Abstract
Fruit diseases brought on by fungus infestation leads to postharvest losses of fresh fruit. Approximately 30% of harvested fruits do not reach consumers’ plates due to postharvest losses. Fungal pathogens play a substantial part in those losses, as they cause the majority of fruit rots and consumer complaints. Understanding fungal pathogenic processes and control measures is crucial for developing disease prevention and treatment strategies. In this review, we covered the presented pathogen entry, environmental conditions for pathogenesis, fruit’s response to pathogen attack, molecular mechanisms by which fungi infect fruits in the postharvest phase, production of mycotoxin, virulence factors, fungal genes involved in pathogenesis, and recent strategies for protecting fruit from fungal attack. Then, in order to investigate new avenues for ensuring fruit production, existing fungal management strategies were then assessed based on their mechanisms for altering the infection process. The goal of this review is to bridge the knowledge gap between the mechanisms of fungal disease progression and numerous disease control strategies being developed for fruit farming.
1. Introduction
Nowadays, eating a diverse and balanced diet is important to people. Because of their high nutritional content, flavor, unique taste, and nutraceutical qualities, as well as their proven health-promoting effects, fruits are being consumed and accepted by more people [1,2]. The advantages of regularly consuming fruit for your health have been well investigated and examined. Fruit is suggested in the dietary standards of many nations, including Argentina, Bolivia, Chile, Colombia, Costa Rica, and others, since it provides a range of vitamins (A, B, B9, C, and D) and minerals that reduce malnutrition, such as calcium (Ca), iron (Fe), magnesium (Mg), iodine (I), manganese (Mn), selenium (Se), and others [3,4,5]. They also provide the bioactive substances that the body needs to function properly [6]. These substances work in a number of ways. The World Health Organization (WHO) 2021 study states that eating fruit is linked to living a healthy lifestyle. Since fruit quality and safety are both essential components in guaranteeing fruit production, interest in high-quality fresh fruit has increased in recent years, mostly as a result of growing customer demand [7]. Globally, 14% of food is wasted from harvest to sale, worth an estimated USD 400 billion (FAO, 2019). A further 17% of waste is generated at the retail and consumer levels (UNEP 2021) [8,9].
Fruits are perishable products [10]. Fruits’ high water content makes them particularly vulnerable to fungus attack [11]. Approximately 25% of all vegetables and fruits are lost each year owing to fungal infections in the context of production and postharvest field [12]. Damage and hazards brought on by pathogenic molds add to the vulnerability of produce. There are various pathogens responsible for blue mold disease among fruit species, such as Penicillium expansum [13], which infects apple, kiwi, pome, and stone fruits, and also P. italicum, which causes infection in the citrus fruits [14]. Additionally, P. digitatum infects citrus fruit and results in green mold disease [15]. In the litchi fruit, various fungal species such as Aspergillus spp., Alternaria spp., Cladosporium spp., Agrostalagmus spp., Colletotrichum spp., Cylindrocarpon tonkinense, Dothiorella sp., Geotrichum ludwigii, G. candidum, Lasiodiplodia theobromae, Mucor spp., Monilia spp.; Neurospora spp., Peronophythora litchi, Pestalotiopsis sp., Diaporthe spp., Stemphylium spp., Trichoderma spp. causes infection [16,17].
Additionally, fruits that are prone to mold contamination caused by fungal infection might release strange odors, such as moldy and earthy odors. The firmness of diseased fruits might range from mild to severe [18]. Generally, fruit antioxidant activity decreases when pathogenic fungi are present [19]. Additionally, the quantity and quality of sugar and nutrients have both significantly decreased [20]. Fruit safety and its derivatives are significantly impacted by molds, which are unhealthy for consumers. Fruits are often more susceptible to contamination by foodborne pathogens when they have fungal infections [21]. In addition, mycotoxins generated by P. expansum (a mycotoxigenic fungus), which produces mycotoxin patulin (PAT), during fruit infection raise safety issues [21,22]. Typical PAT-contaminated fruits include blackcurrants, cranberries, grapes, sour cherries, strawberries, pome, pineapples, and passion fruits [23,24]. PAT, which may be generated by Aspergillus, Byssochlamys, and Penicillium spp., is the major mycotoxin found in fruit [24]. Aspergillus nomius, A. flavus, and A. parasiticus all produce aflatoxins in infected fruits [25]. Fruits and the products they are connected with exhibit the highest concentrations of the aflatoxin B1 (produced by Aspergillus flavus and A. parasiticus) and G1 (produced by some Group II A. flavus and A. parasiticus) types [25,26]. Dates, figs, raisins, and citrus fruits are all contaminated with aflatoxin [27,28]. In dates, Ochratoxin A is also produced by Penicillium and Aspergillus spp. [29].
According to a postharvest report of 2021, more significant postharvest losses occur with fruits and vegetables, with estimates of approx 45% [30]. One of the main causes of these losses is postharvest fruit rotting, which is predominantly brought on by fungal infections after the ripening phase. Like foliar diseases that take place in the field, a number of variables, including fungal-based pathogenesis, response to host, and environment, impact the result of susceptibility or resistance to the host. Fruit ripening, though, is another crucial element that affects fruit resistance in postharvest infections and must be taken into consideration. The fast physiological changes brought on by fungal decay in berries, including color loss, weight loss, hastened softening of tissue, and shorter storage life, considerably reduces the fruit’s market value [31,32]. In addition to such apparent quality attributes, a fungal infection changes the chemical composition and nutritional value of fruits. The chemical alterations include the creation of acid, sugar breakdown, and microbial metabolites. Critically, increasing mycotoxin production often raises questions about food safety because these substances are harmful to human health [33]. According to reports, the process of a fungal infection in fruit involves four major steps, including adhesion of fungal-spores with the surface of the fruit, stabilizing attachment to the host by producing infected structures, invasion into host tissues, and colonization and spread of organisms [34,35]. Fruits’ antifungal defense systems are activated in response to harmful fungi. Fruits’ primary defensive mechanisms include an increase in phytohormone synthesis, an oxidative burst, defense-related enzyme activation, and pathogen-related protein overexpression [36]. This review presents a comprehensive understanding of research progress on the fungal potential entry site for invasion, the genetic and regulatory mechanism of pathogenesis of postharvest fungal infection, and the management approaches of postharvest diseases in fruits.
2. Pathogens, Their Entry, and Transmission
In Plant Pathology, diseases that affect fruits are referred to as “rot,” and the fungi that cause them are referred to as “pathogens” [37]. These changes cause deterioration of the fruit from harvesting to consumption or processing in the postharvest period [37]. In fruit, the cuticular membrane serves as the main layer of defense against fungi [38]. Invasion into the fruit tissue and breach of this defense mechanism are the first steps in a successful infection process. Fruit maturity, tannin content on the fruit’s surface, stage of development, and water activity on the fruit all have an impact on the invasion of fungi into the fruit tissue. Different pathogens enter through numerous entry points along the supply chain and during various phases of fruit production. In farmlands, certain latent diseases start their infectious cycle during fruit and flower growth. When the fruit is in bloom, Botrytis cinerea infects it and then becomes dormant until the berries mature and ripen [39]. The most likely way for a disease to enter fruit is by damage from a vector insect, and enhancement in the diffusion of fungal infections in bunches is attributed to changes in the vector insect population and behavior [40]. Similarly, microcracks in the cuticular membrane of fruits have a significant role in raising disease incidence and mechanical damage in fruit [41]. A fungal infection may also occur in fruits during the supply chain, including the following steps: picking, sorting, packing and transportation, distribution, cold storage, retailing, and consumption [42]. Mishandling and physical and mechanical damage are the leading causes of fruit loss in the supply chain, and these injuries act as entrance routes for pathogenic fungi [43].
3. Postharvest Fungal Infection
By secreting effector proteins, fungi that are biotrophs feed on live cells and weaken the immunological response of their hosts. On the other hand, necrotrophic fungi, also called saprotrophic fungi, have the ability to trigger necrosis and consume the dead cells of the host [12]. Disease signs appear in fruits that have been infected with postharvest fungal infections both after harvest and generally during storage. After harvest, fungi spread and penetrate the host cuticle to access the fruit [44,45]. For this to happen, the fruit cuticle must be damaged, and the invader must enter through natural host perforations and wounds [12]. The fungus frequently stays latent and restricted to the introduction site when those particularly sneaky infections come into touch with immature fruit. They remain hidden from visible inspection until the harvested fruit ripens, which takes months [46]. Numerous fungi have been found to remain dormant in their hosts until the fruits develop, including Alternaria, Botrytis, Colletotrichum, Penicillium, Rhizopus, and Monilinia, (Table 1). As the fruit ripens, postharvest fungal infections grow more active. The fungi are necrotrophs at this aggressive stage, which means that they destroy the host cell and absorb its nutrition to cause deterioration and degraded fruit tissue [46]. However, those fungi survive in a number of ways before reaching this lethal stage. Before the fruit ripens, several fungi, including Alternaria, Colletotrichum, Lasiodiplodia, Phomopsis, and others, begin to cause stem-end rot and colonize the stem end by living an endophytic existence [47]. Fungi, such as Colletotrichum, are categorized as hemibiotrophic since they can exist in unripe fruit cells as biotrophs without harming them [48]. Botrytis cinerea causes grey mold disease in numerous fruit species and varieties [49]. Numerous fungi, including Lasiodiplodia pseudotheobromae, Botryosphaeria dothidea, and Neofusicoccum parvum causes fungal infection in guava, avocado, and persimmon fruit, respectively [50]. Figure 1 demonstrates how fungi Aspergillus spp. infect the guava fruit by secreting mycotoxins (Aflatoxins, Ochratoxin A, and fumonisin) [51] that lead to the elevation of cell wall degradation enzymes, oxidative stress, volatile organic acid, and hormones that damages the fruit.
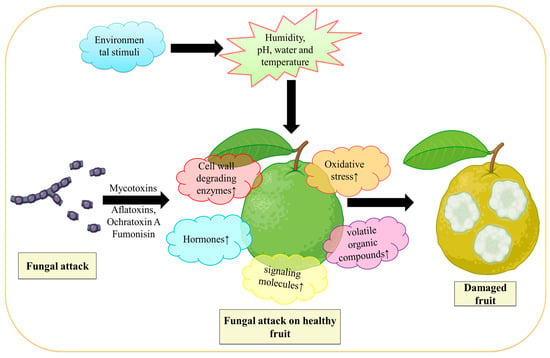
Figure 1.
Demonstrate the action of fungus on fruit. During fungal infection, mycotoxins are released from fungi that cause an increase in oxidative stress, volatile organic compounds, phytohormones (like ethylene), and cell wall hydrolases; all these work together that lead to the deterioration of fruit. Figure created with BioRender.com (https://app.biorender.com/biorender-templates)—accessed on 25 November 2022.
On unripe fruit, Colletotrichum conidia evolve into appressoria, which form an infection peg and pierce the fruit cuticle. When C. gloeosporioides reaches the quiescent stage, two unique structures appear: (1) inflated hyphae that colonize the first epidermal cell layer but stop there and (2) dendritic-like protrusions that grow within the fruit cuticle [52]. Similar to how it works on green fruit, C. gloeosporioides develops on the cuticle of ripe fruit and briefly enters a biotrophic stage. However, this time everything happens far more quickly, and the latent structures start growing necrotrophically right away. This shows that C. gloeosporioides undergo hemibiotrophic growth in response to fruit cuticle. Contrarily, Botrytis spore germlings often enter the epidermis or cuticle of immature fruit through tiny wounds or fissures and remain limited to the lumen of the wounds [53,54]. The necrotrophic method of B. cinerea is used when the hemibiotrophic C. gloeosporioides colonizes tiny wounds of immature fruits, skipping the biotrophic-like stage [52]. Long-term growth of fungal pathogens is restricted to wounds in unripe fruit, and as the fruit ripens, both infections turn necrotrophic, damage host tissues, and produce disease symptoms.

Table 1.
Fruits with postharvest fungal infection.
Table 1.
