation on soil and tree water status. T-sum (accumulated mean daily temperatures above 0 °C) and moisture deficit (evapotranspiration minus effective precipitation) during May 1–September 15 in 2019 and 2020 were obtained from the data sets of weather stations in proximity of the study sites from farmwest.com (Table S1).
4.2. Staining to Characterize Transporting Xylem Tissue
Asymptomatic and severely declining ‘Salish’/M.9 apple trees were excavated from Site A in late September, 2019 (n = 10 trees), and were transported to the greenhouse with roots covered in moist soil in large pots. Trees were watered daily and kept in the greenhouse for 3 days before examination (18–25 °C, 40–60% relative humidity, 16 h sunlight and supplementary lighting). Using a reciprocating saw, a clear cut was made at 10 cm below the graft union. With the tree held in upright position, the cut end was submerged into 200 mL staining solution of 0.5% Safranin O dye (w/v, in 50% ethanol, Sigma-Aldrich) for 16 h to allow the solution to move upwards through xylem transport under transpiration (n = 4 trees) (modified method based on Olmstead et al. [45]). After staining, a stationary band saw was used to cut two cross sections through the center of the graft union and at 10 cm above the graft union, respectively, for the examination of transporting xylem tissues through the graft union and into the scion. Cross sections were smoothed using a belt sander with #120 sand belt prior to photographing. A piece of transparent plastic with a 1 cm ruler mark was placed on top of the cross section; the image of cross section was captured by a NIKON D700 and software Capture NX-D (F-stop f/10, Exposure time ¼ second, and ISO sensitivity 200, with white balance, 35 mm focal length 60 mm, maximum aperture 3.7, image resolution 300 dpi, bit depth 24; Nikon Inc., Melville, NY, USA). The area of the total cross section excluding bark (Area 1) and the area of the stained tissues (Area 2) were measured in Image J [46] (Green channel; threshold setting at 5 to include the entire cross section for Area 1 and at 50–53 to exclude the stained area for Area 3; Area 2 was calculated as Area 1 subtracted by Area 3). The stained tissues indicated where aqueous dye was transported through xylem; therefore, the area of stained tissues represented that of active transporting xylem tissues. The percentage of active transporting xylem tissues to the total cross section was calculated as Area 2 divided by Area 1 at the center of the graft union.
4.3. Tree Water Relations Measurement
On the same day of staining, xylem tissue water potential of graft union (Ψunion) was measured on 6 asymptomatic trees and 6 declining trees sampled from Site A. In the greenhouse, 8-12 pieces of xylem tissue under the bark of the graft union were quickly excised using a dissecting blade (each piece was about 5 mm × 10 mm and 1–1.5 mm thick), immediately placed in a stainless sample cup to cover the cup bottom, and filled to about 1/4–1/3 of the cup, then sealed with a plastic cap and Parafilm® (American Can Co.; Greenwich, CT, USA) along the rim (sample cup and cap were provided by the manufacturer Meter Environment). The samples were brought to the laboratory; immediately after the removal of Parafilm and cap, the sample cup was placed into the sample slot in the WP4C potentiometer (Meter Environment; Pullman, WA, USA) and sealed for sufficient vapor equilibrium for 5 min and then for another 6–10 min before the Ψ measurement was automatically conducted in the fast mode. The −2.19 MPa standard KCl solution was used to calibrate the instrument at 24–25 °C. This protocol was similar to the use of potentiometer in measuring Ψ of fruit flesh [15] and wood tissues [47]; accuracy relies on collecting and sealing samples rapidly, filling at least 1/4 of the sample cup with adequate samples, and allowing sufficient time for vapor equilibrium.
Fruits were sampled at optimal harvest time from asymptomatic and declining trees, at Site S, Site M, and Site C in 2019 and 2020, and at Site P in 2020 (Site S: n = 6 trees, 10 fruits per tree; other sites: n = 10 trees, 5 fruits per tree). Fruits were stored in air at 4 °C for one week, recovered to 23–24 °C in the laboratory and measured for water potential of fruit hypanthium (Ψfruit) using the WP4C potentiometer [15].
