Evaluation of Touchdown Loop-Mediated Isothermal Amplification for the Detection of Giardia duodenalis
Abstract
1. Introduction
2. Materials and Methods
2.1. LAMP Primers
2.2. DNA Preparation and Analytical Sensitivity Measurements
2.3. Reaction Components and LAMP Parameters
2.4. LAMP Protocol
2.5. Gel Electrophoresis and Evaluation of the LAMP Products
2.6. LAMP Repeatability
2.7. Statistical Methods
3. Results
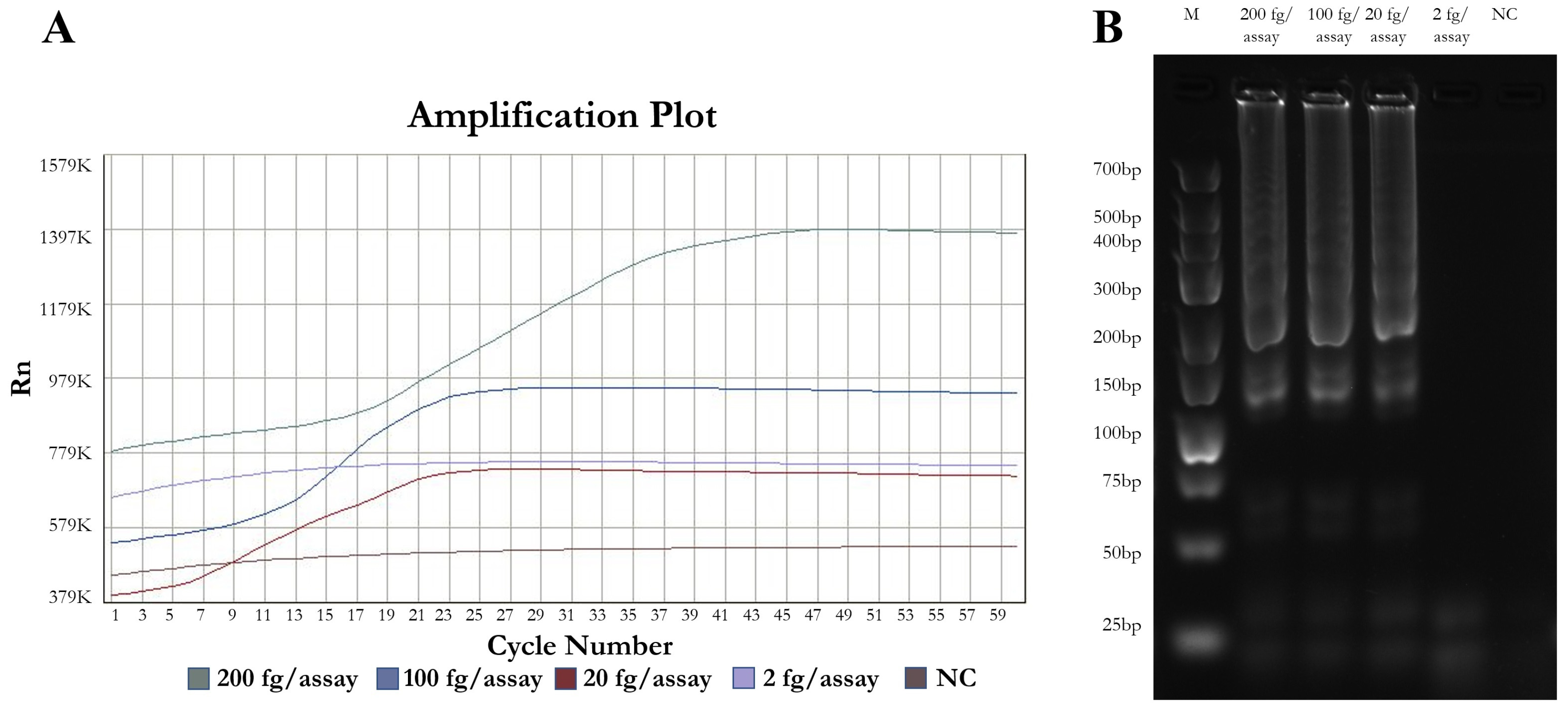