Fruits with postharvest fungal infection.
| Host Crops | Species | Common Names | Symptoms | References |
|---|---|---|---|---|
| Stone fruits and Pome fruits | Alternaria spp. | Alternaria rot | Grey, black, and/or green spore colonies and sunken lesions; over-ripe softer fruits and spots on fruits. | [55] |
| Blueberries | Botrytis cinerea | Gray mold | Tissue softening. | [56] |
| Guava | Botrytis cinerea | Grey mold | Soft rotting; water-soaked parenchyma tissues occur and prolific grey conidiophores with collapsed. | [57] |
| Guava, Citrus species, pome fruits, and mango | Penicillium italicum | Blue mold | Fruit decay; green or blue mold; softens fruits and causes quick senescence. | [58] |
| Citrus | Penicillium digitatum | Green mold | Fruit decay. | [59] |
| Papaya | Stagonosporopsis caricae | Dry or Black rot | Damages fruit peel and causes fruit decay. | [60] |
| Apricots, apples, peaches, pears fruits | Monilia fructiocola | Brown rot | Brown rot; cankers; blossom blight; fruit rots. | [61] |
| Apples, mangoes, avocados, bananas, and pear fruits | Colletotrichum spp. | Anthracnose | Microbial decay; brown lesions. | [62] |
| Variety of papaya (Maradol, Golden, shahi, and Caribbean red) | Colletotrichum okinawense, C. plurivorum, C. capsici, C. truncatum, C. gloeosporioides C. magnum; and C. fructicola | Anthracnose | Brown lesions; microbial decay. | [60] |
| Papaya | Rhizopus stolonifera | Rhizopus rot | Microbial decay, brown lesions on the fruit surface. | [63] |
4. Environmental Influence on Fungal Infection
Environmental parameters, such as intrinsic elements such as water availability, pH, and substrate composition, as well as external ones such as relative humidity, water activity, temperature, and other microbiota living on the fruit, affect pathogenic fungus infection, growth, and conidia generation [64,65]. These environmental conditions have an impact on all phases of fungal development, including spore germination and deposition, sporulation, germ-tube elongation, mycotoxin buildup, and mycelia growth [66]. Several mycotoxins generated by typical postharvest phytopathogens are shown in Table 2. It is challenging to pinpoint a single extrinsic factor that makes postharvest fruit more susceptible to infection. In order to avoid illness and predict fruit storage conditions, research to determine the ideal environmental conditions and the impact of extrinsic variables influencing the fruit infection process is necessary.

Table 2.
Mycotoxins produced by various fungi during fruit-fungal infection.
pH is one of the environmental factors and signals another significant factor that affects all living cells’ ability to grow and survive. Some pathogenic fungi are characterized by their virulence, pathogenesis, and genetic control. The pathogenic fungus either alkalinizes the host fruit by secreting ammonia or acidifies it by secreting organic acids in response to the ambient pH of the host fruit [80]. The postharvest fruit pathogens A. carbonarius, A. niger, B. cinerea, and Penicillium expansum are among several that have an acidic pH. The fruits’ pH of 3.46 ± 0.20 means that it has no impact on the pathogenicity of the acidic fungus. This effect has been confirmed by research into the pathogenic mechanism of PacC mutant B. cinerea strains in grapefruit [81]. PacC, a pH-dependent transcription factor (TF), regulates the pathogenicity, proliferation, and mycotoxin synthesis of pathogenic fungi [82]. As an acidic pathogen, A. carbonarius produces citric acid and D-gluconic acid, which it releases into the growth medium or fruit tissue to further lower the pH of the surrounding environment. In contrast, treating A. carbonarius colonies with sodium bicarbonate to minimize organic acid buildup reduces ochratoxin A (OTA) synthesis and colonization. Reduced ambient pH stimulates OTA-generating genes and fungal colonization [83,84]. Other infections, including Colletotrichum species, create ammonia to convert the acidic environment of the host to an alkaline one [80]. These pathogenic elements influence the pH of the host, promote pathogenesis, and produce mycotoxin.
Uncinula necator, Plasmopara viticola, and B. cinerea spores all sporulate and disseminate in response to humidity, precipitation, temperature, and dew point [85]. Relative humidity (RH) is significant in fruit fungal diseases because it influences mycelial development, spore germination, conidiation, the potential of the pathogen to produce illness, and the formation of mycotoxin, relative humidity (RH) is significant in fruit fungal diseases. It has been demonstrated that a 24 h pre-incubation at 100% RH is sufficient to allow the fungus to colonize and infect grapes incubated at lower RH with Greeneria uvicola and Colletotrichum acutatum fungi [86]. Fruit rot occurs more frequently when the RH is high during cold storage [87]. Fungal spores acquire physiological maturity at high RH levels, break dormancy, and germinate swiftly, with the rate of germination changing depending on the location [88]. Higher RH was said to be crucial for the development of nutrients and bioactive compounds in fruit [89]. RH, therefore, promotes the development of harmful fungus conidia, spore germination, and disease incidence. RH influences rachis browning, mycotoxin buildup, and disease-causing capability in numerous fruits [90]. Careful RH management is strongly advised during fruit handling and storage to minimize fungal diseases.
Water activity (wa) is a significant component that affects the development, colonization, and accumulation of mycotoxin in grapes and grape products. The growth of microbial flora and pathogens is inhibited by wa less than 0.7, although raw grapes have wa larger than 0.95 [91]. Water activity in grapes thus encourages the development of harmful fungi. The ideal water content needed for the development and synthesis of mycotoxin generally varies [92].
5. Fungal Infection Physicological and Physical Process
Spores often enter the fruit surface through the air, soil particles, insect vectors, harvesting tools, containers, operators’ hands, or storage areas. The fruit tissue is subsequently infected by postharvest fungal pathogens in one of three ways: (1) by wounds brought on by abiotic or biotic agents, (2) through naturally occurring holes in plant organs such as the stomata and pedicel-fruit interface, and (3) by directly piercing the fruit cuticle [93].
The correct stimulus is often required for the spore to begin germination. Mature fruit is more vulnerable to infection than unripe fruit as a result of the onset of senescence, which is characterized by diminished defensive systems, weakened tissues, and increased ethylene production [12]. The spore typically “scans” the atmosphere before germination to assess whether it is suitable in terms of excellent pH, temperature, humidity, phytohormone, food availability, topographic feature, excreted enzyme, fruit lipid, hardness, hydrophobicity, and other factors [93].
The fungus conidia stick to and start to germinate on the fruit surface. Typically, modest adhesive forces are employed for attachment, such as hydrophobic interactions between the surfaces of fruit and conidia [94]. For easier access into the fruit host, the germ tubes of Colletotrichum gloeosporioides and Botrytis cinerea create an appressorium, which often forms a penetration peg [31,95]. The germ tube will mechanically penetrate the host fruit once it is long enough, or it may do so by secreting hydrolytic enzymes such as cutinases, polygalacturonases (PGs), and lipases [96,97]. The rupturing of the host surface and the hydrolysis of fruit cell walls often starts a chain of events in both fungus and fruit (defense and attack mechanisms).
An oxidative burst, often known as a typical “war,” between the host fruit and the pathogenic mold is characterized by the exchange of harmful substances [such as the superoxide radical (O2•–) and hydrogen peroxide (H2O2)] and hydrolytic enzymes. For instance, pathogenic mold releases reactive oxygen species (ROS), botrydial, toxins, oxalic acid, and other substances that lead to oxidative stress and harm fruit tissue.
Due to the emergence of systemic resistance, the diseased fruit also produces a number of antimicrobial substances, including H2O2 and different flavonoids [98]. Pathogenic fungi generate a large number of hydrolases, including pectin methylesterases, pectin lyases (PLs), endo and exo-PGs, and cellulases, to hydrolyze the polysaccharides of the host cell [99]. Fungi, which exhibit aggressive growth and invasion as well as the generation of several secondary metabolites, including mycotoxins, will finally follow in their footsteps. In essence, a potential approach to controlling fungal infections is to restrict the ability of pathogenic fungi to encourage fruit ripening. In order to make sense of these ideas, further research is required.
6. Molecular Mechanism of Infection
Producing high-quality fruit and developing fruit handling techniques require an understanding of the intricate molecular pathways behind disease development in fruits brought on by fungus. The basic molecular function of the pathogenic fungus is to suppress or diminish fruit energy metabolism. Glycolysis and the pentose phosphate pathway (PPP) were down-regulated in A. niger-infected fruit, which decreased the synthesis of the tricarboxylic acid (TCA) cycle and ATP. The A. niger infection was made worse by the restriction of ATP generation and sugar metabolism. In fruit infected with A. niger, it was discovered that the rate-limiting genes of the glycolysis route (phosphofructokinase-1, hexokinase, and phosphokinase) and the PPP (6PGDH2 and G6PDH2) were down-regulated [100].
According to Kong An et al. [101], the fungus A. ochraceus increased the quantities of phenolic compounds along with sugar during infection and encouraged the down-regulation of genes involved in several pathways, such as the glycolysis, TCA cycle, phenols pathway, and PPP. According to proteomics analysis by Kupfer et al. [102], fruits with B. cinerea infection had lower levels of pathogenesis-related proteins smaller than 35 kDa. For possible virulence, colonization, and mycotoxin synthesis, A. carbonarius was shown to exhibit external pH signaling and acidification of the growing medium at different stages. A. carbonarius’s pathogenicity and external pH sensing are both mediated by the pH-responsive TF pacC (produced by the pacC gene). A. carbonarius pacC gene deletion strains (AcpacC) did not colonize, sporulate, or generate OTA effectively at neutral or alkaline pH. Furthermore, AcpacC strains expanded regularly in acidic pH environments, much such as wild-type A. carbonarius strains. The OTA production and pathogenicity in peaches and nectarine fruits were also decreased by the AcpacC strains. These results showed that the pacC gene is active at higher pH and is crucial for pathogenicity and the buildup of OTA through regulating fungal secondary metabolism and the infection process [83]. Additionally, it was discovered that the activation of the genes responsible for mycotoxin and virulence was limited by light. In A. carbonarius, the TFs veA and laeA were activated, leading to an increase in OTA accumulation and a decrease in conidiation in the dark condition. However, the TFs veA and laeA were repressed in the presence of light, which led to a considerable decrease in OTA accumulation and an upsurge in conidiation [103]. During plant-pathogen interactions, sugar molecules are transported from plants to infection sites since pathogenic fungi require sugar for growth. Pathogenic fungi rewire fruit molecular networks involved in sugar metabolism and transport, pH change, energy metabolism, sugar transfer, disease resistance, and host immunity as part of the pathogenesis process.
Gene Regulation
Diseases develop as a result of interactions between pathogenic fungi and fruit hosts. Numerous components, including proteins, small RNA, and secreted phytotoxic compounds, support the infection process. In order to trigger necrosis in the host cells early in the infection process, postharvest pathogenic fungi release proteins that promote necrosis. Numerous proteins on the surface of these spores are used to sense environmental and host fruit stimuli. Examples of this include multiprotein structures, signal transduction systems, and receptor tyrosine kinases (RTKs). It is also well known that G protein-coupled receptors (GPCRs) link many signaling pathways, as shown in Figure 2. For a successful infection process, the fungus must be able to perceive signals, which include ethylene, light, and pH detection. In vitro experiments have demonstrated that B. cinerea produces ethylene to support growth. Thus, increased ethylene production was connected to an increase in disease incidence [104]. P. expansum has the capacity to “alter” the ethylene production in apples, which allows it to avoid or inhibit the fruit’s defenses and favors colonization [97].
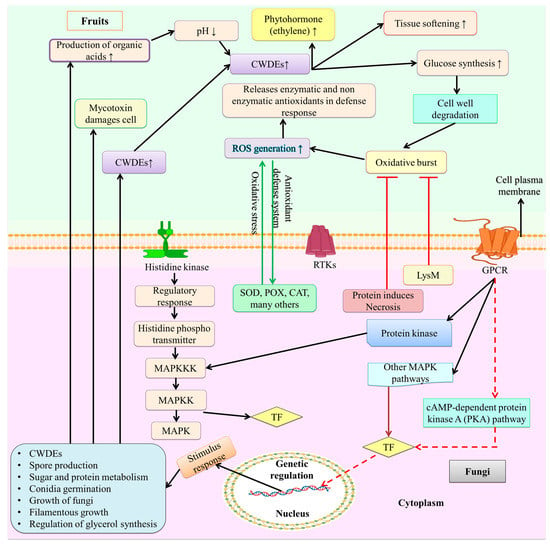
Figure 2.
Fungal infection and signaling mechanism of fruit. On the surface of these spores, several signaling pathways are linked by proteins such as multiprotein complexes, signal transduction systems, receptor tyrosine kinases (RTKs), and G protein–coupled receptors (GPCRs), which are employed to sense environmental and host fruit stimuli. The primary signaling pathways involved in fruit fungal infection include MAPK, cAMP, and protein kinases that causes the activation of TF. These TFs trigger several cell signaling pathways. Similar to fruits cell the toxins produce by these pathway causes oxidative burst by producing ROS, activation on CWHEs, production of organic acid, volatile acid compound, and increases the synthesis of glucose that leads to tissue softening and damages fruit. Figure created with BioRender.com (https://app.biorender.com/biorender-templates)—accessed on 25 November 2022.
