Utilizing Spent Yeast for Tannin Adsorption in Chestnut Shell Treatment Solutions †
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.1.1. Chestnut Shell Tannin Extract
2.1.2. Biosorbent
2.2. Biosorption Studies
2.2.1. Kinetic Assays
2.2.2. Equilibrium Assays
2.3. Analysis by UV-VIS Spectrometry
2.4. Analysis by Fourier Transform Infrared Spectroscopy (FT-IR)
2.5. Statistical Analysis
3. Results and Discussion
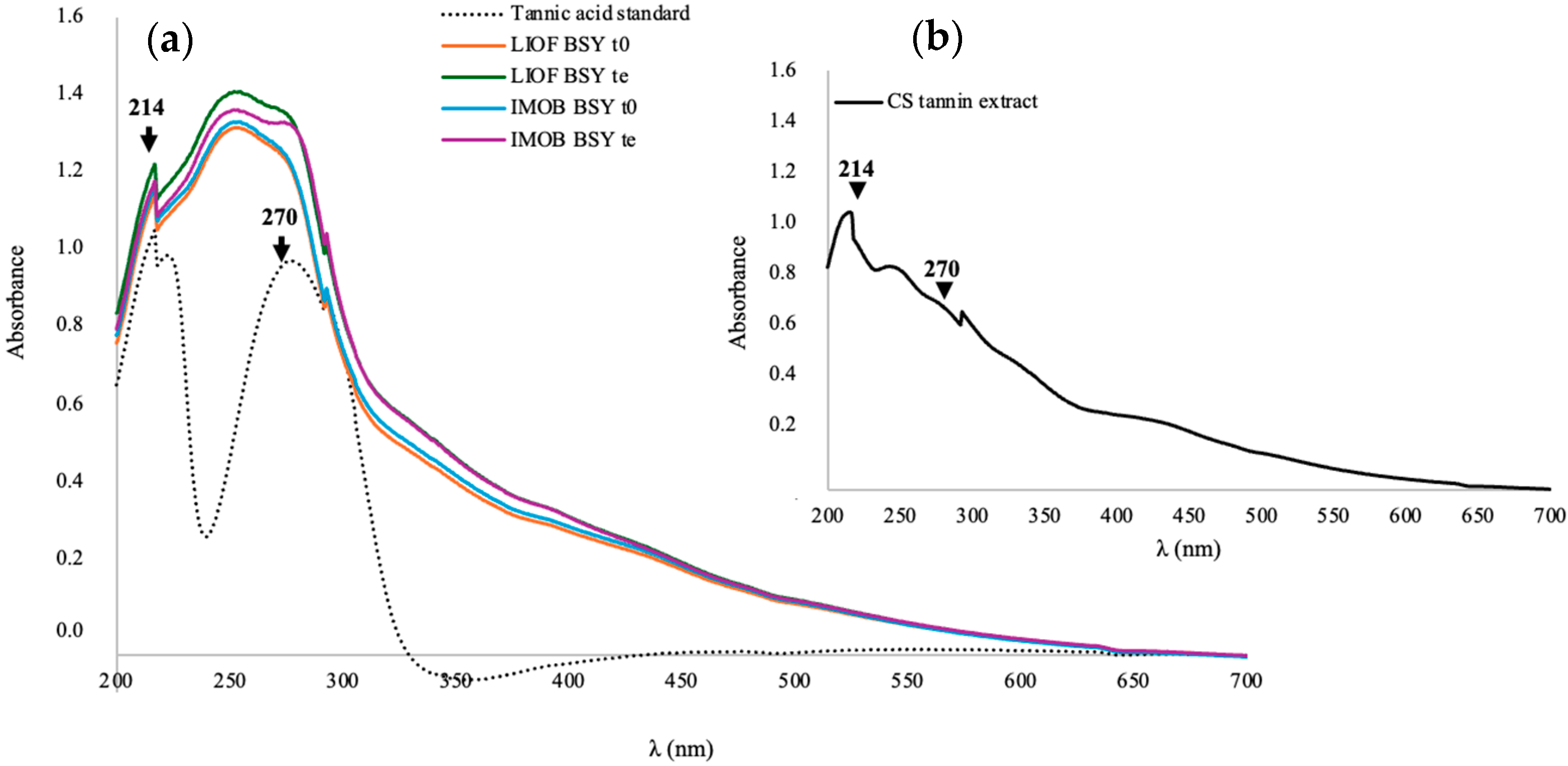
3.1. Characterization of CS Tannin Extract
3.2. Kinetic Studies
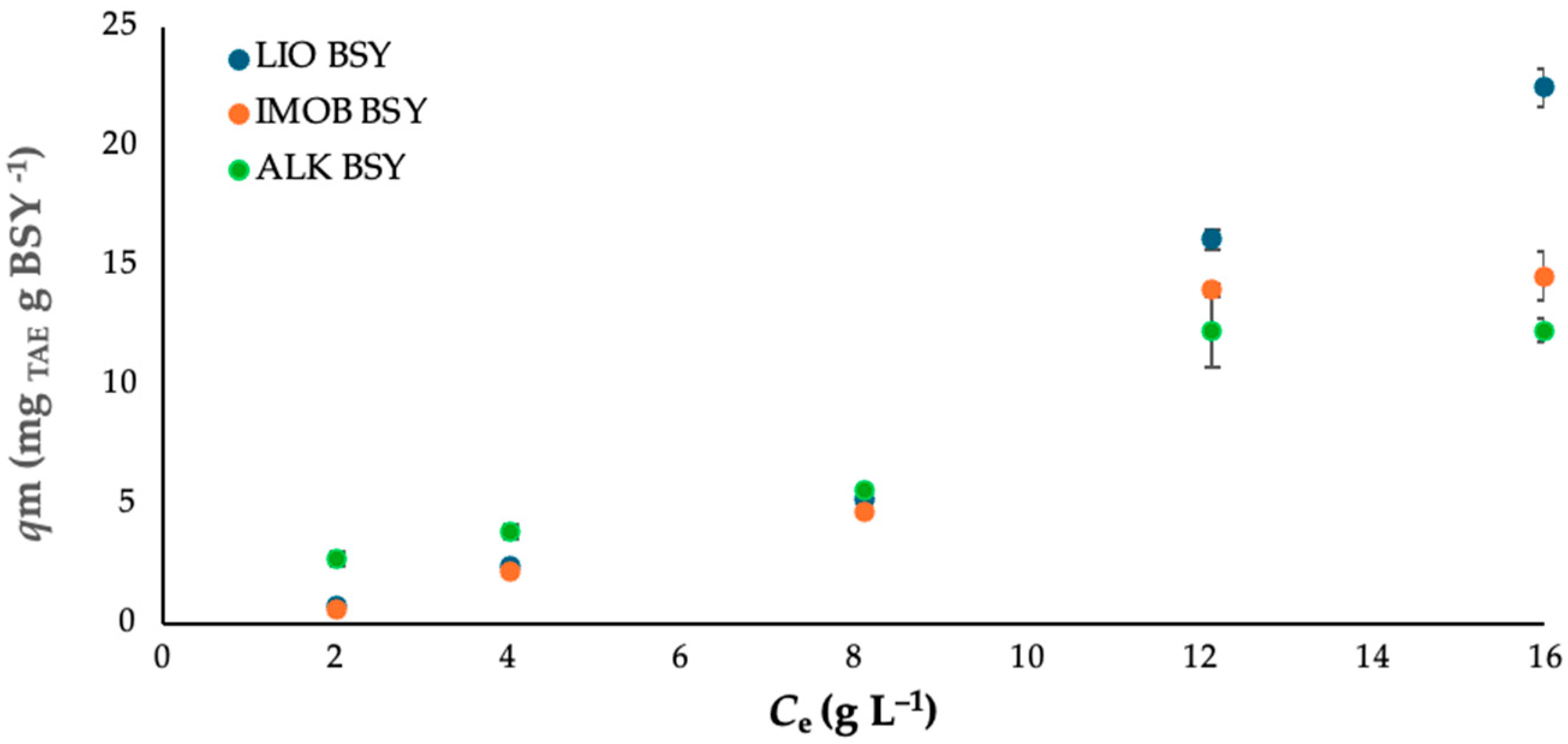
3.3. Equilibrium Studies
3.4. UV-VIS Spectrometry
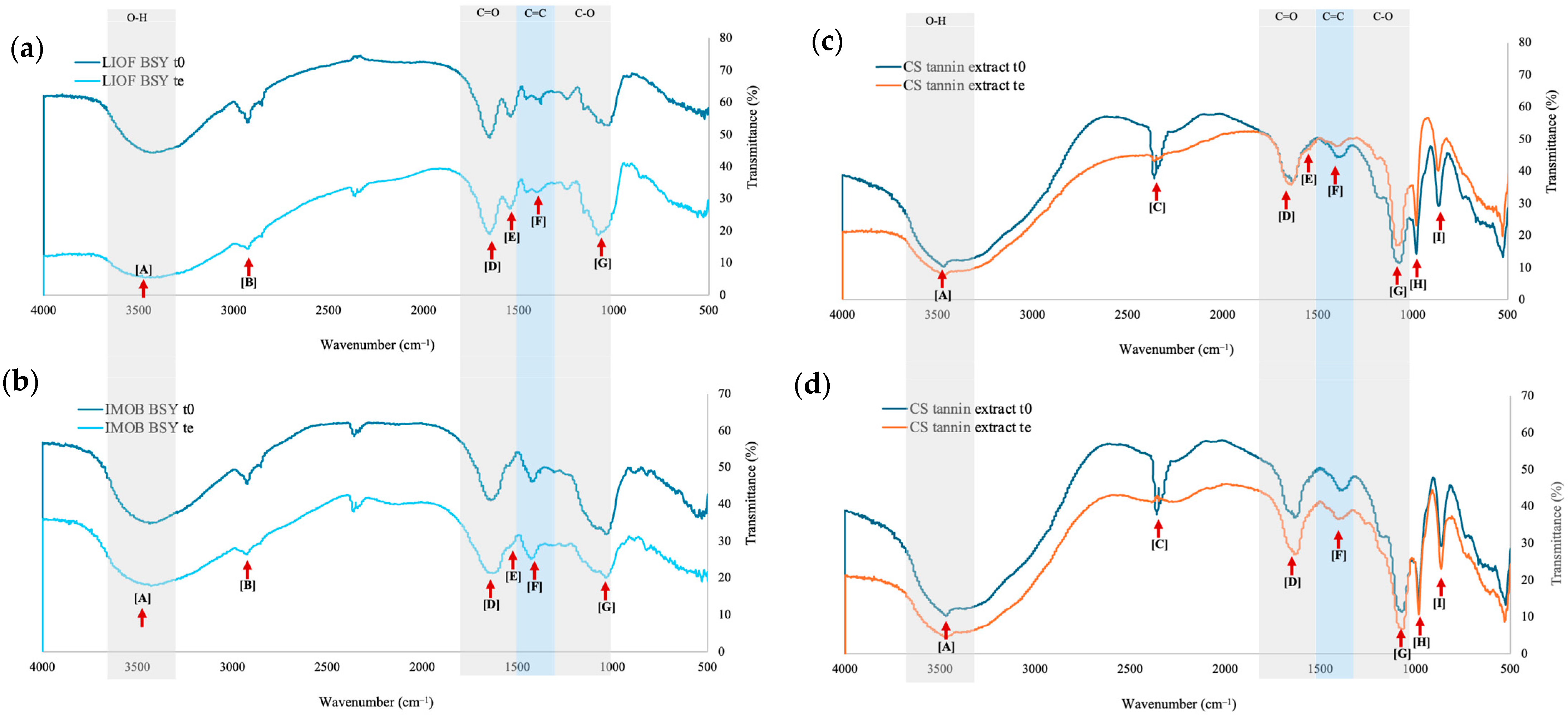
3.5. FT-IR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Ribeiro, V.R.; Fernandes, I.D.A.A.; Mari, I.P.; Stafussa, A.P.; Rossetto, R.; Maciel, G.M.; Haminiuk, C.W.I. Bringing together Saccharomyces cerevisiae and bioactive compounds from plants: A new function for a well-known biosorbent. J. Funct. Foods 2019, 60, 103433. [Google Scholar] [CrossRef]
- Jilani, H.; Cilla, A.; Barberá, R.; Hamdi, M. Biosorption of green and black tea polyphenols into Saccharomyces cerevisiae improves their bioaccessibility. J. Funct. Foods 2015, 17, 11–21. [Google Scholar] [CrossRef]
- Ribeiro, V.R.; Maciel, G.M.; Fachi, M.M.; Pontarolo, R.; Fernandes I de, A.A.; Stafussa, A.P.; Haminiuk, C.W.I. Improvement of phenolic compound bioaccessibility from yerba mate (Ilex paraguariensis) extracts after biosorption on Saccharomyces cerevisiae. Food Res. Int. 2019, 126, 108623. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, R.; Maciel, G.M.; Bortolini, D.G.; Ribeiro, V.R.; Haminiuk, C.W.I. Acai pulp and seeds as emerging sources of phenolic compounds for enrichment of residual yeasts (Saccharomyces cerevisiae) through biosorption process. LWT-Food Sci. Technol. 2020, 128, 109447. [Google Scholar] [CrossRef]
- Maroldi, W.V.; Maciel, G.M.; Rossetto, R.; Bortolini, D.G.; Fernandes I de, A.A.; Haminiuk, C.W.I. Biosorption of phenolic compounds from Plinia cauliflora seeds in residual yeast: Kinetic, equilibrium, and bioaccessibility studies. J. Food Process Preserv. 2022, 46, e17156. [Google Scholar] [CrossRef]
- Jilani, H.; Cilla, A.; Barberá, R.; Hamdi, M. Improved bioaccessibility and antioxidant capacity of olive leaf (Olea euro-paea L.) polyphenols through biosorption on Saccharomyces cerevisiae. Ind. Crops Prod. 2016, 84, 131–138. [Google Scholar] [CrossRef]
- Rubio, F.T.V.; Maciel, G.M.; da Silva, M.V.; Corrêa, V.G.; Peralta, R.M.; Haminiuk, C.W.I. Enrichment of waste yeast with bioactive compounds from grape pomace as an innovative and emerging technology: Kinetics, isotherms and bioaccessibility. Innov. Food Sci. Emerg. Technol. 2018, 45, 18–28. [Google Scholar] [CrossRef]
- Mirmahdi, R.S.; Mahoozi, T.; Zoghi, A.; Montazeri, N.; Khosravi-Darani, K. The roles of Saccharomyces cerevisiae on the bioaccessibility of phenolic compounds. World J. Microbiol. Biotechnol. 