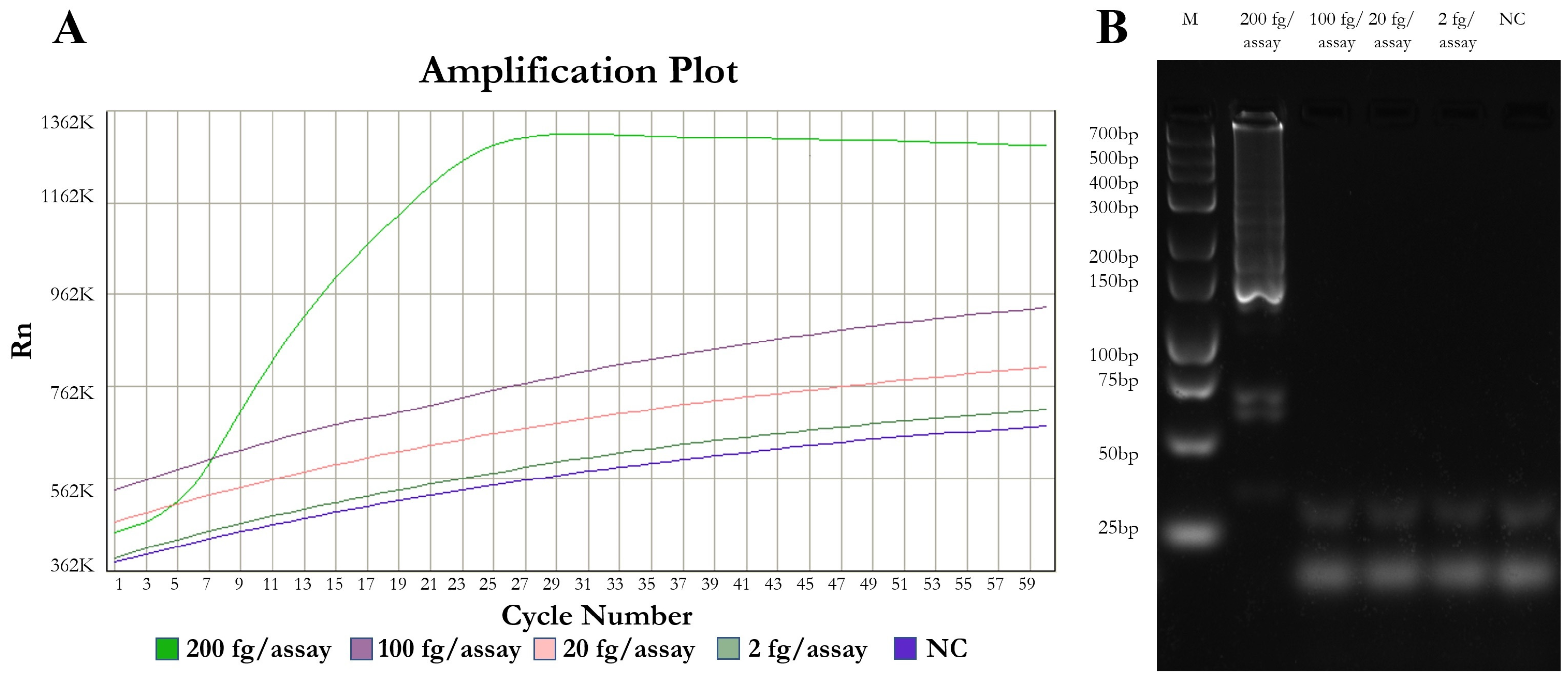
3.1. Optimal Parameters, Analytical Sensitivity
3.2. Repeatability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAMP | Loop-mediated isothermal amplification |
References
- Fever, B. Giardia Enteritis, Lambliasis. 2012. Available online: https://scholar.google.hu/scholar?hl=hu&as_sdt=0%2C5&q=fever+giardia+enteritis%2C+lambliasis+2012&btnG=https://abrakadabraenvironmental.com/wp-content/uploads/2021/05/Giardiasis.pdf (accessed on 23 March 2025).