Through intricate molecular pathways, fungal spores integrate the infection signal in response to the relevant environmental stimuli. Several signaling routes are generally used within the cell to transmit signals (Figure 2). According to Martnez-Soto and Ruiz-Herrera [105], the primary signaling pathways involved in fruit fungal infection include mitogen-activated protein kinase (MAPK), cyclic adenosine monophosphate (cAMP), AMP-activated protein kinase/sucrose non-fermenting 1 (AMPK/SFN1), and high osmolarity glycerol (HOG). Protein chains known as the MAPK pathways typically assist in signal transduction from the cell surface to the nucleus [106]. GPCRs change in conformation when a signal from an extracellular compartment is identified [105,107]. This conversion will result in the dissociation of the trimeric G proteins into GTP-Gα and Gβ-Gγ. Following this dissociation, GTP-Gα will phosphorylate the protein kinases in the downstream pathway in the form of MAPK kinase (MAPKK), which helps to activate (phosphorylate) the MAPK protein. Following the hydrolysis of the GTP, the heterodimer Gβ-Gγ is created [105]. MAPK activation regulates gene expression, which in turn regulates the outcome of infection processes (Figure 2).
Adenylyl cyclase, an enzyme that catalyzes the conversion of ATP into cyclic cAMP, is activated by the cAMP-dependent pathway, which helps the body intercept external signals. PKA, a protein kinase that regulates the metabolism of lipids and glycogen as well as other cellular functions, is activated by cAMP [105]. In order to understand the importance of the cAMP signaling system during fungal infection, the cAMP signaling system was studied. It was observed that it might influence the energy metabolism of the mold, lessening the severity of the illness. A well-known family of protein kinases called SNF1/AMPK is present in both fungi and humans. SNF1 controls a variety of nutrient-sensitive cellular functions, including the transcription of genes and the activity of metabolic enzymes [105,108]. Researchers looked into the SNF1 gene’s function during infection because of how important it is for cellular functions. Figure 2 depicts the fungal infection and signaling process of fruit.
To ensure the efficiency of transcription, TFs must determine the sequences of the genes to be expressed before they can be transcribed. Particular TFs that can be triggered by several cell signaling pathways are necessary for certain cell functions. Expression disruption of the calcineurin-responsive TF crz1 in P. digitatum may have an impact on complete virulence, conidiation, and demethylation inhibitor (DMI) fungicide tolerance [109]. Fungi must have their environment’s pH under control to be completely pathogenic. The pH signaling TF pacC gene in P. digitatum was damaged, which had an impact on the expression of the PL pnl1 gene and PG pg2 gene and reduced the fungal virulence of citrus [109]. Sterol regulatory element-binding proteins (SREBPs) are fundamental helix-loop-helix transcription regulators that control the expression of the genes involved in sterol biosynthesis, which is found in fungi. Essential TFs play a significant role in how different MAPK pathways interact with one another and with other signaling pathways that are engaged in crucial physiological functions. Most likely, these TFs serve as the link between different cellular signaling pathways. Thus, a crucial component of infection control techniques is focusing on crucial TFs to achieve maximum inhibition of the infection process [110]. Key TFs are phosphorylated and activated to stop extracellular signals that are conveyed via MAPK pathways. These crucial TFs will, in turn, control the transcription of other TFs important for the perceived extracellular signal response as well as gene expression. DNA is compressed into chromatin in the nucleus of eukaryotic cells by certain proteins known as histones. The cell modifies histones to alter chromosomal shape and control gene expression. Recently, a virulence deficiency and a decrease in PAT and citrinin accumulation (CIT) were caused by the loss of the epigenetic reader SntB. Late LaeA, PacC, and CreA TFs, which are crucial regulators of virulence and secondary metabolism, were controlled by SntB in a crucial way [111]. Many cellular activities that are activated in response to infection stimulation work to speed up the fruit’s infection process. The fungal genes implicated in the emergence of postharvest fungal infections are shown in Table 3.

Table 3.
Fungal genes involved in postharvest fungal infections.
Reactive oxygen species in living things, such as singlet oxygen (1O2), superoxide anion (O2−), hydroxyl radical (OH−), and hydrogen (H2O2), are often formed as byproducts of metabolic activities [131,132,133,134]. Numerous studies have revealed that ROS are essential for fruit-microbe interactions [98,135,136]. Around the infectious site, fruit cells may quickly build up a significant quantity of ROS when fungi attack fruits [137]. NADPH oxidase (Nox) complex is the most significant enzyme for ROS generation in fungi. By employing NADPH as an electron donor, Nox, which is present in the plasma membrane or endoplasmic reticulum membrane, transfers electrons across membranes to convert oxygen molecules to •O2 [138]. NoxR is the common regulatory component that has additive effects for NoxA and NoxB, and the two catalytic subunits, NoxA and NoxB, are involved in B. cinerea at various phases of infection [138,139]. NoxR deletion in B. cinerea reduced virulence, vegetative growth, and conidiation in a range of hosts [140]. The production of ROS during fruit fungal infection and ROS signaling in the interaction of the fungal pathogens with their plant host is depicted in Figure 3.
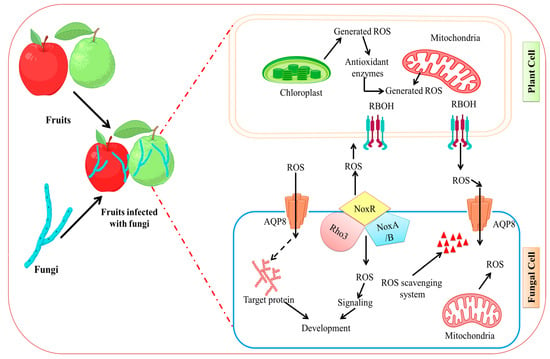
Figure 3.
Fungal infection induced ROS generation in fruit. ROS signaling in fungal infections’ interactions with their plant hosts. In response to pathogen detection, plant cells release ROS via RBOHs in the plasma membrane and several intracellular organelles. Fungal hyphae generate ROS via Nox complexes, which are primarily found at the plasma membrane or endoplasmic reticulums and stimulate an oxidative burst response within the pathogen. Scavenging systems, which include both enzymatic and non-enzymatic systems, work together to maintain intra and extracellular redox homeostasis in both plants and pathogens. Figure created with BioRender.com (https://app.biorender.com/biorender-templates)—accessed on 25 November 2022.
7. Modern Methods for Securing Fruit Yield
The discovery of key proteins and genes that are targets for antifungal medications has come about as a result of research into the molecular underpinnings of postharvest fruit infections. One of the most popular techniques for reducing harmful fungus infection involves treating fruits with fungicides.
7.1. Use of Cell Wall, Membrane Degrading Enzymes, and Antifungal Proteins (AFP)
For pathogenic fungi to infect fruit, the plasma membrane and cell wall must be intact. Degradation of the polysaccharides in fungi’s cell walls can halt fruit infection. Peptidases and chitinases have been reported to be effective against postharvest fungal infections [141]. Peptidase may harm membrane proteins and cell walls, in contrast to chitinases which can break down the chitin in the fungal cell wall. It has been demonstrated by da Silva [141] that these enzymes can stop spore germination successfully. Recombinant alkaline peptidase made from the yeast Aureobasidium pullulans proved particularly effective at controlling the development of B. cinerea in vitro. Alkaline serine peptidase was discovered to be efficient against B. cinerea and Monilinia fructicola infection in apples. This peptidase restricted B. cinerea infection in apples by causing aberrant enlargement of the hyphae at a concentration of 62.5 ng/L [141].
The most recent advance in bio-fungicides is the AFP generated by filamentous ascomycetes. Small cationic proteins called AFPs, which fungi synthesize and exude into the culture medium, are related to defensin. Direct inhibition of harmful fungi in plants and fruits is a property of AFPs. PAF (P. chrysogenum), PgAFP (A. giganteus), Pc-Arctin (A. giganteus), AcAMP (A. clavatus), AcAFP (A. clavatus), and AFPB (P.digitatum) are a few of the molecules that are present [142]. They are biosynthesized in the host cell in their pre-programmed state to remain inactive. Most of these AFPs are known to function internally, permeabilize the plasma membrane, and prevent chitin formation. To make AFPs on a large scale, Pichia or Saccharomyces have been utilized as biofactories [142].
7.2. Use of Bio-Inhibitors
The process of fruit infection is significantly influenced by several pathogenic fungal proteins. One of the most important tactics for controlling the infection process is to inhibit these enzymes. Biochemical compounds called “bio-inhibitors” aim for and deactivate important proteins that have a role in infection. A protein called phaseolus vulgaris protein 2 prevents the synthesis of PG (PGIP). A PGIP isolated from healthy apples that had been kept was similarly evaluated in vivo and in vitro against a C. acutatum endo-PG. However, additional study is required to comprehend the biochemical characteristics of PGIP and assess how much it reduces soft fruit rot brought on by C. acutatum [143]. The invasion and growth of B. cinerea inside the fruit have been connected to siderophores. They may work by trapping the cofactors of infectious fungus enzyme activity. As potential inhibitors of B. cinerea laccase (LC) and PG, Sansone et al. [144] proposed calcium chloride (CaCl2), ethylenediaminetetraacetic acid, enterochelin (siderophore produced by Rahnella aquatilis), and rhodotorulic acid (siderophore produced by Rhodotorula glutinis). As a result, these products have all been linked to preventive and curative effects against B. cinerea infections. Enterochelin and CaCl2 were good in treating infections that have existed, although rhodotorulic acid and EDTA were better at preventing infections. In addition, it was shown that CaCl2 and enterochelin might stop the release of PG and LC [144]. Researchers have also looked at how different chelators and protease inhibitors affect the virulence of P. digitatum [145]. The foundation for the creation of innovative alternative therapies to combat postharvest infections is being laid by studies such as these and others.
7.3. Use of Natural Molecules—Plant Extracts, Essential Oils, Other Solvent Extracted Plant Molecules, and Molecules of Animal Origin
Plant extracts provide several benefits, including low phytotoxicity, systemic mode of action, low environmental toxicity, antifungal activity, and decomposability. The capacity of essential oils (EOs) and other plant compounds that have been extracted using solvents to prevent pathogenic fungi in fruits has drawn a lot of interest. The biological management of postharvest infection by bacterial species is shown in Table 4.

Table 4.
Postharvest disease biological control employing bacterial species.
EOs are the most significant chemicals generated in various plant parts in terms of their antifungal effects. Examples of EOs include phenolic components, sesquiterpenes, terpenes, aldehydes, and ketones [156]. Because of developments in bioinformatics analysis and the rise of cutting-edge analytical approaches such as transcriptome and proteome analysis, their mechanism of action is about to be completely known [157]. They work by altering the cellular metabolism of postharvest fungal infections to prevent fruits from becoming infected. Due to the synergistic effects of their many volatile components, they are safe for consumers and the environment, and postharvest pathogenic fungi are unlikely to develop resistance [158]. A number of postharvest fungal diseases, including B. cinerea, P. digitatum, C. gloeosporioides, and Molininia fruticola have been demonstrated to be inhibited and controlled by essential oils of lemongrass, oregano, thyme, citrus, tea tree, along with citronella oil [159]. The most likely mechanisms by which EOs exert their effects are as follows: (i) by inhibiting enzymes and altering intracellular processes by forming hydrogen bonds; (ii) by interacting with membrane enzymes and reducing cell wall firmness and integrity; (iii) by accumulating in the cell membrane due to its molecular structure, causing damage to and destabilizing the cell membrane; (iv) by causing cell starvation; and (v) by changing cytoplasmic membrane permeability and granulation [157].
Due to aqueous and organic solvent extraction, the plant extract is prevalent in secondary antifungal metabolites, such as alkaloids, flavonoids, tannins, quinones, benzyl alcohol, phenyl propanoids, saponins, terpenes, sterols, acetaldehyde, ethyl benzoate, ethylbenzaldehyde formate, methyl salicylate and ethanol [131,160]. Citrus P. digitatum infection has also been demonstrated to be resistant to methanolic extracts of Inula viscosa L. and Cinnamomum cassia L. [161]. Inhibiting the biosynthesis of nucleic acid by altering DNA gyrase, altering the flow of substances between cells, impeding energy metabolism, disrupting cell respiration, changing cell structure, causing oxidative stress, and changing the functions of genetic material are all possible effects of plant extracts [59,162,163]. Anthraquinones, phenols, and flavonoids from plants have been associated with antifungal properties [161].
