Independent Predictors of Mycoplasma pneumoniae Infection: A Retrospective Cohort Study Among Hospitalized Adults in an East Texas Health Facility
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Source
2.2. Detection of Mycoplasma pneumoniae Infection
2.3. Study Population
2.4. Study Variables
2.5. Ethical Approval
2.6. Statistical Analysis
3. Results
3.1. Baseline and Clinical Characteristics of Mycoplasma pneumoniae Infection
3.2. Regression Analysis of Factors Associated with Mycoplasma pneumoniae Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bajantri, B.; Venkatram, S.; Diaz-Fuentes, G. Mycoplasma pneumoniae: A Potentially Severe Infection. J. Clin. Med. Res. 2018, 10, 535–544. [Google Scholar] [CrossRef]
- Sauteur, P.M.M.; Beeton, M.L.; Uldum, S.A.; Bossuyt, N.; Vermeulen, M.; Loens, K.; Pereyre, S.; Bébéar, C.; Keše, D.; Day, J.; et al. Mycoplasma pneumoniae detections before and during the COVID-19 pandemic: Results of a global survey, 2017 to 2021. Eurosurveillance 2022, 27, 2100746. [Google Scholar] [CrossRef] [PubMed]
- Parrott, G.L.; Kinjo, T.; Fujita, J. A Compendium for Mycoplasma pneumoniae. Front. Microbiol. 2016, 7, 513. [Google Scholar] [CrossRef] [PubMed]
- Marchello, C.; Dale, A.; Thai, T.N.; Han, D.S.; Ebell, M.H. Prevalence of Atypical Pathogens in Patients with Cough and Community-Acquired Pneumonia: A Meta-Analysis. Ann. Fam. Med. 2016, 14, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Prevention and Control. Mycoplasma pneumoniae Respiratory Illness—Two Rural Counties, West Virginia. 2011. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6141a3.htm (accessed on 6 August 2023).
- Atkinson, T.P.; Balish, M.F.; Waites, K.B. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol. Rev. 2008, 32, 956–973. [Google Scholar] [CrossRef]
- Thurman, K.A.; Walter, N.D.; Schwartz, S.B.; Mitchell, S.L.; Dillon, M.T.; Baughman, A.L.; Deutscher, M.; Fulton, J.P.; Tongren, J.E.; Hicks, L.A.; et al. Comparison of Laboratory Diagnostic Procedures for Detection of Mycoplasma pneumoniae in Community Outbreaks. Clin. Infect. Dis. 2009, 48, 1244–1249. [Google Scholar] [CrossRef]
- Hyde, T.B.; Gilbert, M.; Schwartz, S.B.; Zell, E.R.; Watt, J.P.; Thacker, W.L.; Talkington, D.F.; Besser, R.E. Azithromycin Prophylaxis during a Hospital Outbreak of Mycoplasma pneumoniae Pneumonia. J. Infect. Dis. 2001, 183, 907–912. [Google Scholar] [CrossRef]
- Díaz, A.; Barria, P.; Niederman, M.; Restrepo, M.I.; Dreyse, J.; Fuentes, G.; Couble, B.; Saldias, F. Etiology of Community-Acquired Pneumonia in Hospitalized Patients in Chile: The Increasing Prevalence of Respiratory Viruses Among Classic Pathogens. Chest 2007, 131, 779–787. [Google Scholar] [CrossRef]