- Heyworth, M.F. Giardia duodenalis genetic assemblages and hosts. Parasite 2016, 23, 13. [Google Scholar] [CrossRef]
- Plutzer, J.; Karanis, P. Rapid identification of Giardia duodenalis by loop-mediated isothermal amplification (lamp) from faecal and environmental samples and comparative findings by PCR and real-time PCR methods. Parasitol. Res. 2009, 104, 1527–1533. [Google Scholar] [CrossRef]
- Efstratiou, A.; Ongerth, J.; Karanis, P. Evolution of monitoring for Giardia and Cryptosporidium in water. Water Res. 2017, 123, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Devleesschauwer, B.; Bouwknegt, M.; Dorny, P.; Gabriël, S.; Havelaar, A.H.; Quoilin, S.; Robertson, L.J.; Speybroeck, N.; Torgerson, P.R.; van der Giessen, J.W.; et al. Risk ranking of foodborne parasites: State of the art. Food Waterborne Parasitol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bouwknegt, M.; Devleesschauwer, B.; Graham, H.; Robertson, L.J.; van der Giessen, J.W.; The Euro-Fbp Workshop Participants. Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance 2018, 23, 17-00161. [Google Scholar] [CrossRef] [PubMed]
- Plutzer, J.; Lassen, B.; Jokelainen, P.; Djurković-Djaković, O.; Kucsera, I.; Dorbek-Kolin, E.; Šoba, B.; Sréter, T.; Imre, K.; Omeragić, J.; et al. Review of Cryptosporidium and Giardia in the eastern part of Europe, 2016. Eurosurveillance 2018, 23, 16-00825. [Google Scholar] [CrossRef]
- Olson, M.; Ceri, H.; Morck, D. Giardia vaccination. Parasitol. Today 2000, 16, 213–217. [Google Scholar] [CrossRef]
- Wolfe, M.S. Giardiasis. Clin. Microbiol. Rev. 1992, 5, 93–100. [Google Scholar] [CrossRef]
- Vicente, B.; De Freitas, A.; Freitas, M.; Midlej, V. Systematic review of diagnostic approaches for human giardiasis: Unveiling optimal strategies. Diagnostics 2024, 14, 364. [Google Scholar] [CrossRef]
- ISO 18744:2016; Microbiology of the Food Chain—Detection and Enumeration of Cryptosporidium and Giardia in Fresh Leafy Green Vegetables and Berry Fruits. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 15553; Water Quality—Isolation and Identification of Cryptosporidium Oocysts and Giardia Cysts from Water. International Organization for Standardization: Geneva, Switzerland, 2006.