2024, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Maros, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Rodrigues, F.; Silva, A.; Costa, P.; Delerue-Matos, C.; Vieira, E.F. Reinforcement of starch film with Castanea sativa shells polysaccharides: Optimized formulation and characterization. Food Chem. 2022, 396, 133609. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.; Gúllon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Aimone, C.; Grillo, G.; Boffa, L.; Giovando, S.; Cravotto, G. Tannin extraction from chestnut wood waste: From lab scale to semi-industrial plant. Appl. Sci. 2023, 13, 2494. [Google Scholar] [CrossRef]
- De Rossi, A.; Rigueto, C.V.T.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Synthesis, characterization, and application of Saccharomyces cerevisiae/alginate composites beads for adsorption of heavy metals. J. Environ. Chem. Eng. 2020, 8, 104009. [Google Scholar] [CrossRef]
- Stafussa, A.P.; Maciel, G.M.; Da Silva Anthero, A.G.; Da Silva, M.V.; Zielinski, A.A.F.; Haminiuk, C.W.I. Biosorption of anthocyanins from grape pomace extracts by waste yeast: Kinetic and isotherm studies. J. Food Eng. 2016, 169, 53–60. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
| Model | Parameters | LIOF BSY | IMOB BSY | ALK BSY | ACID BSY |
|---|---|---|---|---|---|
| Pseudo 1st Order | qe exp. (mg TAE g BSY−1) | 22.87 ± 0.48 | 13.92 ± 0.29 | 22.87 ± 0.48 | 7.96 ± 0.56 |
| qe (mg TAE g BSY−1) | 23.01 ± 0.41 | 14.02 ± 0.10 | 16.01 ± 0.12 | — | |
| k1 (g BSY mg TAE−1 min−1) | 2.51 ± 0.50 | 1.80 ± 0.76 | 2.52 ± 0.12 | — | |
| Adjusted R2 | 0.9335 | 0.8424 | 0.8426 | 0.2231 | |