- Onozuka, D.; Hashizume, M.; Hagihara, A. Impact of weather factors on Mycoplasma pneumoniae pneumonia. Thorax 2009, 64, 507–511. [Google Scholar] [CrossRef]
- Cillóniz, C.; Torres, A.; Niederman, M.; van der Eerden, M.; Chalmers, J.; Welte, T.; Blasi, F. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016, 42, 1374–1386. [Google Scholar] [CrossRef]
- Lui, G.; To, H.K.W.; Lee, N.; Chan, R.W.Y.; Li, T.; Wong, R.Y.K.; Hui, D.S.C.; Ip, M. Adherence to Treatment Guideline Improves Patient Outcomes in a Prospective Cohort of Adults Hospitalized for Community-Acquired Pneumonia. Open Forum Infect. Dis. 2020, 7, ofaa146. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y. Thrombosis associated with Mycoplasma pneumoniae infection (Review). Exp. Ther. Med. 2021, 22, 967. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Huang, C.-C.; Chan, W.-L.; Chen, Y.-C.; Chen, T.-J.; Lin, S.-J.; Chen, J.-W.; Leu, H.-B. Association Between Mycoplasma pneumonia and Increased Risk of Ischemic Stroke. Stroke 2011, 42, 2940–2943. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, Y.Y. Predictive Factors of the Responses to Treatment of Mycoplasma pneumoniae Pneumonia. J. Clin. Med. 2021, 10, 1154. [Google Scholar] [CrossRef]
- Diaz, M.H.; Winchell, J.M. Detection of Mycoplasma pneumoniae and Chlamydophila pneumoniae directly from respiratory clinical specimens using a rapid real-time polymerase chain reaction assay. Diagn. Microbiol. Infect. Dis. 2012, 73, 278–280. [Google Scholar] [CrossRef]
- Bjarnason, A.; Westin, J.; Lindh, M.; Andersson, L.-M.; Kristinsson, K.G.; Löve, A.; Baldursson, O.; Gottfredsson, M. Incidence, Etiology, and Outcomes of Community-Acquired Pneumonia: A Population-Based Study. Open Forum Infect. Dis. 2018, 5, ofy010. [Google Scholar] [CrossRef]
- Nisar, N.; Guleria, R.; Kumar, S.; Chawla, T.C.; Biswas, N.R. Mycoplasma pneumoniae and its role in asthma. Postgrad. Med. J. 2007, 83, 100–104. [Google Scholar] [CrossRef][Green Version]
- Hong, S.-J. The Role of Mycoplasma pneumoniae Infection in Asthma. Allergy Asthma Immunol. Res. 2012, 4, 59–61. [Google Scholar] [CrossRef]
- Raeven, V.M.; Spoorenberg, S.M.C.; Boersma, W.G.; van de Garde, E.M.W.; Cannegieter, S.C.; Voorn, G.P.P.; Bos, W.J.W.; van Steenbergen, J.E.; On behalf of the Alkmaar study group; On behalf of the Ovidius study group. Atypical aetiology in patients hospitalised with community-acquired pneumonia is associated with age, gender and season; a data-analysis on four Dutch cohorts. BMC Infect. Dis. 2016, 16, 299. [Google Scholar] [CrossRef]