- Koehler, A.V.; Jex, A.R.; Haydon, S.R.; Stevens, M.A.; Gasser, R.B. Giardia/giardiasis-a perspective on diagnostic and analytical tools. Biotechnol. Adv. 2014, 32, 280–289. [Google Scholar] [CrossRef]
- Soares, R.; Tasca, T. Giardiasis: An update review on sensitivity and specificity of methods for laboratorial diagnosis. J. Microbiol. Methods 2016, 129, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Momoda, T.; Kojima, T.; Ikedo, M.; Izumiyama, S.; Endo, T. Sensitive and rapid detection of Cryptosporidium and Giardia by loop-mediated isothermal amplification. J. Jpn. Soc. Water Environ. 2009, 32, 321–324. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Zhang, A.; Zhang, P.; Alsarakibi, M.; Li, G. Sensitive and rapid detection of Giardia lamblia infection in pet dogs using loop-mediated isothermal amplification. Korean J. Parasitol. 2013, 51, 237. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Todt, C.; Mikkelsen, N.T.; Lieb, B. Fast evolving 18S rRNA sequences from Solenogastres (Mollusca) resist standard PCR amplification and give new insights into mollusk substitution rate heterogeneity. BMC Evol. Biol. 2010, 10, 70. [Google Scholar] [CrossRef]
- Brynildsrud, O.; Tysnes, K.R.; Robertson, L.J.; Debenham, J.J. Giardia duodenalis in primates: Classification and host specificity based on phylogenetic analysis of sequence data. Zoonoses Public Health 2018, 65, 637–647. [Google Scholar] [CrossRef]
- Lund, A.; Knudsen, S.; Vissing, H.; Clark, B.; Tommerup, N. Assignment of Human Elongation Factor 1α Genes: EEF1AMaps to Chromosome 6q14 andEEF1A2to 20q13. 3. Genomics 1996, 36, 359–361. [Google Scholar] [CrossRef]
- Lass, A.; Szostakowska, B.; Korzeniewski, K.; Karanis, P. Detection of Giardia intestinalis in water samples collected from natural water reservoirs and wells in northern and north-eastern Poland using lamp, real-time PCR and nested PCR. J. Water Health 2017, 15, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Brewster, J.; Paul, M.; Tomasula, P. Two methods for increased specificity and sensitivity in loop-mediated isothermal amplification. Molecules 2015, 20, 6048–6059. [Google Scholar] [CrossRef]
- Guy, R.A.; Payment, P.; Krull, U.J.; Horgen, P.A. Real-time PCR for quantification of Giardia and Cryptosporidium in environmental water samples and sewage. Appl. Environ. Microbiol. 2003, 69, 5178–5185. [Google Scholar] [CrossRef]
- [Dataset] Inc., New England Biolabs. Typical Lamp Protocol (M0275). 2019. Available online: https://international.neb.com/protocols/2014/11/21/typical-lamp-protocol-m0275 (accessed on 14 March 2019).
- Thompson, R.C.A.; Ash, A. Molecular epidemiology of Giardia and Cryptosporidium infections. Infect. Genet. Evol. 2016, 40, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ozdemir, P. Microfluidic DNA amplification—A review. Anal. Chim. Acta 2009, 638, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Fohlerova, Z.; Chang, H.; Iliescu, C.; Neuzil, P. LAMP-on-a-chip: Revising microfluidic platforms for loop-mediated DNA amplification. TrAC Trends Anal. Chem. 2019, 113, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.; Bridle, H. Microfluidics for effective concentration and sorting of waterborne protozoan pathogens. J. Microbiol. Methods 2016, 126, 8–11. [Google Scholar] [CrossRef]