At Site S, mid-day stem water potential (Ψstem) and stomatal conductance (gs) were measured in asymptomatic and declining trees during the growing season of 2019 (n = 6), using pressure chamber (PMS 1505D; PMS Instrument Company, Albany, OR, USA) [48] and leaf porometer (Decagon SC-1), respectively. Measurements were conducted on one sunlit and non-fruiting extension shoot of approximately 10–15 cm in length with 3–5 fully expanded leaves on each sample tree, for 6 asymptomatic trees and 6 declining trees, between 10:00 AM and 2 PM on sunny days (specific dates were shown in Figure 2).
Leaf tissue was collected in June (Site M), July (Site S), and August (Sites A and C). Leaves were dried for 24 h at 65 °C and ball milled prior to determination of 13C using an IsoPrime continuous-flow mass spectrometer (GV Instruments, Manchester, UK) linked to a EuroVector elemental analyzer (Milan, Italy). A two-point linear normalization procedure, using two in-house working standards, was used to standardize the isotopic analysis. The working standards were calibrated to V-PDB through the National Institute of Standards and Technology reference materials sucrose RM-8542 and polyethylene RM-8540.
4.4. Soil Organic Matter and Soil pH
Soil samples were collected from all five sites in the summer of 2019. At each site, pairs of asymptomatic and declining trees were identified. Four subsamples were collected to a depth of 15 cm directly adjacent to each identified tree, and composited (one composite soil sample per tree). Composite samples were sieved (2 mm), air-dried, and sent for standard analysis at a commercial laboratory (Western Canada Test Package + Particle Size Analysis; A&L laboratories, London, Ontario). There were no differences in the properties of soils adjacent to asymptomatic and declining trees; thus, average soil properties were calculated by site using data from all sample locations (Table 1).
4.5. Apple Fruit Quality Assessment
Fruit mass, dry matter content (DMC%), firmness and soluble solids content (SSC%) were measured for at-harvest fruit quality assessment. Fruit mass was measured using a compact bench scale (Ohaus R71MHD35 Ranger 7000, Parsippany, NJ, USA); DMC at the sunlit-shaded transition zone was estimated using Felix-750 Produce Quality Meter (Felix Instruments Inc., Camas, WA, USA) (For Site M, C and P: 10 trees, 5 fruits per tree; for Site S: 6 trees, 10 fruits per tree). Fruit firmness was measured using Fruit Texture Analyzer (FTA-G25, GÜSS Manufacturing Ltd., Strand, South Africa) (10 trees and 3 fruits per tree in 2019, 10 trees and 1 fruits per tree in 2020). Soluble solids content was measured using a portable refractometer (30P, Mettler Toledo, Columbus, OH, USA) (For Site M, C and P: each juice sample consisted of juice from 5 fruits per tree, 2 slices per fruit; for Site S: each juice sample consisted of juice from 10 fruits per tree, 1 slice per fruit).
4.6. Data Analysis
Data plotting, linear regression and statistical significance analysis (ANOVA, Tukey’s test, p ≤ 0.05) was conducted using OriginPro 8.0 (OriginLab, Northampton, MA, USA).
5. Conclusions
This investigation showed the potential relevance of hydraulic dysfunctions to the recent apple tree decline. Examination of water transporting tissues at the graft union helped to elucidate the relation between tree mortality and catastrophic hydraulic failure due to the massive loss of vascular tissues. The measurements of gs and Ψstem revealed the dynamics of transient water relations during the progression of tree decline, whereas δ13C‰ and Ψfruit reflected the average plant water status over a longer period of time.
The results showed that abrupt loss of hydraulic function at the graft union was fatal at Site A. Gradual hydraulic decline concurred with the decrease in fruit dry matter accumulation, implying the risk of carbohydrate depletion over time at Sites C and M. Tree decline could be alleviated when water and carbohydrates in the hydraulic systems were replenished under a favorable irrigation regime and moderate crop load, as shown at Site S. Further study is required to identify the physiological thresholds for xylem hydraulic loss and carbohydrate depletion that lead to irreversible tree decline; this information could be used to identify horticultural mitigation practices that can prevent tree mortality, alleviate crop stresses and enhance orchard resilience.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/stresses3010019/s1, Table S1. T-sum and moisture during May 1—September 15 in 2019 and 2020 in the weather stations in proximity of the study sites.