- Momiyama, Y.; Ohmori, R.; Taniguchi, H.; Nakamura, H.; Ohsuzu, F. Association of Mycoplasma pneumoniae infection with coronary artery disease and its interaction with chlamydial infection. Atherosclerosis 2004, 176, 139–144. [Google Scholar] [CrossRef]
- Abdul-Wahab, O.M.S.; Alshehri, A.H.; Assiri, A.S.; Almasswary, A.; Benahmed, F. Association of Mycoplasma pneumoniae Infection with Ischemic Heart Diseases. Am. J. Immunol. 2012, 8, 117–122. [Google Scholar] [CrossRef]
- Weiss, T.; Schumacher, S.; Kaun, C.; Katsaros, K.; Trøseid, M.; Huber, K.; Maurer, G.; Wojta, J. Association between smoking and presence of Mycoplasma pneumoniae in circulating leukocytes. Swiss Med. Wkly. 2010, 140, w13105. [Google Scholar] [CrossRef]
- Kornum, J.B.; Thomsen, R.W.; Riis, A.; Lervang, H.-H.; Schønheyder, H.C.; Sørensen, H.T. Type 2 Diabetes and Pneumonia Outcomes: A population-based cohort study. Diabetes Care 2007, 30, 2251–2257. [Google Scholar] [CrossRef]
- Brunetti, V.C.; Ayele, H.T.; Yu, O.H.Y.; Ernst, P.; Filion, K.B. Type 2 diabetes mellitus and risk of community-acquired pneumonia: A systematic review and meta-analysis of observational studies. CMAJ Open 2021, 9, E62–E70. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Corica, B.; Tartaglia, F.; D’Amico, T.; Romiti, G.F.; Cangemi, R. Sex and gender differences in community-acquired pneumonia. Intern. Emerg. Med. 2022, 17, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Papaetis, G.; Anastasakou, E.; Tselou, T.; Sotiriou, A.; Rarra, V.; Roussou, P.; Karakatsani, A.; Orphanidou, D. Serological evidence of Mycoplasma pneumoniae infection in patients with acute exacerbation of COPD: Analysis of 100 hospitalizations. Adv. Med. Sci. 2010, 55, 235–241. [Google Scholar] [CrossRef]
- Baskaran, V.; Murray, R.L.; Hunter, A.; Lim, W.S.; McKeever, T.M.; Ho, P.L. Effect of tobacco smoking on the risk of developing community acquired pneumonia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0220204. [Google Scholar] [CrossRef]
- Kariya, C.; Chu, H.W.; Huang, J.; Leitner, H.; Martin, R.J.; Day, B.J. Mycoplasma pneumoniae infection and environmental tobacco smoke inhibit lung glutathione adaptive responses and increase oxidative stress. Infect. Immun. 2008, 76, 4455–4462. [Google Scholar] [CrossRef]
- Guo, Z.-Q.; Gu, S.-Y.; Tian, Z.-H.; Du, B.-Y. A comprehensive review of Mycoplasma pneumoniae infection in chronic lung diseases: Recent advances in understanding asthma, COPD, and bronchiectasis. Front. Med. 2024, 11, 1437731. [Google Scholar] [CrossRef]