A phytopolyphenolic pigment called curcumin, commonly referred to as turmeric longa, is usually derived from the Curcuma longa L. plant and has a wide range of antifungal properties. Three MAPK genes, sak1, bmp1, and bmp3, which are involved in vegetative development, pathogenicity, and osmotic stress response, were shown to be targeted and controlled by curcumin. According to studies on the impact of curcumin on grey mold (B. cinerea) degradation in kiwi, curcumin greatly inhibits mycelial growth, germ tube elongation, and spore germination. Mycelium that has been given curcumin was more susceptible to osmotic stress. The ability of this mycelium to break down plant cell walls was reduced. A 400 mg/L dosage of curcumin in kiwifruit can greatly slow the onset of illness [164].
Chitosan (CS) and lactoferrin are two compounds with significant antifungal actions that were derived from animals [165]. The exoskeletons of crustaceans include CS. Its mode of action entails interfering with nutrition and mineral uptake as well as modifying spore germination through contact with the spore wall. Chitosan interferes with the fungal cell’s genetic material to obstruct protein production, permeabilizes the fungal membrane by altering the function of a particular recognition site on the fungal surface, and induces stress, which results in cell leakage (Figure 4).
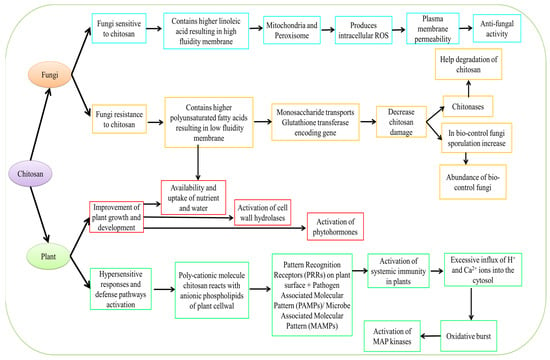
Figure 4.
Mechanism of action of CS derived from plant and fungal sources.
Pathogenesis-related protein (PR1 and PR5) levels were raised after CS nanoparticles (CSNP) treatment [166]. The maximal peroxidase (POD), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL) activity were increased by 1.10, 1.10, and 1.08, respectively, by CSNPs. Zen et al. [167] reported increases in POD, superoxide dismutase (SOD) activity, glutathione (GSH), and H2O2 levels in Navel oranges following postharvest treatment of fruit with CS. POD and PPO are responsible for activating plant defense. In comparison to the control, CS treatment greatly boosted POD activity and gene expression.
In Rhizopus stolonifera, high CS concentrations can lead to fast potassium efflux, which lowers H+-ATPase, accumulating protons inside the cell and altering the H+/K+ exchange transit across the membrane negatively [168]. The mechanism of action of CS has been excellently elucidated in Figure 4. The lowest disease incidence (10.08%) was obtained by the researcher after 90 DAT (days after treatment) using a combination CS treatment (1% seed treatment + 0.5% foliar spray) [169]. As a result, ROS are generated, which causes cell death [170]. Chitosan largely increased plasma membrane, oxidoreductase, and transport activity, according to RNAseq data and gene ontology (GO) analysis [171]. Additionally, CS enhances oxidative metabolism, respiration, and GO transport activities in the model yeast plasma membrane. The cell wall integrity genes and stress response in Saccharomyces cerevisiae have also been demonstrated to be induced by CS [172].
7.4. Use of Hot Water Treatment (HWT)
Hot water treatment (HWT) is frequently utilized to prevent fruit infections caused by pathogenic fungi [173]. HWT stresses the fruit, resulting in a number of physicochemical alterations. The principal method by which HWTs restrict the spread of infection is by the disinfection of fruit specimens. Similar to phytoalexins, heat shock proteins, and PR proteins, hot water encourages the development of secondary metabolites that play a role in fruit resistance to fungus [174]. HWT encourages the buildup of lignin in diseased fruit, which serves as a barrier against fungal infection and delays the fruit’s decomposition for a long time. A significant investigation on the development of resistance in mangoes following HWT was carried out by Luria et al. [175]. An examination of the mango peel transcriptome revealed that HWT boosted the expression of genes involved in disease resistance, as well as genes involved not only in flavonoid and sugar metabolism but also in chlorophyll degradation [175].
7.5. Use of Irradiation
Ionizing radiation has an antibacterial effect by rupturing the pathogen’s cell membrane, which causes the pathogen to lose its cytoplasmic nutrients. Ionizing radiation can also harm people directly or indirectly [176]. Ionizing radiation primarily harms a pathogen’s nucleic acids by damaging its genetic makeup. The forms of DNA damage induced by irradiation include hydrolytic damage, oxidative damage brought on by direct contact with ionizing radiation with DNA molecules, and alkylating chemicals. Bases can also be incorrectly incorporated during the replication process [177]. Multiple breaks render the virus non-viable, even if single-strand breaks may not be fatal or may result in a mutation. Ionizing radiation’s indirect impact on infections is caused by interactions with other atoms or molecules inside the pathogens. Water molecules lose one electron as a result of radiation, going from H2O to H2O and +e. Molecular hydrogen and oxygen, H2O2, OH-, hydrogen radicals, and H2O2 are created when these byproducts react with one another or other water molecules [177]. Ionizing radiation can also have an impact on proteins, plasmids, or enzymes that are crucial for the development of infections.
The three forms of non-ionizing radiation are UV-A (315 to 400 nm), UV-B (280 to 315 nm), and UV-C (100 to 280 nm) [178]. Fruits include photoreceptors that allow UV light to affect vegetative tissues and control metabolic processes. The fruit quality might suffer from excessive UV-C exposure. Citrus fruits’ overall quality is less affected by UV-B than by UV-C irradiation [179]. Lemon fruits’ cell walls are said to thicken when exposed to UV-B light, improving the fruit’s defense against harmful fungus. The synthesis of secondary antifungal metabolites such as polyphenols and phytoalexins is accelerated by UV radiation, which might reduce the spread of dangerous fungi [179]. By encouraging phytochemical processes that are affected by the length and temperature of incubation, UV-C radiation serves as an antifungal agent [180].
7.6. Use of Microbial Antagonists
Microbial antagonists can prevent infections from attaching directly to fruit, compete with the fruit for resources and space, disable fungal inoculum, and produce enzymes that break down fungi or cause fruit resistance [119]. They quickly deplete the nutrients on the fruit’s surface, lowering the number of nutrients that are available to pathogens. Yeasts also create extracellular polysaccharides that help them survive and stop pathogens from spreading. The least influence on these creatures is caused by pesticides put on harvested fruits. Figure 5 represents the mechanism of biocontrol mechanism in fruit and pathogen.
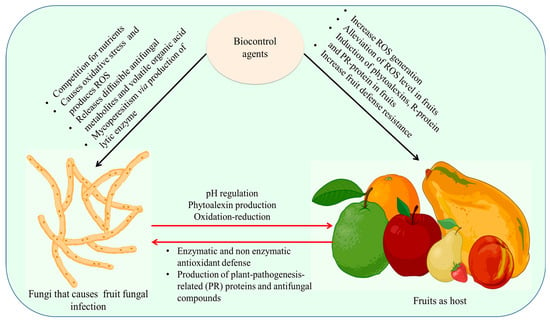
Figure 5.
Mechanism of biocontrol mechanism in fruit and pathogen. The biocontrol agents affect fungal pathogens by causing oxidative stress that triggers ROS, releases diffusible antifungal metabolites and volatile organic acid and Mycoperesitism via the production of lytic enzyme. In fruits, increase ROS generation, induction of phytoalexins, R-protein, and PR-protein in fruits led to an increase in fruit defense resistance by antioxidant enzymes. Figure created with BioRender.com (https://app.biorender.com/biorender-templates)—accessed on 25 November 2022.
Limitations in nutrients and available space, the creation of different bioactive compounds, the generation of antibiotic activity/CWDE, and the induction of resistance are all potential defense strategies employed by antagonistic bacteria (mainly Bacillus sp.) against pathogenic fungus (Lasiodiplodia theobromae, C. gloeosporioides, and Penicillium digitatum). Contrarily, the effectiveness of Bacillus biocontrol may be influenced by a number of variables, such as the kind of host/pathogen, strain type (endophytes or epiphytes), administration methods (pre or post-storage/harvest), and others [181,182,183,184].
8. Future Perspectives
The relevance of signaling pathways and molecular processes involved in the sense of exterior changes during fruit infection by pathogenic mold was validated by this review. Pathogenic mold infection and proliferation mostly depend on mechanisms involved in the control of fungal physiology as well as those involved in the production of specialized structures such as sclerotia or appressorium. However, further investigation is required to determine how mycotoxins affect the pathogenicity and virulence of mold. Emerging omics approaches and CRISPR-Cas9-mediated gene editing technologies could be widely exploited to examine the functions of genes and fungal proteins that are involved in fruit infection. It may be possible to clarify the fundamental principles underlying eukaryotic cell signaling systems by studying the molecular mechanisms of fruit infection, especially MAPK modules. The possible development of novel biotechnological strategies for the management or prevention of fruit infections, including the use of bio-inhibitors, hydrolytic enzymes, natural products, and biological control agents, is also important to take into account. The mechanism of pathogenic fungus infection is shown to be closely tied to biological control techniques. An adequate application of plant extracts with acceptable biocontrol agents should be made after HWT and UV irradiation in order to produce an efficient antifungal management system. The greatest method for preventing fungal infections is the selective breeding of disease-tolerant fruit cultivars using agricultural biotechnology. An interesting advance that can help in the selection of genotypes with higher disease tolerance is the identification of molecular markers and genetic networks connected to disease resistance. By creating a hostile environment, harmful fungi are prevented from growing and causing as many diseases in the field and storage. Computational biology and whole-genome sequencing of pathogenic fungi will make it easier to evaluate antifungal drugs that target particular infections, leading to a more effective approach to disease control.
9. Conclusions
Researchers are looking at the connections between fruit-pathogenic fungi to solve the issue of rising fungal infections in fruits. A wide range of physiological, chemical, environmental, and genetic variables can affect fruit fungal pathology. In recent years, our knowledge of fungal pathogenesis has improved. Fruits that have suffered from biotic or abiotic stress are more prone to fungal diseases. Fruits are susceptible to fungal disease due to water activity, relative humidity, ideal temperature, pH, and substrate concentration. These factors also affect the pathogenic fungi’s quiescent and necrotrophic life stages. Changes in host pH, the development of cell wall-degrading enzymes, the formation of mycotoxins, and the down-regulation of fruit primary and secondary metabolism are some of the pathogenic processes involved in the fungal pathogenesis of fruits. Phytohormone synthesis is altered, antifungal compounds are produced, defense-related enzyme production is increased, primary and secondary metabolite production is increased, and cellular signal transduction is all part of a fruit’s defensive mechanism against pathogenic invaders. It is still unknown exactly what circumstances lead to successful illness or infection tolerance. Therefore, additional investigation into the genetic control of fruit disease immunity and resistance is required in order to create innovative, secure, and high-quality fruit production techniques.
Author Contributions
A.B. and A.G.: Conceptualization, A.B., A.G. and M.K.; formal analysis, writing—original draft preparation, A.B., A.G., M.R.P. and M.K.; reviewing and editing and finalizing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Semwal, P.; Painuli, S.; Jamloki, A.; Rauf, A.; Rahman, M.M.; Olatunde, A.; Hemeg, H.A.; Abu-Izneid, T.; Naz, S.; Punia Bangar, S.; et al. Himalayan Wild Fruits as a Strong Source of Nutraceuticals, Therapeutics, Food and Nutrition Security. Food Rev. Int. 2022. [Google Scholar] [CrossRef]
- Gâtlan, A.-M.; Gutt, G. Sea Buckthorn in Plant Based Diets. An Analytical Approach of Sea Buckthorn Fruits Composition: Nutritional Value, Applications, and Health Benefits. Int. J. Environ. Res. Public Health 2021, 18, 8986. [Google Scholar] [CrossRef]
- Rong, S.; Liao, Y.; Zhou, J.; Yang, W.; Yang, Y. Comparison of Dietary Guidelines among 96 Countries Worldwide. Trends Food Sci. Technol. 2021, 109, 219–229. [Google Scholar] [CrossRef]
- Jaglan, P.; Buttar, H.S.; Al-bawareed, O.A.; Chibisov, S. Potential Health Benefits of Selected Fruits: Apples, Blueberries, Grapes, Guavas, Mangos, Pomegranates, and Tomatoes. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 359–370. [Google Scholar] [CrossRef]
- Vicente, A.R.; Manganaris, G.A.; Darre, M.; Ortiz, C.M.; Sozzi, G.O.; Crisosto, C.H. Compositional Determinants of Fruit and Vegetable Quality and Nutritional Value. In Postharvest Handling: A Systems Approach; Academic Press: Cambridge, MA, USA, 2022; pp. 565–619. [Google Scholar] [CrossRef]
- Da Silva, L.M.R.; de Figueiredo, E.A.T.; Ricardo, N.M.P.S.; Vieira, I.G.P.; de Figueiredo, R.W.; Brasil, I.M.; Gomes, C.L. Quantification of Bioactive Compounds in Pulps and By-Products of Tropical Fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef]
- World Health Organization. Food Safety. Available online: https://www.who.int/health-topics/food-safety (accessed on 29 November 2022).