Author Contributions
Data collection: xylem staining, stomatal conductance, stem water potential—D.E. and H.X.; fruit analysis—D.E., J.L.M. and H.X.; 13C analysis and soil analysis—K.D.H. and J.L.M. Formal analysis, data visualization and interpretation: K.D.H., J.L.M. and H.X. Writing: original draft—H.X.; review & editing—K.D.H., J.L.M. and H.X. Project administration—J.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the research project J-002199 (Emerging fruit tree decline), Agriculture and Agri-Food Canada.
Data Availability Statement
All the data are available upon request.
Acknowledgments
This study was part of the research project “Emergence of rapid tree fruit decline”, supported by Agriculture and Agri-Food Canada (Project ID J-002199). We are grateful to Megan Lowe, Duncan McGregor, Shawn Kuchta, Istvan Losso, Andrew Midwood and Sebastian Damin for their excellent technical support, to Michael Weis for advising on cross section preparation and photographing, to the four collaborating growers for granting us with access to their apple orchards and crops, to Tom Forge and Paige Munro for assisting the tree health inspection at Site S, to Summerland RDC Field Service for maintaining Site S, and to Oualid Ellouz for coordinating the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosenberger, D. Sudden Apple Decline: Trunk-Related Problems in Apples. Cornell University. 2018. Available online: http://www.hort.cornell.edu/expo/proceedings/2017/TreeFruitPestMGMT.AppleTrunkDisorders.Rosenberger.2017.pdf (accessed on 17 November 2022).
- Villani, S.M.; Calvin, J.; Kreis, R.; Schoof, S.; Walgenbach, J.F. Defining factors associated with rapid apple decline in the Southeastern United States. In Proceedings of the International Congress of Plant Pathology (ICPP) 2018: Plant Health in A Global Economy, St. Paul, MN, USA, 29 July–3 August 2018. [Google Scholar]
- Singh, J.; Silva, K.J.P.; Fuchs, M.; Khan, A. Potential role of weather, soil and plant microbial communities in rapid decline of apple trees. PLoS ONE 2019, 14, e0213293. [Google Scholar] [CrossRef] [PubMed]
- Stokstad, E. Rapid apple decline has researchers stumped. Science 2019, 363, 1259. [Google Scholar] [CrossRef]
- MacDonald, J.L.; Hannam, K.; Xu, H. Signs and symptoms of sudden apple decline in British Columbia, impacts on tree physiology, and the potential role of environmental stressors. In Proceedings of the Canadian Phytopathological Society Annual Meeting, Virtual, 4–8 July 2022. [Google Scholar]
- Xiao, H.; Hao, W.; Storoschuk, G.; MacDonald, J.L.; Sanfaçon, H. Characterizing the Virome of Apple Orchards Affected by Rapid Decline in the Okanagan and Similkameen Valleys of British Columbia (Canada). Pathogens 2022, 11, 1231. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, J.; Waring, R.; Ryan, M. Water relations in tree physiology: Where to from here? Tree Physiol. 2017, 37, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Naor, A.; Bennink, J.; Grava, A.; Tyree, M. Hydraulic resistance components of mature apple trees on rootstocks of different vigours. J. Exp. Bot. 2007, 58, 4213–4224. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, T.L.; Centinari, M.; Bauerle, W.L. Shifts in xylem vessel diameter and embolisms in grafted apple trees of differing rootstock growth potential in response to drought. Planta 2011, 234, 1045–1054. [Google Scholar] [CrossRef]
- Olien, W.; Lakso, A.N. Effect of rootstock on apple (Malus domestica) tree water relations. Physiol. Plant. 1986, 67, 421–430. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Webster, A.D.; Vaughan, S.; Lucas, A.S. Effects of root restriction on the physiology of apple tree growth. Acta Hortic. 1997, 451, 587–598. [Google Scholar] [CrossRef]
- Jones, H.G. How do rootstocks control shoot water relations? New. Phytol. Found. 2012, 194, 301–303. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Effects of size-controlling apple rootstocks on growth, abscisic acid, and hydraulic conductivity of scion of different vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Marini, R.P.; Fazio, G. Apple rootstocks: History, physiology, management and breeding—Stresses influencing rootstock performance. In Horticultural Reviews; Warrington, I., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 2018; Volume 45, pp. 225–258. [Google Scholar]