- Yeh, J.-J.; Lin, C.-L.; Kao, C.-H.; Plavec, D. Associations among chronic obstructive pulmonary disease with asthma, pneumonia, and corticosteroid use in the general population. PLoS ONE 2020, 15, e0229484. [Google Scholar] [CrossRef]
- Centers for Disease Prevention and Control. Mycoplasma pneumoniae Infection Surveillance and Trends. November 2024. Available online: https://www.cdc.gov/mycoplasma/php/surveillance/index.html (accessed on 11 January 2025).
- Hausmann, L.R.; Ibrahim, S.A.; Mehrotra, A.; Nsa, W.; Bratzler, D.W.; Mor, M.K.; Fine, M.J. Racial and ethnic disparities in pneumonia treatment and mortality. Med. Care 2009, 47, 1009–1017. [Google Scholar] [CrossRef]
- Donaldson, S.V.; Thomas, A.N.; Gillum, R.F.; Mehari, A. Geographic Variation in Racial Disparities in Mortality From Influenza and Pneumonia in the United States in the Pre-Coronavirus Disease 2019 Era. Chest 2021, 159, 2183–2190. [Google Scholar] [CrossRef]
- Nowalk, M.P.; Wateska, A.R.; Lin, C.J.; Schaffner, W.; Harrison, L.H.; Zimmerman, R.K.; Smith, K.J. Racial Disparities in Adult Pneumococcal Vaccination Indications and Pneumococcal Hospitalizations in the U.S. J. Natl. Med. Assoc. 2019, 111, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Kumar, N.; Malik, Y.S.; Everett, D.; Saluja, D.; Launey, T.; Kaushik, R. Critical insights from recent outbreaks of Mycoplasma pneumoniae: Decoding the challenges and effective interventions strategies. Int. J. Infect. Dis. 2024, 147, 107200. [Google Scholar] [CrossRef]
- Kutty, P.K.; Jain, S.; Diaz, M.H.; Self, W.H.; Williams, D.; Zhu, Y.; Grijalva, C.G.; Edwards, K.M.; Wunderink, R.G.; Winchell, J.; et al. Clinical and Epidemiologic Features of Mycoplasma pneumoniae Infection Among Adults Hospitalized with Community-acquired Pneumonia. Int. J. Med. Sci. 2024, 21, 3003–3009. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-Acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
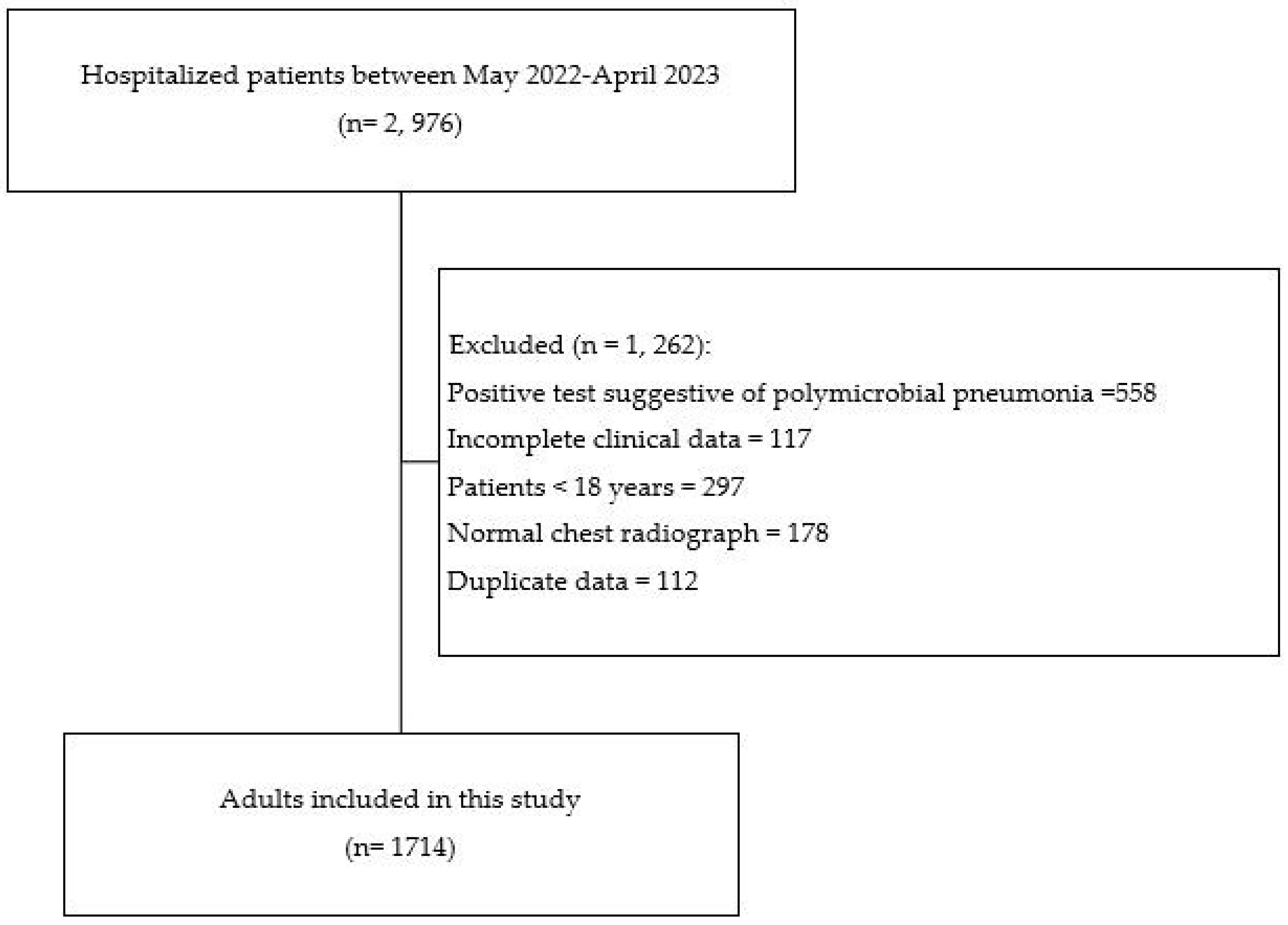
| Inclusion | Exclusion |
|---|---|
|
|
|
|
|
| Total | Mycoplasma Pneumonia Positive | Mycoplasma Pneumonia Negative | ||
|---|---|---|---|---|
| Variables | (n = 1714) | (n = 297) | (n = 1417) | p-value |
| N (%) | N (%) | N (%) | ||
| Race | 0.0013 | |||
| White non-Hispanic | 1294 (75.50) | 199 (67.00) | 1095 (77.28) | |
| Hispanics | 88 (5.13) | 24 (8.08) | 64 (4.52) | |
| Black non-Hispanic | 324 (18.90) | 73 (24.58) | 251 (17.71) | |
| Asian | 8 (0.47) | 1 (0.34) | 7 (0.49) | |
| Sex | 0.0127 | |||
| Male | 805 (46.97) | 120 (40.40) | 685 (48.34) | |
| Female | 909 (53.03) | 177 (59.60) | 732 (51.66) | |
| Age | <0.0001 | |||
| 18–34 years | 82 (4.78) | 33 (11.11) | 49 (3.46) | |
| 35–54 years | 294 (17.15) | 69 (23.23) | 225 (15.88) | |