- Fang, X.; Chen, H.; Xu, L.; Jiang, X.; Wu, W.; Kong, J. A portable and integrated nucleic acid amplification microfluidic chip for identifying bacteria. Lab A Chip 2012, 12, 1495–1499. [Google Scholar] [CrossRef]
- Hsieh, K.; Patterson, A.S.; Ferguson, B.S.; Plaxco, K.W.; Soh, H.T. Rapid, sensitive, and quantitative detection of pathogenic DNA at the point of care through microfluidic electrochemical quantitative loop-mediated isothermal amplification. Angew. Chem. Int. Ed. 2012, 51, 4896–4900. [Google Scholar] [CrossRef]
- Wang, C.-H.; Lien, K.-Y.; Wang, T.-Y.; Chen, T.-Y.; Lee, G.-B. An integrated microfluidic loop-mediated-isothermal-amplification system for rapid sample pre-treatment and detection of viruses. Biosens. Bioelectron. 2011, 26, 2045–2052. [Google Scholar] [CrossRef]
| Giardia Target Gene | Giardia Species and Assemblage Accession Number | Primer Name | Primer Sequences (5′-3′) | The Size of the Product | |
|---|---|---|---|---|---|
| Plutzer et al. (2009) [3] | EF1α (elongation factor 1 Alfa) | G. duodenalis assemblage B, accession number: AF069570 | F3 B3 FIP BIP FLP BLP | GCCGGGATCTCGAAGGAC TCGGGATGTAGTCGAACTCC TGACCTGGCCGTCGTCCATC TTGCGACGCTCGCGAACA GTACTCGAAGGAGCGCTACG ACGCCTTCTTCCAGCCGATG GACGGCCAGACGCGCGAG GCGGAGGGGCTTGTCGGTC | 18 20 38 40 18 19 |
| sum: | 153 | ||||
| Momoda et al. (2009) [16] | 18S rRNA gene | G. intestinalis assemblage n.a., accession number: AF473852 | F3 B3 FIP BIP FLP BLP | GCCGGGGGCTAGAAGG CGCGTTGAGTCAGATTGAGC CGGTTTCCCTGGGCGGCAGA CACCACCGTATTCCCG CTCTGGGGGGAGTATGGCCC ACGTCTGGTGGTACCC GGCGGCACCGTTTACG AGGCTGAAACTTGAAGGCAT | 16 20 36 36 16 20 |
| sum: | 144 | ||||
| Li et al. (2013) [17] | EF1α (elongation factor 1 Alfa) | G. duodenalis assemblage D, accession number: n.a. | F3 B3 FIP BIP FLP BLP | ATGGACGACGGCCAGG CCCTCGTACCAGGGCATC AGCCGATGTTCTTGAGCTGC TTGTACTCGAAGGAGCGCTA CG GAAGAAGGCCGAGGAGTTCG TTGTCGGACCTCTCCATGA CTGGACCGGGGACAACA ATCATCTCGCCCTTGATCTCG | 16 18 42 39 17 21 |
| sum: | 153 |
| Reaction Components | Plutzer et al., 2009 [3] | Li et al., 2013 [17] | Momoda et al., 2009 [16] | Typical LAMP Protocol | Present Study |
|---|---|---|---|---|---|
| FIP/BIP | 1.6 µM | 1.6 µM | n.a. | 1.6 µM | 1.6 µM |
| FLP/BLP | 0.8 µM | 0.8 µM | n.a. | 0.4 µM | 0.4 µM |
| F3/B3 | 0.2 µM | 0.2 µM | n.a. | 0.2 µM | 0.2 µM |
| dNTP | 2.8 µM | 2.5 µM | 2.5 mM | 1.4 mM | 1.4 mM |
| Betaine | 1.6 M | 1.6 µM | 0.8 M | - | 0.8 M or nothing |
| Tris-HCl (pH 8.8) | 20 mM | - | 20 mM | - | - |
| KCl | 10 mM | - | 10 mM | - | - |
| (NH4)2SO4 | 10 mM | - | 10 mM | - | - |
| Tween 20 | 0.2% | - | 0.1% | - | - |
| MgSO4 | 8 mM | 8 mM | 8 mM | 6 mM (8 mM total) | 6 mM (8 mM total in the 1× final) |
| SYBR Green I | - | 400x | - | - | 1× final |
| Bst DNA polymerase buffer | - | 2.5 mM | - | 1× (contains 2 mM MgSO4) | 1× (contains 2 mM MgSO4) |
| Bst DNA polymerase | 8 U | 8 U | 8 U | 8 U | 8 U |