- UNEP DTU Partnership and United Nations Environment Programme. Reducing Consumer Food Waste Using Green and Digital Technologies. Copenhagen and Nairobi. Copyright IEA. 2021. Available online: https://unepccc.org/wp-content/uploads/2022/03/reducing-consumer-food-waste-using-green-and-digital-technologies.pdf (accessed on 22 November 2022).
- World Health Organization. Safety Evaluation of Certain Contaminants in Food: Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Pirozzi, A.; Ferrari, G.; Donsì, F. The Use of Nanocellulose in Edible Coatings for the Preservation of Perishable Fruits and Vegetables. Coatings 2021, 11, 990. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Kumar, S.A.; Meraj, T.; Rauf, H.T.; Alnuaim, A.A.; Alkhayyal, M.A. Guava Disease Detection Using Deep Convolutional Neural Networks: A Case Study of Guava Plants. Appl. Sci. 2021, 12, 239. [Google Scholar] [CrossRef]
- Petrasch, S.; Silva, C.J.; Mesquida-Pesci, S.D.; Gallegos, K.; van den Abeele, C.; Papin, V.; Fernandez-Acero, F.J.; Knapp, S.J.; Blanco-Ulate, B. Infection Strategies Deployed by Botrytis cinerea, Fusarium acuminatum, and Rhizopus stolonifera as a Function of Tomato Fruit Ripening Stage. Front. Plant Sci. 2019, 10, 223. [Google Scholar] [CrossRef]
- Luciano-Rosario, D.; Keller, N.P.; Jurick, W.M., II. Penicillium expansum: Biology, Omics, and Management Tools for a Global Postharvest Pathogen Causing Blue Mould of Pome Fruit. Mol. Plant Pathol. 2020, 21, 1391–1404. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Zhang, M.; Yang, Y.; Peng, L. Identification of Pathogenicity-Related Effector Proteins and the Role of PIWSC1 in the Virulence of Penicillium italicum on Citrus Fruits. J. Fungi 2022, 8, 646. [Google Scholar] [CrossRef]
- Liu, S.; Du, Y.; Zhang, D.; Yang, F.; He, X.; Long, C. Aluminum Sulfate Inhibits Green Mold by Inducing Chitinase Activity of Penicillium digitatum and Enzyme Activity of Citrus Fruit. Food Control 2022, 136, 108854. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, L.; Tan, J.; Zheng, K.; Jiang, Y. Effects of Chitosan Coating on Quality and Shelf Life of Peeled Litchi Fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Nath, V.; Lal, N.; Singh, S.K.; Pandey, S.; Prakash, K. Seventy Five Years of Research and Development in Litchi. Int. J. Innov. Hortic. 2022, 11, 47–61. [Google Scholar] [CrossRef]
- Johansen, K.S. Lytic Polysaccharide Monooxygenases: The Microbial Power Tool for Lignocellulose Degradation. Trends Plant Sci. 2016, 21, 926–936. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, C.; Huang, R.; He, M.; Duan, X.; Jiang, Y.; Li, T. Enhanced Resistance in ‘Shatang’ Mandarin Fruit against Penicillium italicum Caused by 2-Methoxy-1,4-Naphthoquinone. Physiol. Mol. Plant Pathol. 2022, 119, 101828. [Google Scholar] [CrossRef]
- Tang, N.; Chen, N.; Hu, N.; Deng, W.; Chen, Z.; Li, Z. Comparative Metabolomics and Transcriptomic Profiling Reveal the Mechanism of Fruit Quality Deterioration and the Resistance of Citrus Fruit against Penicillium digitatum. Postharvest Biol. Technol. 2018, 145, 61–73. [Google Scholar] [CrossRef]
- Graça, A.; Esteves, E.; Nunes, C.; Abadias, M.; Quintas, C. Microbiological Quality and Safety of Minimally Processed Fruits in the Marketplace of Southern Portugal. Food Control 2017, 73, 775–783. [Google Scholar] [CrossRef]
- Rodrigues, M.H.P.; Furlong, E.B. Fungal Diseases and Natural Defense Mechanisms of Tomatoes (Solanum lycopersicum): A Review. Physiol. Mol. Plant Pathol. 2022, 122, 101906. [Google Scholar] [CrossRef]
- Mushtaq, M. Extraction of Fruit Juice: An Overview. In Fruit Juices: Extraction, Composition, Quality and Analysis; Academic Press: Cambridge, MA, USA, 2018; pp. 131–159. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in Food: A Mycotoxin Concern for Human Health and Its Management Strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus Species and Mycotoxins: Occurrence and Importance in Major Food Commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Marwa, A.Y.; Embaby, E.M.; Abeer, A.F. Control of the Toxigenic Fungi Affecting Fig Fruits Quality. Egypt. J. Chem. 2022, 65, 339–347. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Yan, Z.; Li, Z.; Cheng, Y.; Farooq, S. Multi-Mycotoxin Exposure and Risk Assessments for Chinese Consumption of Nuts and Dried Fruits. J. Integr. Agric. 2018, 17, 1676–1690. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ahmed, F.; Kamal, M.; Khan, S.; Aghar, M.A. Effectiveness of Citrus Fruit Peel as a Biosorbent for the Mitigation of Aflatoxins In Vitro. Food Addit. Contam. Part A 2022, 39, 1987–2001. [Google Scholar] [CrossRef]
- Nikolchina, I.; Rodrigues, P. A Preliminary Study on Mycobiota and Ochratoxin a Contamination in Commercial Palm Dates (Phoenix dactylifera). Mycotoxin Res. 2021, 37, 215–220. [Google Scholar] [CrossRef]
- The Top Food Loss and Waste Statistics of 2022. Available online: https://www.postharvest.com/blog/top-food-waste-statistics-of-2021/ (accessed on 22 November 2022).
- Alkan, N.; Fortes, A.M. Insights into Molecular and Metabolic Events Associated with Fruit Response to Post-Harvest Fungal Pathogens. Front. Plant Sci. 2015, 6, 889. [Google Scholar] [CrossRef]
- Cesarino, I. Killing Me Softly: A Pathogen Accelerates Fruit Ripening and Softening to Cause Disease. Plant Physiol. 2022, 191, 21–23. [Google Scholar] [CrossRef]
- Welke, J.E. Fungal and Mycotoxin Problems in Grape Juice and Wine Industries. Curr. Opin. Food Sci. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Filippovich, S.Y.; Bachurina, G.P. Antifungal Surfaces. Appl. Biochem. Microbiol. 2022, 58, 507–517. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Gu, X.; Zhang, Q.; Zhao, L.; Zheng, X.; Zhang, H. The Infection of Grapes by Talaromyces rugulosus O1 and the Role of Cell Wall-Degrading Enzymes and Ochratoxin A in the Infection. Physiol. Mol. Plant Pathol. 2019, 106, 263–269. [Google Scholar] [CrossRef]
- Apaliya, M.T.; Zhang, H.; Yang, Q.; Zheng, X.; Zhao, L.; Kwaw, E.; Mahunu, G.K. Hanseniaspora uvarum Enhanced with Trehalose Induced Defense-Related Enzyme Activities and Relative Genes Expression Levels against Aspergillus tubingensis in Table Grapes. Postharvest Biol. Technol. 2017, 132, 162–170. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P.H.J. Latent Postharvest Pathogens of Pome Fruit and Their Management: From Single Measures to a Systems Intervention Approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Arya, G.C.; Sarkar, S.; Manasherova, E.; Aharoni, A.; Cohen, H. The Plant Cuticle: An Ancient Guardian Barrier Set Against Long-Standing Rivals. Front. Plant Sci. 2021, 12, 663165. [Google Scholar] [CrossRef] [PubMed]
- Esterio, M.; Osorio-Navarro, C.; Carreras, C.; Azócar, M.; Copier, C.; Estrada, V.; Rubilar, M.; Auger, J. Botrytis prunorum Associated to Vitis vinifera Blossom Blight in Chile. Plant Dis. 2020, 104, 2324–2329. [Google Scholar] [CrossRef] [PubMed]
- Machota, R., Jr.; Bortoli, L.C.; Cavalcanti, F.R.; Botton, M.; Grützmacher, A.D. Assessment of Injuries Caused by Anastrephafraterculus (Wied.) (Diptera: Tephritidae) on the Incidence of Bunch Rot Diseases in Table Grape. Neotrop. Entomol. 2016, 45, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Muñoz, R.; Heredia, A.; Domínguez, E. The Role of Cuticle in Fruit Shelf-Life. Curr. Opin. Biotechnol. 2022, 78, 102802. [Google Scholar] [CrossRef] [PubMed]
- Jianying, F.; Bianyu, Y.; Xin, L.; Dong, T.; Weisong, M. Evaluation on Risks of Sustainable Supply Chain Based on Optimized BP Neural Networks in Fresh Grape Industry. Comput. Electron. Agric. 2021, 183, 105988. [Google Scholar] [CrossRef]
- Zhao, P.; Ndayambaje, J.P.; Liu, X.; Xia, X. Microbial Spoilage of Fruits: A Review on Causes and Prevention Methods. Food Rev. Int. 2020, 38, 225–246. [Google Scholar] [CrossRef]
- Kankam, F.; Larbi-Koranteng, S.; Adomako, J.; Kwowura Kwodaga, J.; Boatey Akpatsu, I.; Danso, Y.; Nortaa Kunedeb Sowley, E. Anthracnose Disease of Mango: Epidemiology, Impact and Management Options. In Current and Emerging Challenges in the Diseases of Trees [Working Title]; InTech Open: London, UK, 2022. [Google Scholar] [CrossRef]
- Strano, M.C.; Altieri, G.; Allegra, M.; Di Renzo, G.C.; Paterna, G.; Matera, A.; Genovese, F. Postharvest Technologies of Fresh Citrus Fruit: Advances and Recent Developments for the Loss Reduction during Handling and Storage. Horticulturae 2022, 8, 612. [Google Scholar] [CrossRef]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and Necrotrophic Lifestyle Choice during Postharvest Disease Development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef]
- Diskin, S.; Feygenberg, O.; Maurer, D.; Droby, S.; Prusky, D.; Alkan, N. Microbiome Alterations Are Correlated with Occurrence of Postharvest Stem-End Rot in Mango Fruit. Phytobiomes J. 2017, 1, 117–127. [Google Scholar] [CrossRef]
- Moreira, V.; Ferronato, B.; de Benedetti, F.; González-Barrios, P.; Mondino, P.; Alaniz, S. Incidence of Colletotrichum Latent Infections during Olive Fruit Development under Uruguayan Environmental Conditions. Int. J. Pest Manag. 2022, 68, 286–294. [Google Scholar] [CrossRef]
- Rhouma, A.; Hajji-Hedfi, L.; Ben Othmen, S.; Kumari Shah, K.; Matrood, A.A.A.; Okon, O.G.; Pant, D. Strawberry Grey Mould, a Devastating Disease Caused by the Airborne Fungal Pathogen Botrytis cinerea. Egypt. J. Phytopathol. 2022, 50, 44–50. [Google Scholar] [CrossRef]
- Navarro, B.L.; Edwards Molina, J.P.; Nogueira, A.F., Jr. Penetration by Botryosphaeriaceae Species in Avocado, Guava and Persimmon Fruit during Postharvest. J. Phytopathol. 2021, 170, 57–68. [Google Scholar] [CrossRef]
- Embaby, E.; Hassan, M.K. Decay of guava fruit (Psidium guajava Linn.) quality caused by some mold fungi. Int. J. Agric. Technol. 2015, 11, 713–730. [Google Scholar]
- Alkan, N.; Friedlander, G.; Ment, D.; Prusky, D.; Fluhr, R. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New Phytol. 2014, 205, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The Cause of Grey Mould Disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.; Vicente, A.R.; Greve, L.C.; Dewey, F.M.; Bennett, A.B.; Labavitch, J.M.; Powell, A.L.T. The Intersection between Cell Wall Disassembly, Ripening, and Fruit Susceptibility to Botrytis cinerea. Proc. Natl. Acad. Sci. USA 2008, 105, 859–864. [Google Scholar] [CrossRef]
- Chen, D.; Förster, H.; Adaskaveg, J.E. Baseline Sensitivities of Major Citrus, Pome, and Stone Fruits Postharvest Pathogens to Natamycin and Estimation of the Resistance Potential in Penicillium digitatum. Plant Dis. 2021, 105, 2114–2121. [Google Scholar] [CrossRef]