| 55–64 years | 364 (21.24) | 60 (20.20) | 304 (21.45) | |
| 65–79 years | 638 (37.22) | 100 (33.67) | 538 (37.97) | |
| 80–99 years | 336 (19.60) | 35 (11.78) | 301 (21.24) | |
| Season | 0.0652 | |||
| Respiratory season | 975 (56.88) | 156 (52.53) | 819 (57.80) | |
| Non-respiratory season | 739 (43.12) | 141 (47.47) | 598 (42.20) | |
| Asthma | 0.2555 | |||
| Present | 362 (21.12) | 70 (23.57) | 292 (20.61) | |
| Absent | 1352 (78.88) | 227 (76.43) | 1125 (79.39) | |
| Cancer | 0.7153 | |||
| Present | 118 (6.88) | 19 (6.40) | 99 (6.99) | |
| Absent | 1596 (93.12) | 278 (93.60) | 1318 (93.01) | |
| Tobacco use | 0.0275 | |||
| Current user | 486 (28.35) | 88 (29.63) | 398 (28.09) | |
| Former user | 584 (34.07) | 96 (32.32) | 488(34.44) | |
| Non-user | 644 (37.57) | 113 (38.05) | 531 (37.47) | |
| Stroke | 0.4519 | |||
| Present | 303 (17.68) | 57 (19.19) | 246 (17.36) | |
| Absent | 1411 (82.32) | 240 (80.81) | 1171 (82.64) | |
| Coronary artery disease | 0.0068 | |||
| Present | 504 (29.40) | 68 (22.90) | 436 (30.77) | |
| Absent | 1210 (70.60) | 229 (77.10) | 981 (69.23) | |
| Chronic kidney disease | 0.2402 | |||
| Present | 593 (34.60) | 94 (31.65) | 499 (35.22) | |
| Absent | 1121 (65.40) | 203 (68.35) | 918 (64.78) | |
| Chronic obstructive pulmonary disease | 0.2041 | |||
| Present | 802 (46.79) | 136 (45.79) | 666 (47.00) | |
| Absent | 912 (53.21) | 161 (54.21) | 751 (53.00) | |
| Diabetes | 0.6396 | |||
| Present | 667 (38.91) | 112 (37.71) | 555 (39.17) | |
| Absent | 1047 (61.09) | 185 (62.29) | 862 (60.83) | |
| Heart Failure | 0.3744 | |||
| Present | 709 (41.37) | 116 (39.06) | 593 (41.85) | |
| Absent | 1005 (58.63) | 181 (60.94) | 824 (58.15) | |
| Hyperlipidemia | 0.0016 | |||
| Present | 1155 (67.39) | 177 (59.60) | 978 (69.02) | |
| Absent | 559 (32.61) | 120 (40.40) | 439 (30.98) | |
| Hypertension | <0.0001 | |||
| Present | 1416 (82.61) | 221 (74.41) | 1195 (84.33) | |
| Absent | 298 (17.39) | 76 (25.59) | 222 (15.67) |
| Variables | COR (95% CI) | AOR (95% CI) |
|---|---|---|
| Race | ||
| Hispanics | 2.06 (1.26–3.38) | 1.60 (1.13–2.74) |
| Black non-Hispanic | 1.60 (1.18–2.16) | 1.50 (1.09–2.08) |
| Asian | 0.80 (0.10–6.42) | 0.69 (0.08–6.34) |
| White non-Hispanic | Reference | Reference |
| Sex | ||
| Male | 0.73 (0.56–0.93) | 0.69 (0.52–0.89) |
| Female | Reference | Reference |
| Age | ||
| 18–34 years | Reference | Reference |
| 35–54 years | 0.46 (0.27–0.76) | 0.51 (0.29–0.89) |
| 55–64 years | 0.29 (0.17–0.49) | 0.32 (0.17–0.60) |
| 65–79 years | 0.28 (0.17–0.45) | 0.32 (0.17–0.57) |
| 80–99 years | 0.17 (0.10–0.30) | 0.20 (0.10–0.40) |