- Xu, H.; Ediger, D. Rootstocks with different vigor influenced scion–water relations and stress responses in AmbrosiaTM apple trees (Malus domestica var. Ambrosia). Plants 2021, 10, 614. [Google Scholar] [CrossRef]
- Xu, H.; Watanabe, Y.; Ediger, D.; Yang, X.; Iritani, D. Characteristics of sunburn browning fruit and rootstock-dependent damage-free yield of Ambrosia™ apple after sustained summer heat events. Plants 2022, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Blatt, S.; Ediger, D. Tools for climate resilience in tree fruit I: Large-dwarfing rootstocks can alleviate sunburn damage in “Buckeye Gala” apple. Can. J. Plant Sci. 2022. e-First. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.; Hassan, G.; Baba, T.; Alyemeni, M.; Alsahli, A.; El-Serehy, H.; Paray, B.; Ahmad, P. Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Klein, T.; Hoch, G.; Yakir, D.; Körner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Priestley, C.A. Carbohydrate reserves in deciduous fruit trees. Hort. Rev. 1988, 10, 403–430. [Google Scholar]
- Cailleret, M.; Jansen, S.; Robert, E.M.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Change Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef]
- Robinson, T. Apple Rootstock Performance—An Eastern Perspective. SCRI Apple Root to Fruit Webinar on Rootstock and Nutrition 2022. Virtual. March 1 2022. Available online: https://youtube.com/playlist?list=PLYLbxsK4pTXXoyNLVkWZjKprOVOkkoQCL (accessed on 20 March 2022).
- Pacific Northwest Pest Management Handbooks. Insect/Tree Fruit Crops/Apple Pest—Apple-Shothole Borer. A Pacific northwest Extension Publication. Available online: https://pnwhandbooks.org/insect/tree-fruit/apple/apple-shothole-borer (accessed on 30 December 2022).
- Úrbez-Torres, J.R.; Boule, J.; O’Gorman, D.T. First report of Diplodia seriata and D. mutila causing apple dieback in British Columbia. Plant Dis. 2016, 100, 1243. [Google Scholar] [CrossRef]
- Trifilò, P.; Kiorapostolou, N.; Petruzzellis, F.; Vitti, S.; Petit, G.; Gullo, M.A.L.; Nardini, A.; Casolo, V. Hydraulic recovery from xylem embolism in excised branches of twelve woody species: Relationships with parenchyma cells and non-structural carbohydrates. Plant Physiol. Biochem. 2019, 139, 513–520. [Google Scholar] [CrossRef]
- Choi, B.H.; Bhusal, N.; Jeong, W.T.; Park, I.H.; Han, S.G.; Yoon, T.M. Waterlogging tolerance in apple trees grafted on rootstocks from G, CG, and M series. Hort. Environ. Biotech. 2020, 61, 685–692. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Z.; Xu, H.; Ali, A.; Zhang, X.; Liu, X.; Zhang, Y.; Zhou, X.; Lu, Z. Thirst or Malnutrition: The impacts of invasive insect Agrilus mali on the physiological status of wild apple trees. Forests 2020, 11, 440. [Google Scholar] [CrossRef]
- Farquar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Casagrande Biasuz, E.; Kalcsits, L.A. Apple rootstocks affect functional leaf traits with consequential effects on carbon isotope composition and vegetative vigour. AoB Plants 2022, 14, plac020. [Google Scholar] [CrossRef]
- Kalcsits, L.; Valverdi, N.; Reid, M. Timing of water limitations affect source to sink differences in δ13C composition in apple. Acta Hortic. 2022, 1335, 437–444. [Google Scholar] [CrossRef]
- Valverdi, N.A.; Kalcsits, L. Apple rootstock genotype affects scion responses to water limitations under field conditions. Acta Physiol. Plantatum 2022, 43, 97. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Neilsen, G.H.; Hogue, E.; Neilsen, D. Influence of organic waste on selected soil physical and chemical properties. Can. J. Soil Sci. 1999, 79, 501–504. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Hogue, E.J.; Forge, T.; Neilsen, D. Surface application of mulches and biosolids affect orchard soil properties after 7 years. Can. J. Soil Sci. 2003, 83, 131–137. [Google Scholar] [CrossRef]
- Environment Canada. Station Results. Historic Data; Station: Summerland, BC. Available online: https://climate.weather.gc.ca/historical_data/search_historic_data_e.html (accessed on 5 April 2021).