- Saito, S.; Wang, F.; Xiao, C.-L. Natamycin as a Postharvest Treatment to Control Gray Mold on Stored Blueberry Fruit Caused by Multi-Fungicide Resistant Botrytis cinerea. Postharvest Biol. Technol. 2022, 187, 111862. [Google Scholar] [CrossRef]
- Abdel-Rahim, I.R.; Abo-Elyousr, K.A.M. Using of Endophytic Saccharomycopsis fibuligera and Thyme Oil for Management of Gray Mold Rot of Guava Fruits. Biol. Control 2017, 110, 124–131. [Google Scholar] [CrossRef]
- Schirra, M.; D’Aquino, S.; Cabras, P.; Angioni, A. Control of Postharvest Diseases of Fruit by Heat and Fungicides: Efficacy, Residue Levels, and Residue Persistence. A Review. J. Agric. Food Chem. 2011, 59, 8531–8542. [Google Scholar] [CrossRef]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-Chemical Treatments for Preventing the Postharvest Fungal Rotting of Citrus Caused by Penicillium digitatum (Green Mold) and Penicillium italicum (Blue Mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; de Souza Coelho, C.C.; Soares, A.G.; Freitas-Silva, O. Current Technologies to Control Fungal Diseases in Postharvest Papaya (Carica papaya L.). Biocatal. Agric. Biotechnol. 2021, 36, 102128. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, S.; Du, J.; Wang, X.; Xu, W.; Luo, C. Monilinia fructicola on Loquat: An Old Pathogen Invading a New Host. J. Integr. Agric. 2021, 20, 2009–2014. [Google Scholar] [CrossRef]
- Ciofini, A.; Negrini, F.; Baroncelli, R.; Baraldi, E. Management of Post-Harvest Anthracnose: Current Approaches and Future Perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef]
- Cruz-Lachica, I.; Marquez-Zequera, I.; Allende-Molar, R.; Sañudo-Barajas, J.A.; Leon-Felix, J.; Ley-Lopez, N.; Garcia-Estrada, R.S. Diversity of Mucoralean Fungi in Soils of Papaya (Carica papaya L.) Producing Regions in Mexico. Fungal Biol. 2018, 122, 810–816. [Google Scholar] [CrossRef]
- Ciliberti, N.; Fermaud, M.; Roudet, J.; Languasco, L.; Rossi, V. Environmental Effects on the Production of Botrytis cinerea Conidia on Different Media, Grape Bunch Trash, and Mature Berries. Aust. J. Grape Wine Res. 2016, 22, 262–270. [Google Scholar] [CrossRef]
- Jiao, W.; Liu, X.; Li, Y.; Li, B.; Du, Y.; Zhang, Z.; Chen, Q.; Fu, M. Organic Acid, a Virulence Factor for Pathogenic Fungi, Causing Postharvest Decay in Fruits. Mol. Plant Pathol. 2021, 23, 304–312. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Gao, C.; Gao, Q.; Cheng, Y.; Zhao, M.; Guan, J. Mycotoxin Production and the Relationship between Microbial Diversity and Mycotoxins in Pyrus bretschneideri Rehd Cv. Huangguan Pear. Toxins 2022, 14, 699. [Google Scholar] [CrossRef]
- He, Y.; Cox, R.J. The Molecular Steps of Citrinin Biosynthesis in Fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef]
- Costa, J.; Rodríguez, R.; Garcia-Cela, E.; Medina, A.; Magan, N.; Lima, N.; Battilani, P.; Santos, C. Overview of Fungi and Mycotoxin Contamination in Capsicum Pepper and in Its Derivatives. Toxins 2019, 11, 27. [Google Scholar] [CrossRef]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.-D. Aflatoxin Biosynthesis and Genetic Regulation: A Review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef]
- Ghuffar, S.; Irshad, G.; Ahmed, M.Z.; Zeshan, M.A.; Ali, R.; ul Haq, E.; Anwaar, H.A.; Abdullah, A.; Ahmad, F.; Haque, K. First Report of Aspergillus flavus Causing Fruit Rot of Grapes (Vitis vinifera) in Pakistan. Plant Dis. 2020, 104, 3062. [Google Scholar] [CrossRef]
- Troncoso-Rojas, R.; Tiznado-Hernández, M.E. Alternaria alternata (Black Rot, Black Spot). In Postharvest Decay: Control Strategies; Academic Press: Cambridge, MA, USA, 2014; pp. 147–187. [Google Scholar] [CrossRef]
- Wenderoth, M.; Garganese, F.; Schmidt-Heydt, M.; Soukup, S.T.; Ippolito, A.; Sanzani, S.M.; Fischer, R. Alternariol as Virulence and Colonization Factor of Alternaria alternata during Plant Infection. Mol. Microbiol. 2019, 112, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Jurick, W.M., II; Kou, L.P.; Gaskins, V.L.; Luo, Y.G. First Report of Alternaria alternata Causing Postharvest Decay on Apple Fruit During Cold Storage in Pennsylvania. Plant Dis. 2014, 98, 690. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Liu, Y.; Shujian, H.; Moosa, A. First Record of Alternaria alternata Causing Postharvest Fruit Rot of Sweet Cherry (Prunus avium) in China. Plant Dis. 2020, 104, 2030. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.N.; Zhao, Y.; Xing, F.; Yin, W.-B.; et al. A Consensus Ochratoxin A Biosynthetic Pathway: Insights from the Genome Sequence of Aspergillus ochraceus and a Comparative Genomic Analysis. Appl. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef] [PubMed]
- Tannous, J.; El Khoury, R.; Snini, S.P.; Lippi, Y.; El Khoury, A.; Atoui, A.; Lteif, R.; Oswald, I.P.; Puel, O. Sequencing, Physical Organization and Kinetic Expression of the Patulin Biosynthetic Gene Cluster from Penicillium expansum. Int. J. Food Microbiol. 2014, 189, 51–60. [Google Scholar] [CrossRef]
- Jurick, W.M., II; Peng, H.; Beard, H.S.; Garrett, W.M.; Lichtner, F.J.; Luciano-Rosario, D.; Macarisin, O.; Liu, Y.; Peter, K.A.; Gaskins, V.L.; et al. Blistering1 Modulates Penicillium expansum Virulence Via Vesicle-Mediated Protein Secretion. Mol. Cell. Proteom. 2020, 19, 344–361. [Google Scholar] [CrossRef]
- Brito, A.C.Q.; Mello, J.F.; Vieira, J.C.B.; Câmara, M.P.S.; Bezerra, J.D.P.; Souza-Motta, C.M.; Machado, A.R. First Report of Penicillium expansum Causing Postharvest Fruit Rot on Black Plum (Prunus domestica) in Brazil. Plant Dis. 2020, 104, 576. [Google Scholar] [CrossRef]
- Ballester, A.-R.; Marcet-Houben, M.; Levin, E.; Sela, N.; Selma-Lázaro, C.; Carmona, L.; Wisniewski, M.; Droby, S.; González-Candelas, L.; Gabaldón, T. Genome, Transcriptome, and Functional Analyses of Penicillium expansum Provide New Insights Into Secondary Metabolism and Pathogenicity. Mol. Plant-Microbe Interact. 2015, 28, 232–248. [Google Scholar] [CrossRef]
- Alkan, N.; Espeso, E.A.; Prusky, D. Virulence Regulation of Phytopathogenic Fungi by pH. Antioxid. Redox Signal. 2013, 19, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Rascle, C.; Dieryckx, C.; Dupuy, J.W.; Muszkieta, L.; Souibgui, E.; Droux, M.; Bruel, C.; Girard, V.; Poussereau, N. The pH Regulator PacC: A Host-Dependent Virulence Factor in Botrytis cinerea. Environ. Microbiol. Rep. 2018, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, L.; Qi, F.; Htwe, N.M.P.S.; Li, Q.; Zhang, D.; Lin, F.; Shang-Guan, K.; Liang, Y. The Receptor-like Cytoplasmic Kinase RIPK Regulates Broad-Spectrum ROS Signaling in Multiple Layers of Plant Immune System. Mol. Plant 2021, 14, 1652–1667. [Google Scholar] [CrossRef] [PubMed]
- Barda, O.; Maor, U.; Sadhasivam, S.; Bi, Y.; Zakin, V.; Prusky, D.; Sionov, E. The pH-Responsive Transcription Factor PacC Governs Pathogenicity and Ochratoxin A Biosynthesis in Aspergillus carbonarius. Front. Microbiol. 2020, 11, 210. [Google Scholar] [CrossRef]
- Maor, U.; Sadhasivam, S.; Zakin, V.; Prusky, D.; Sionov, E. The Effect of Ambient pH Modulation on Ochratoxin A Accumulation by Aspergillus carbonarius. World Mycotoxin J. 2017, 10, 339–348. [Google Scholar] [CrossRef]
- Martínez-Bracero, M.; Alcázar, P.; Velasco-Jiménez, M.J.; Galán, C. Fungal Spores Affecting Vineyards in Montilla-Moriles Southern Spain. Eur. J. Plant Pathol. 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Steel, C.C.; Greer, L.A.; Savocchia, S. Studies on Colletotrichum acutatum and Greeneria uvicola: Two Fungi Associated with Bunch Rot of Grapes in Sub-Tropical Australia. Aust. J. Grape Wine Res. 2007, 13, 23–29. [Google Scholar] [CrossRef]
- Makule, E.; Dimoso, N.; Tassou, S.A. Precooling and Cold Storage Methods for Fruits and Vegetables in Sub-Saharan Africa—A Review. Horticulturae 2022, 8, 776. [Google Scholar] [CrossRef]
- Yi, T.; Hong, Y.; Li, M.; Li, X. Examination and Characterization of Key Factors to Seasonal Epidemics of Downy Mildew in Native Grape Species Vitis davidii in Southern China with the use of Disease Warning Systems. Eur. J. Plant Pathol. 2019, 154, 389–404. [Google Scholar] [CrossRef]
- Amiri, A.; Mortazavi, S.M.H.; Ramezanian, A.; Mahmoodi Sourestani, M.; Mottaghipisheh, J.; Iriti, M.; Vitalini, S. Prevention of Decay and Maintenance of Bioactive Compounds in Strawberry by Application of UV-C and Essential Oils. J. Food Meas. Charact. 2021, 15, 5310–5317. [Google Scholar] [CrossRef]
- Pinto, J.A.V.; Schorr, M.R.W.; Thewes, F.R.; Ceconi, D.L.; Both, V.; Brackmann, A.; Fronza, D. Relative Humidity during Cold Storage on Postharvest Quality of “Niagara Rosada” Table Grapes. Ciênc. Rural 2015, 45, 386–391. [Google Scholar] [CrossRef]
- Igo, M.J.; Schaffner, D.W. Models for Factors Influencing Pathogen Survival in Low Water Activity Foods from Literature Data Are Highly Significant but Show Large Unexplained Variance. Food Microbiol. 2021, 98, 103783. [Google Scholar] [CrossRef]
- Kogkaki, E.A.; Natskoulis, P.I.; Magan, N.; Panagou, E.Z. Effect of Interaction between Aspergillus carbonarius and Non-Ochratoxigenic Grape-Associated Fungal Isolates on Growth and Ochratoxin A Production at Different Water Activities and Temperatures. Food Microbiol. 2015, 46, 521–527. [Google Scholar] [CrossRef]
- Van Kan, J.A.L. Licensed to Kill: The Lifestyle of a Necrotrophic Plant Pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, R.; Fang, X.; Wu, W.; Han, Y.; Chen, H.; Xu, F.; Gao, H. Botrytis cinerea Infection Affects Wax Composition, Content and Gene Expression in Blueberry Fruit. Postharvest Biol. Technol. 2022, 192, 112020. [Google Scholar] [CrossRef]
- Choquer, M.; Fournier, E.; Kunz, C.; Levis, C.; Pradier, J.-M.; Simon, A.; Viaud, M. Botrytis cinerea Virulence Factors: New Insights into a Necrotrophic and Polyphageous Pathogen. FEMS Microbiol. Lett. 2007, 277, 1–10. [Google Scholar] [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Nuñez, C.; Arce-Johnson, P. Grapevine Pathogenic Microorganisms: Understanding Infection Strategies and Host Response Scenarios. Front. Plant Sci. 2016, 7, 382. [Google Scholar] [CrossRef]
- Vilanova, L.; Vall-llaura, N.; Torres, R.; Usall, J.; Teixidó, N.; Larrigaudière, C.; Giné-Bordonaba, J. Penicillium expansum (Compatible) and Penicillium digitatum (Non-Host) Pathogen Infection Differentially alter Ethylene Biosynthesis in Apple Fruit. Plant Physiol. Biochem. 2017, 120, 132–143. [Google Scholar] [CrossRef]
- Deng, B.; Wang, W.; Deng, L.; Yao, S.; Ming, J.; Zeng, K. Comparative RNA-Seq Analysis of Citrus Fruit in Response to Infection with Three Major Postharvest Fungi. Postharvest Biol. Technol. 2018, 146, 134–146. [Google Scholar] [CrossRef]