| Season | ||
| Non-respiratory season | 1.24 (1.19–1.60) | 1.25 (1.10–1.61) |
| Respiratory season | Reference | Reference |
| Asthma | ||
| Present | 1.19 (0.88–1.50) | 1.03 (0.75–1.41) |
| Absent | Reference | Reference |
| Cancer | ||
| Present | 1.00 (0.55–1.51) | 1.01 (0.60–1.70) |
| Absent | Reference | Reference |
| Tobacco use | ||
| Non-user | 0.78 (0.38–0.72) | 0.64 (0.48–0.75) |
| Former | 0.89 (0.64- 0.88) | 0.72 (0.69–0.85) |
| Current | Reference | Reference |
| Stroke | ||
| Present | 1.13 (0.82–1.37) | 1.40 (0.99–1.95) |
| Absent | Reference | Reference |
| Coronary artery disease | ||
| Present | 0.67 (0.50–0.87) | 0.83 (0.60–1.16) |
| Absent | Reference | Reference |
| Chronic kidney disease | ||
| Present | 0.90 (0.65–1.11) | 1.02 (0.75–1.39) |
| Absent | Reference | Reference |
| Chronic obstructive pulmonary disease | ||
| Present | 1.05 (0.93–1.22) | 1.25 (0.95–1.45) |
| Absent | Reference | Reference |
| Diabetes | ||
| Present | 1.00 (0.72–1.21) | 1.07 (0.81–1.44) |
| Absent | Reference | Reference |
| Heart failure | ||
| Present | 0.90 (0.69–1.15) | 1.06 (0.79–1.43) |
| Absent | Reference | Reference |
| Hyperlipidemia | ||
| Present | 0.66 (0.51–0.86) | 0.87 (0.64–1.18) |
| Absent | Reference | Reference |
| Hypertension | ||
| Present | 0.54 (0.40–0.72) | 0.74 (0.51–1.08) |
| Absent | Reference | Reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoli, M.L.; Okoli, I.C.; Okoli, A.C.; Umezurike, I.; Ishiekwene, C. Independent Predictors of Mycoplasma pneumoniae Infection: A Retrospective Cohort Study Among Hospitalized Adults in an East Texas Health Facility. J. Respir. 2025, 5, 13. https://doi.org/10.3390/jor5030013
Okoli ML, Okoli IC, Okoli AC, Umezurike I, Ishiekwene C. Independent Predictors of Mycoplasma pneumoniae Infection: A Retrospective Cohort Study Among Hospitalized Adults in an East Texas Health Facility. Journal of Respiration. 2025; 5(3):13. https://doi.org/10.3390/jor5030013
Chicago/Turabian StyleOkoli, Menkeoma Laura, Ibuchim Chinemerem Okoli, Abuoma Chisom Okoli, Ikechukwu Umezurike, and Celestine Ishiekwene. 2025. "Independent Predictors of Mycoplasma pneumoniae Infection: A Retrospective Cohort Study Among Hospitalized Adults in an East Texas Health Facility" Journal of Respiration 5, no. 3: 13. https://doi.org/10.3390/jor5030013
APA StyleOkoli, M. L., Okoli, I. C., Okoli, A. C., Umezurike, I., & Ishiekwene, C. (2025). Independent Predictors of Mycoplasma pneumoniae Infection: A Retrospective Cohort Study Among Hospitalized Adults in an East Texas Health Facility. Journal of Respiration, 5(3), 13. https://doi.org/10.3390/jor5030013