- O’Leary, M.H. Carbon isotopes in photosynthesis. Bioscience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Suni, M.; Nyman, M.; Eriksson, N.A.; Björk, L.; Björck, I. Carbohydrate composition and content of organic acids in fresh and stored apples. J. Sci. Food Agric. 2000, 80, 1538–1544. [Google Scholar] [CrossRef]
- Andrews, P.K.; Marquez, C.S. Graft incompatibility. Hortic. Rev. 2010, 15, 183–232. [Google Scholar]
- Kalcsits, L.; Asteggiano, L.; Schmidt, T.; Musacchi, S.; Serra, S.; Layne, D.R.; Mupambi, G. Shade netting reduces sunburn damage and soil moisture depletion in ‘Granny Smith’ apples. Acta Hortic. 2018, 1228, 85–90. [Google Scholar] [CrossRef]
- Mupambi, G.; Anthony, B.M.; Layne, D.R.; Musacchi, S.; Serra, S.; Schmidt, T.; Kalcsits, L.A. The influence of protective netting on tree physiology and fruit quality of apple: A review. Sci. Hortic. 2018, 236, 60–72. [Google Scholar]
- Boini, A.; Manfrini, L.; Morandi, B.; Corelli Grappadelli, L.; Predieri, S.; Daniele, G.M.; López, G. High levels of shading as a sustainable application for mitigating drought, in modern apple production. Agronomy 2021, 11, 422. [Google Scholar] [CrossRef]
- Lulane, E.B.; Dzikiti, S.; Volschenk, T.; Lötze, E.; Midgley, S.J.E. Quantifying water saving benefits of fixed white protective netting in irrigated apple orchards under Mediterranean-type climate conditions in South Africa. Sci. Hortic. 2022, 305, 111439. [Google Scholar] [CrossRef]
- Naschitz, S.; Naor, A.; Genish, S.; Wolf, S.; Goldschmidt, E.E. Internal management of non-structural carbohydrate resources in apple leaves and branch wood under a broad range of sink and source manipulations. Tree Physiol. 2010, 30, 715–727. [Google Scholar] [CrossRef]
- Tomasella, M.; Petrussa, E.; Petruzzellis, F.; Nardini, A.; Casolo, V. The possible role of non-structural carbohydrates in the regulation of tree hydraulics. Int. J. Mol. Sci. 2019, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, J.; Hwang, I. Investigating water transport through the xylem network in vascular plants. J. Exp. Bot. 2014, 65, 1895–1904. [Google Scholar] [CrossRef]
- Olmstead, M.A.; Lang, N.S.; Lang, G.A.; Ewers, F.W.; Owens, S.A. Examining the vascular pathway of sweet cherries grafted onto dwarfing rootstocks. HortScience 2006, 41, 674–679. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Fields, J.S.; Fonteno, W.C.; Jackson, B.E.; Heitman, J.L.; Owen Jr, J.S. The Use of Dewpoint Hygrometry to Measure Low Water Potentials in Soilless Substrate Components and Composites. Agronomy 2020, 10, 1393. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).