| Pseudo 2nd Order | qe exp. (mg TAE g BSY−1) | 22.96 ± 0.08 | 13.58 ± 0.05 | 16.96 ± 0.08 | 7.09 ± 0.04 |
| qe (mg TAE g BSY−1) | 23.00 ± 0.05 | 13.60 ± 0.15 | 16.0 ± 0.05 | — | |
| k2 (g BSY mg TAE−1 min−1) | 5.0 ± 0.40 | 5.5 ± 2.04 | 5.2 ± 0.76 | — | |
| Adjusted R2 | 0.9672 | 0.9368 | 0.8991 | 0.6533 | |
| Elovich | α (g BSY mg TAE−1 min−1) | 1.0 × 1021 ± 1.0 × 108 | 2.1 × 1017 ± 0.97 | 2.3 × 1012 ± 0.66 | — |
| β (mg TAE g BSY−1) | 2.3 ± 0.63 | 3.1 ± 060 | 2.3 ± 0.65 | — | |
| Adjusted R2 | 0.9120 | 0.8806 | 0.8526 | 0.0250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, E.F.; Amaral, T.; Ferraz, R.; Delerue-Matos, C. Utilizing Spent Yeast for Tannin Adsorption in Chestnut Shell Treatment Solutions. Eng. Proc. 2025, 87, 21. https://doi.org/10.3390/engproc2025087021
Vieira EF, Amaral T, Ferraz R, Delerue-Matos C. Utilizing Spent Yeast for Tannin Adsorption in Chestnut Shell Treatment Solutions. Engineering Proceedings. 2025; 87(1):21. https://doi.org/10.3390/engproc2025087021
Chicago/Turabian StyleVieira, Elsa F., Tomás Amaral, Ricardo Ferraz, and Cristina Delerue-Matos. 2025. "Utilizing Spent Yeast for Tannin Adsorption in Chestnut Shell Treatment Solutions" Engineering Proceedings 87, no. 1: 21. https://doi.org/10.3390/engproc2025087021
APA StyleVieira, E. F., Amaral, T., Ferraz, R., & Delerue-Matos, C. (2025). Utilizing Spent Yeast for Tannin Adsorption in Chestnut Shell Treatment Solutions. Engineering Proceedings, 87(1), 21. https://doi.org/10.3390/engproc2025087021