- Lorrai, R.; Ferrari, S. Host Cell Wall Damage during Pathogen Infection: Mechanisms of Perception and Role in Plant-Pathogen Interactions. Plants 2021, 10, 399. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Han, T.; Jiang, L.; Xi, P.; Wang, Q.; Jiang, Z.; Gao, L. In Vitro and in Vivo Effectiveness of Phenolic Compounds for the Control of Postharvest Gray Mold of Table Grapes. Postharvest Biol. Technol. 2018, 139, 106–114. [Google Scholar] [CrossRef]
- Kong, Q.; An, P.; Xu, Z.; Zhang, R.; Qi, J.; Ren, X. New Insights into the Alleviating Role of Melaleuca alternifolia Oil on Metabolites Pathway Disorder of Grapes Caused by Aspergillus niger, Verified by Corresponding Key Genes Expression. Food Chem. 2020, 327, 127083. [Google Scholar] [CrossRef]
- Kupfer, V.M.; Vogt, E.I.; Ziegler, T.; Vogel, R.F.; Niessen, L. Comparative Protein Profile Analysis of Wines Made from Botrytis cinerea Infected and Healthy Grapes Reveals a Novel Biomarker for Gushing in Sparkling Wine. Food Res. Int. 2017, 99, 501–509. [Google Scholar] [CrossRef]
- Crespo-Sempere, A.; Marín, S.; Sanchis, V.; Ramos, A.J. VeA and LaeA Transcriptional Factors Regulate Ochratoxin A Biosynthesis in Aspergillus carbonarius. Int. J. Food Microbiol. 2013, 166, 479–486. [Google Scholar] [CrossRef]
- Zhu, P.; Xu, L.; Zhang, C.; Toyoda, H.; Gan, S.-S. Ethylene Produced by Botrytis cinerea Can Affect Early Fungal Development and Can Be Used as a Marker for Infection during Storage of Grapes. Postharvest Biol. Technol. 2012, 66, 23–29. [Google Scholar] [CrossRef]
- Martínez-Soto, D.; Ruiz-Herrera, J. Functional Analysis of the MAPK Pathways in Fungi. Rev. Iberoam. Micol. 2017, 34, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, X.; Liu, H.; Xu, J.-R. Mitogen-Activated Protein Kinase Signaling in Plant Pathogenic Fungi. PLoS Pathog. 2018, 14, e1006875. [Google Scholar] [CrossRef]
- Zhao, X.; Mehrabi, R.; Xu, J.-R. Mitogen-Activated Protein Kinase Pathways and Fungal Pathogenesis. Eukaryot. Cell 2007, 6, 1701–1714. [Google Scholar] [CrossRef]
- Hedbacker, K. SNF1/AMPK Pathways in Yeast. Front. Biosci. 2008, 13, 2408. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Q.; Sun, X.; Li, H. The Calcineurin-Responsive Transcription Factor Crz1 Is Required for Conidation, Full Virulence and DMI Resistance in Penicillium digitatum. Microbiol. Res. 2013, 168, 211–222. [Google Scholar] [CrossRef]
- Bushweller, J.H. Targeting Transcription Factors in Cancer—From Undruggable to Reality. Nat. Rev. Cancer 2019, 19, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Tannous, J.; Barda, O.; Luciano-Rosario, D.; Prusky, D.B.; Sionov, E.; Keller, N.P. New Insight Into Pathogenicity and Secondary Metabolism of the Plant Pathogen Penicillium expansum Through Deletion of the Epigenetic Reader SntB. Front. Microbiol. 2020, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Voß, B.; Kirschhöfer, F.; Brenner-Weiß, G.; Fischer, R. Alternaria alternata Uses Two Siderophore Systems for Iron Acquisition. Sci. Rep. 2020, 10, 3587. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, X.; Shi, T.; Li, C.; Huang, G. The Colletotrichum gloeosporioides Perilipin Homologue CAP 20 Regulates Functional Appressorial Formation and Fungal Virulence. J. Phytopathol. 2017, 166, 216–225. [Google Scholar] [CrossRef]
- Bi, F.; Ment, D.; Luria, N.; Meng, X.; Prusky, D. Mutation of AREA Affects Growth, Sporulation, Nitrogen Regulation, and Pathogenicity in Colletotrichum gloeosporioides. Fungal Genet. Biol. 2017, 99, 29–39. [Google Scholar] [CrossRef]
- Chudasama, K.S.; Monpara, J.K.; Thaker, V.S. Identification and Characterization of Pectin Lyase Gene as a Virulence Factor in Colletotrichum gloeosporioides. Physiol. Mol. Plant Pathol. 2021, 116, 101706. [Google Scholar] [CrossRef]
- Yakoby, N.; Beno-Moualem, D.; Keen, N.T.; Dinoor, A.; Pines, O.; Prusky, D. Colletotrichum gloeosporioides pelB Is an Important Virulence Factor in Avocado Fruit-Fungus Interaction. Mol. Plant-Microbe Interact. 2001, 14, 988–995. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Y.; Ma, Z. Involvement of BcVeA and BcVelB in Regulating Conidiation, Pigmentation and Virulence in Botrytis cinerea. Fungal Genet. Biol. 2013, 50, 63–71. [Google Scholar] [CrossRef]
- Archana, S.; Raguchander, T.; Prabakar, K. Detection of -Tubulin Gene from Benomyl Sensitive Isolates of Colletotrichum gloeosporioides Causing Anthracnose Disease in Mango. Afr. J. Microbiol. Res. 2018, 12, 806–814. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Yang, Q.; Apaliya, M.T. Recent Developments in the Enhancement of Some Postharvest Biocontrol Agents with Unconventional Chemicals Compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Sánchez-Torres, P.; Vilanova, L.; Ballester, A.R.; López-Pérez, M.; Teixidó, N.; Viñas, I.; Usall, J.; González-Candelas, L.; Torres, R. Unravelling the Contribution of the Penicillium expansum PeSte12 Transcription Factor to Virulence during Apple Fruit Infection. Food Microbiol. 2018, 69, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ngea, G.L.N.; Godana, E.A.; Shi, Y.; Lanhuang, B.; Zhang, X.; Zhao, L.; Yang, Q.; Wang, S.; Zhang, H. Recent Advances in Penicillium expansum Infection Mechanisms and Current Methods in Controlling P. expansum in Postharvest Apples. Critical Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- De Ramón-Carbonell, M.; Sánchez-Torres, P. The Transcription Factor PdSte12 Contributes to Penicillium digitatum Virulence during Citrus Fruit Infection. Postharvest Biol. Technol. 2017, 125, 129–139. [Google Scholar] [CrossRef]
- Wang, M.; Chen, C.; Zhu, C.; Sun, X.; Ruan, R.; Li, H. Os2 MAP Kinase-Mediated Osmostress Tolerance in Penicillium digitatum Is Associated with Its Positive Regulation on Glycerol Synthesis and Negative Regulation on Ergosterol Synthesis. Microbiol. Res. 2014, 169, 511–521. [Google Scholar] [CrossRef]
- Vilanova, L.; López-Pérez, M.; Ballester, A.-R.; Teixidó, N.; Usall, J.; Lara, I.; Viñas, I.; Torres, R.; González-Candelas, L. Differential Contribution of the Two Major Polygalacturonases from Penicillium digitatum to Virulence towards Citrus Fruit. Int. J. Food Microbiol. 2018, 282, 16–23. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, W.; Wang, M.; Ruan, R.; Sun, X.; He, M.; Mao, C.; Li, H. Deletion of PdMit1, a Homolog of Yeast Csg1, Affects Growth and Ca2+ Sensitivity of the Fungus Penicillium digitatum, but Does Not Alter Virulence. Res. Microbiol. 2015, 166, 143–152. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Wang, J.; Zhu, C.; Chung, K.-R.; Li, H. Adenylyl Cyclase Is Required for cAMP Production, Growth, Conidial Germination, and Virulence in the Citrus Green Mold Pathogen Penicillium digitatum. Microbiol. Res. 2016, 192, 11–20. [Google Scholar] [CrossRef]
- Ruan, R.; Wang, M.; Liu, X.; Sun, X.; Chung, K.-R.; Li, H. Functional Analysis of Two Sterol Regulatory Element Binding Proteins in Penicillium digitatum. PLoS ONE 2017, 12, e0176485. [Google Scholar] [CrossRef]
- De Ramón-Carbonell, M.; Sánchez-Torres, P. Involvement of Penicillium digitatum PdSUT1 in Fungicide Sensitivity and Virulence during Citrus Fruit Infection. Microbiol. Res. 2017, 203, 57–67. [Google Scholar] [CrossRef]
- De Ramón-Carbonell, M.; López-Pérez, M.; González-Candelas, L.; Sánchez-Torres, P. PdMFS1 Transporter Contributes to Penicilliun digitatum Fungicide Resistance and Fungal Virulence during Citrus Fruit Infection. J. Fungi 2019, 5, 100. [Google Scholar] [CrossRef]
- De Ramón-Carbonell, M.; Sánchez-Torres, P. PdSlt2 Penicillium digitatum Mitogen-Activated-Protein Kinase Controls Sporulation and Virulence during Citrus Fruit Infection. Fungal Biol. 2017, 121, 1063–1074. [Google Scholar] [CrossRef]
- Bano, A.; Gupta, A.; Rai, S.; Sharma, S.; Pathak, N. Elucidation of Bioactive Potential of Two Commonly Grown North Indian Psidium guajava Viz., Lalit and Shweta against Pathogenic Foodborne and MDR Bacteria. Biointerface Res. Appl. Chem. 2021, 11, 14090–14102. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic Insights of Plant Growth Promoting Bacteria Mediated Drought and Salt Stress Tolerance in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Bano, A.; Gupta, A.; Rai, S.; Fatima, T.; Sharma, S.; Pathak, N. Mechanistic Role of Reactive Oxygen Species and Its Regulation via the Antioxidant System under Environmental Stress. In Plant Stress Physiology: Perspectives in Agriculture; InTech Open: London, UK, 2022. [Google Scholar] [CrossRef]
- Gupta, A.; Bano, A.; Rai, S.; Mishra, R.; Singh, M.; Sharma, S.; Pathak, N. Mechanistic Insights of Plant-Microbe Interaction towards Drought and Salinity Stress in Plants for Enhancing the Agriculture Productivity. Plant Stress 2022, 4, 100073. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; Chen, T.; Li, B.-Q.; Qin, G.-Z.; Tian, S.-P. Molecular Basis of Pathogenesis of Postharvest Pathogenic Fungi and Control Strategy in Fruits: Progress and Prospect. Mol. Hortic. 2021, 1, 2. [Google Scholar] [CrossRef]
- Ezzat, A.; Szabó, S.; Szabó, Z.; Hegedűs, A.; Berényi, D.; Holb, I.J. Temporal Patterns and Inter-Correlations among Physical and Antioxidant Attributes and Enzyme Activities of Apricot Fruit Inoculated with Monilinia laxa under Salicylic Acid and Methyl Jasmonate Treatments under Shelf-Life Conditions. J. Fungi 2021, 7, 341. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, B.; Chen, T.; Tian, S. Reactive Oxygen Species: A Generalist in Regulating Development and Pathogenicity of Phytopathogenic Fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3344–3349. [Google Scholar] [CrossRef]
- Bedard, K.; Lardy, B.; Krause, K. NOX Family NADPH Oxidases: Not Just in Mammals. Biochimie 2007, 89, 1107–1112. [Google Scholar] [CrossRef]
- Segmüller, N.; Kokkelink, L.; Giesbert, S.; Odinius, D.; van Kan, J.; Tudzynski, P. NADPH Oxidases Are Involved in Differentiation and Pathogenicity in Botrytis cinerea. Mol. Plant-Microbe Interact. 2008, 21, 808–819. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; He, C.; Qin, G.; Tian, S. Comparative Proteomics Reveals the Potential Targets of BcNoxR, a Putative Regulatory Subunit of NADPH Oxidase of Botrytis cinerea. Mol. Plant-Microbe Interact. 2016, 29, 990–1003. [Google Scholar] [CrossRef]
- Da Silva, R.R. Enzyme Technology in Food Preservation: A Promising and Sustainable Strategy for Biocontrol of Post-Harvest Fungal Pathogens. Food Chem. 2019, 277, 531–532. [Google Scholar] [CrossRef]