| DNA template | 2 µL | 2 µL | 2 µL | 2 µL | 1 µL |
| Reaction Step | TLS1 (°C) | TLS2 (°C) | TLS3 (°C) | TLS4 (°C) | Time | Betaine | Polymerase |
|---|---|---|---|---|---|---|---|
| Denaturation * | 90 | 2 min | +/− | Bst 2.0 Warmstart DNA Polymerase and Bst.3.0 DNA Polymerase (New England Biolabs Inc.) | |||
| Touchdown Activation 1 | 66 | 68 | 70 | 72 | 5 min | +/− | |
| Touchdown Activation 2 | 64 | 66 | 68 | 70 | 5 min | +/− | |
| Touchdown Activation 3 | 62 | 64 | 66 | 68 | 5 min | +/− | |
| Incubation | 60 | 62 | 64 | 66 | 60 min | +/− | |
| Dissociation measurement | 95 | 15 s | +/− | ||||
| 60 | 60 s | +/− | |||||
| 95 | 15 s | +/− | |||||
| Unit | Plutzer et al., 2009 [3] | Plutzer et al., 2009 [3] | Momoda et al., 2009 [16] | Li et al., 2013 [17] | ||
|---|---|---|---|---|---|---|
| Assemblage | A | B | unknown | C/D | ||
| Detection limit | fg DNA/tube | 800 | 548 | 156 | 100 | |
| Detection time | min | 129 | 129 | 60 | 70 | |
| After optimization: | ||||||
| Assemblage | A | B | A | B | A/B | |
| Detection limit | fg DNA/tube | 200 | 195 | 20 | 19.5 | - |
| Detection time | min | 19–43 | 23–41 | 25–46 | 25–35 | - |
| Optimal reaction conditions * | TLS2 | TLS2 | TLS1 | TLS1 | ||
| Amount of DNA/LAMP Assay | Variability of Threshold Time Value | ||||
|---|---|---|---|---|---|
| G. duodenalis Assemblage A | |||||
| Primer Set | fg/Assay | Threshold Times | Mean | SD | CV (%) |
| Momoda et al., 2009 [16] | 200 | 26; 38; 22; 25 | 27.75 | 7.04 | 25.37 |
| 100 | 42; 43; n.a.; 30 | 38.33 | 7.23 | 18.87 | |
| 20 | 34; 46; 25; 31 | 34 | 8.83 | 25.98 | |
| Plutzer et al., 2009 [3] | 200 | 19; 43; 19 | 27 | 13.86 | 51.32 |
| G. duodenalis Assemblage B | |||||
| Primer set | fg/assay | Threshold times | Mean | SD | CV (%) |
| Momoda et al., 2009 [16] | 195 | 19; 18; 23 | 20 | 2.65 | 13.23 |
| 97.5 | 18; 25; 21 | 21.33 | 3.51 | 16.46 | |
| 19.5 | 29; 25; 35; 33 | 30.5 | 4.43 | 14.54 | |
| Plutzer et al., 2009 [3] | 195 | 23; 25; 41 | 29.66 | 9.87 | 33.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartdégen, M.; Laki, A.J.; Farkasvölgyi, K.; Iván, K.; Plutzer, J. Evaluation of Touchdown Loop-Mediated Isothermal Amplification for the Detection of Giardia duodenalis. Parasitologia 2025, 5, 25. https://doi.org/10.3390/parasitologia5020025
Hartdégen M, Laki AJ, Farkasvölgyi K, Iván K, Plutzer J. Evaluation of Touchdown Loop-Mediated Isothermal Amplification for the Detection of Giardia duodenalis. Parasitologia. 2025; 5(2):25. https://doi.org/10.3390/parasitologia5020025
Chicago/Turabian StyleHartdégen, Márton, András József Laki, Kolos Farkasvölgyi, Kristóf Iván, and Judit Plutzer. 2025. "Evaluation of Touchdown Loop-Mediated Isothermal Amplification for the Detection of Giardia duodenalis" Parasitologia 5, no. 2: 25. https://doi.org/10.3390/parasitologia5020025
APA StyleHartdégen, M., Laki, A. J., Farkasvölgyi, K., Iván, K., & Plutzer, J. (2025). Evaluation of Touchdown Loop-Mediated Isothermal Amplification for the Detection of Giardia duodenalis. Parasitologia, 5(2), 25. https://doi.org/10.3390/parasitologia5020025