- Leiter, É.; Gáll, T.; Csernoch, L.; Pócsi, I. Biofungicide Utilizations of Antifungal Proteins of Filamentous Ascomycetes: Current and Foreseeable Future Developments. BioControl 2016, 62, 125–138. [Google Scholar] [CrossRef]
- Gregori, R.; Mari, M.; Bertolini, P.; Barajas, J.A.S.; Tian, J.B.; Labavitch, J.M. Reduction of Colletotrichum acutatum Infection by a Polygalacturonase Inhibitor Protein Extracted from Apple. Postharvest Biol. Technol. 2008, 48, 309–313. [Google Scholar] [CrossRef]
- Sansone, G.; Rezza, I.; Fernández, G.; Calvente, V.; Benuzzi, D.; Sanz, M.I. Inhibitors of Polygalacturonase and Laccase of Botrytis cinerea and Their Application to the Control of This Fungus. Int. Biodeterior. Biodegrad. 2011, 65, 243–247. [Google Scholar] [CrossRef]
- Ballester, A.-R.; López-Pérez, M.; de la Fuente, B.; González-Candelas, L. Functional and Pharmacological Analyses of the Role of Penicillium digitatum Proteases on Virulence. Microorganisms 2019, 7, 198. [Google Scholar] [CrossRef]
- Hernandez Montiel, L.G.; Zulueta Rodriguez, R.; Angulo, C.; Rueda Puente, E.O.; Quiñonez Aguilar, E.E.; Galicia, R. Marine Yeasts and Bacteria as Biological Control Agents against Anthracnose on Mango. J. Phytopathol. 2017, 165, 833–840. [Google Scholar] [CrossRef]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest Biocontrol Ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on Stone Fruit. Postharvest Biol. Technol. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Fu, X.; Li, Y.; Wang, Q. Isolation and Characterization of Bacillus amyloliquefaciens PG12 for the Biological Control of Apple Ring Rot. Postharvest Biol. Technol. 2016, 115, 113–121. [Google Scholar] [CrossRef]
- Wallace, R.L.; Hirkala, D.L.; Nelson, L.M. Mechanisms of Action of Three Isolates of Pseudomonas fluorescens Active against Postharvest Grey Mold Decay of Apple during Commercial Storage. Biol. Control 2018, 117, 13–20. [Google Scholar] [CrossRef]
- Khleekorn, S.; Wongrueng, S. Evaluation of antagonistic bacteria inhibitory to Colletotrichum musae on banana. J. Agric. Technol. 2014, 10, 383–390. [Google Scholar]
- Fu, G.; Huang, S.; Ye, Y.; Wu, Y.; Cen, Z.; Lin, S. Characterization of a Bacterial Biocontrol Strain B106 and Its Efficacies on Controlling Banana Leaf Spot and Post-Harvest Anthracnose Diseases. Biol. Control 2010, 55, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Zhu, P.; Liu, Y.; Zhang, Z.; Mastuda, Y.; Toyoda, H.; Xu, L. Postharvest biological control of melon pathogens using Bacillus subtilis EXWB1. J. Plant Pathol. 2010, 92, 645–652. [Google Scholar]
- Kasfi, K.; Taheri, P.; Jafarpour, B.; Tarighi, S. Identification of Epiphytic Yeasts and Bacteria with Potential for Biocontrol of Grey Mold Disease on Table Grapes Caused by Botrytis cinerea. Span. J. Agric. Res. 2018, 16, e1002. [Google Scholar] [CrossRef]
- Kim, Y.S.; Balaraju, K.; Jeon, Y. Effects of Rhizobacteria Paenibacillus polymyxa APEC136 and Bacillus subtilis APEC170 on Biocontrol of Postharvest Pathogens of Apple Fruits. J. Zhejiang Univ. Sci. B 2016, 17, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Calvo, H.; Marco, P.; Blanco, D.; Oria, R.; Venturini, M.E. Potential of a New Strain of Bacillus amyloliquefaciens BUZ-14 as a Biocontrol Agent of Postharvest Fruit Diseases. Food Microbiol. 2017, 63, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.; Li, Y.; Zhang, J.; Bi, S. Chemical Composition and Biological Activity of the Essential Oil from Rhododendronanwheiense Flowers. Chem. Nat. Compd. 2022, 58, 947–950. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Bi, Y.; Han, Y.; Zong, Y.; Prusky, D. Cuminal Inhibits Trichothecium roseum Growth by Triggering Cell Starvation: Transcriptome and Proteome Analysis. Microorganisms 2020, 8, 256. [Google Scholar] [CrossRef]
- Tripathi, P.; Dubey, N.K. Exploitation of Natural Products as an Alternative Strategy to Control Postharvest Fungal Rotting of Fruit and Vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A Review on the Use of Essential Oils for Postharvest Decay Control and Maintenance of Fruit Quality during Storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Sompila, A.W.G.T.; Mabika, A.B.M.; Pambou-Tobi, N.P.G.; Gouollaly, T.; Moussounga, J.E.; N’simba, G.L.L.B.; Nguie, R.; Matos, L. Evaluation of Some Secondary Metabolites and Determination of the Antioxidant Potential of Different Extracts from the Plant of Pteridium aquilinum. Am. J. Anal. Chem. 2021, 12, 506–519. [Google Scholar] [CrossRef]
- Kanan, G.; Al-Najar, R. In Vitro and In Vivo Activity of Selected Plant Crude Extracts and Fractions Against Penicillium italicum. J. Plant Prot. Res. 2009, 49, 341–352. [Google Scholar] [CrossRef]
- Pangallo, S.; Li Destri Nicosia, M.G.; Raphael, G.; Levin, E.; Ballistreri, G.; Cacciola, S.O.; Rapisarda, P.; Droby, S.; Schena, L. Elicitation of Resistance Responses in Grapefruit and Lemon Fruits Treated with a Pomegranate Peel Extract. Plant Pathol. 2016, 66, 633–640. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A Structure–Activity Relationship Study of Flavonoids as Inhibitors of E. coli by Membrane Interaction Effect. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Kai, K.; Wang, X.; Shi, W.; Zhang, D.; Liu, Y. Curcumin Inhibits Gray Mold Development in Kiwifruit by Targeting Mitogen-Activated Protein Kinase (MAPK) Cascades in Botrytis cinerea. Postharvest Biol. Technol. 2019, 151, 152–159. [Google Scholar] [CrossRef]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.P.V.; Olika Keyata, E. Potentials of Natural Preservatives to Enhance Food Safety and Shelf Life: A Review. Sci. World J. 2022, 2022, 9901018. [Google Scholar] [CrossRef]
- Siddaiah, C.N.; Prasanth, K.V.H.; Satyanarayana, N.R.; Mudili, V.; Gupta, V.K.; Kalagatur, N.K.; Satyavati, T.; Dai, X.-F.; Chen, J.-Y.; Mocan, A.; et al. Chitosan Nanoparticles Having Higher Degree of Acetylation Induce Resistance against Pearl Millet Downy Mildew through Nitric Oxide Generation. Sci. Rep. 2018, 8, 2485. [Google Scholar] [CrossRef]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of Disease Resistance and ROS Metabolism in Navel Oranges by Chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- García-Rincón, J.; Vega-Pérez, J.; Guerra-Sánchez, M.G.; Hernández-Lauzardo, A.N.; Peña-Díaz, A.; Velázquez-Del Valle, M.G. Effect of Chitosan on Growth and Plasma Membrane Properties of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Pestic. Biochem. Physiol. 2010, 97, 275–278. [Google Scholar] [CrossRef]
- Akter, J.; Jannat, R.; Hossain, M.M.; Ahmed, J.U.; Rubayet, T. Chitosan for Plant Growth Promotion and Disease Suppression against Anthracnose in Chilli. Int. J. Environ. Agric. Biotechnol. 2018, 3, 806–817. [Google Scholar] [CrossRef]
- Gupta, A.; Bano, A.; Rai, S.; Kumar, M.; Ali, J.; Sharma, S. ACC deaminase producing Plant Growth Promoting Rhizobacteria enhance salinity stress tolerance in Pisum sativum. 3 Biotech 2021, 11, 514. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Kowbel, D.; Nueda, M.J.; Palma-Guerrero, J.; Glass, N.L.; Lopez-Llorca, L.V. Neurospora crassa Transcriptomics Reveals Oxidative Stress and Plasma Membrane Homeostasis Biology Genes as Key Targets in Response to Chitosan. Mol. BioSyst. 2016, 12, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Jaime, M.D.; Lopez-Llorca, L.V.; Conesa, A.; Lee, A.Y.; Proctor, M.; Heisler, L.E.; Gebbia, M.; Giaever, G.; Westwood, J.T.; Nislow, C. Identification of Yeast Genes That Confer Resistance to Chitosan Oligosaccharide (COS) Using Chemogenomics. BMC Genom. 2012, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Placì, N.; Scialanga, B.; Ceredi, G.; Baraldi, E. Ripe Indexes, Hot Water Treatments, and Biocontrol Agents as Synergistic Combination to Control Apple Bull’s Eye Rot. Biocontrol Sci. Technol. 2022, 32, 1016–1026. [Google Scholar] [CrossRef]
- Ngolong Ngea, G.L.; Qian, X.; Yang, Q.; Dhanasekaran, S.; Ianiri, G.; Ballester, A.R.; Zhang, X.; Castoria, R.; Zhang, H. Securing fruit production: Opportunities from the elucidation of the molecular mechanisms of postharvest fungal infections. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2508–2533. [Google Scholar] [CrossRef]
- Luria, N.; Sela, N.; Yaari, M.; Feygenberg, O.; Kobiler, I.; Lers, A.; Prusky, D. De-Novo Assembly of Mango Fruit Peel Transcriptome Reveals Mechanisms of Mango Response to Hot Water Treatment. BMC Genom. 2014, 15, 957. [Google Scholar] [CrossRef]
- Jeong, M.-A.; Jeong, R.-D. Applications of Ionizing Radiation for the Control of Postharvest Diseases in Fresh Produce: Recent Advances. Plant Pathol. 2017, 67, 18–29. [Google Scholar] [CrossRef]
- Rothkamm, K.; Barnard, S.; Moquet, J.; Ellender, M.; Rana, Z.; Burdak-Rothkamm, S. DNA Damage Foci: Meaning and Significance. Environ. Mol. Mutagen. 2015, 56, 491–504. [Google Scholar] [CrossRef]
- Loconsole, D.; Santamaria, P. UV Lighting in Horticulture: A Sustainable Tool for Improving Production Quality and Food Safety. Horticulturae 2021, 7, 9. [Google Scholar] [CrossRef]
- Kaewsuksaeng, S.; Urano, Y.; Aiamla-or, S.; Shigyo, M.; Yamauchi, N. Effect of UV-B Irradiation on Chlorophyll-Degrading Enzyme Activities and Postharvest Quality in Stored Lime (Citrus latifolia Tan.) Fruit. Postharvest Biol. Technol. 2011, 61, 124–130. [Google Scholar] [CrossRef]
- Ruiz, V.E.; Cerioni, L.; Zampini, I.C.; Cuello, S.; Isla, M.I.; Hilal, M.; Rapisarda, V.A. UV-B Radiation on Lemons Enhances Antifungal Activity of Flavedo Extracts against Penicillium digitatum. LWT Food Sci. Technol. 2017, 85, 96–103. [Google Scholar] [CrossRef]
- Usall, J.; Ippolito, A.; Sisquella, M.; Neri, F. Physical Treatments to Control Postharvest Diseases of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 2016, 122, 30–40. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient Biotic Strategy to Control Postharvest Diseases of Fruits and Vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bano, A.; Pandey, N.; Rai, S.; Sharma, S.; Pathak, N. Recent Advances in Biotic and Abiotic Stresses of Solanum melongena L. In Solanum melongena: Production, Cultivation and Nutrition; Nova Science Publishers: Hauppauge, NY, USA, 2021; pp. 151–171. ISBN 978-1-68507-311-4. [Google Scholar]
- Gupta, A.; Aamina, S.; Bano, A.; Rai, S.; Bansal, P.; Sharma, S. Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Plant growth under biotic stress condition. In Recent Advances and Approaches for Crop Improvement and Sustainable Agriculture; Aargon Press: New Delhi, India, 2022; pp. 1–12. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).