Discrimination of Different Human Cell Lines by Using FT-IR Spectra Spectroscopy †
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line Growth Conditions
2.2. FT-IR Spectroscopy Measurements
2.3. Data Analysis
3. Results and Discussion
3.1. FT-IR Spectra Analysis of SH-SY5Y, HepG2, and MCF-10A Cell Lines
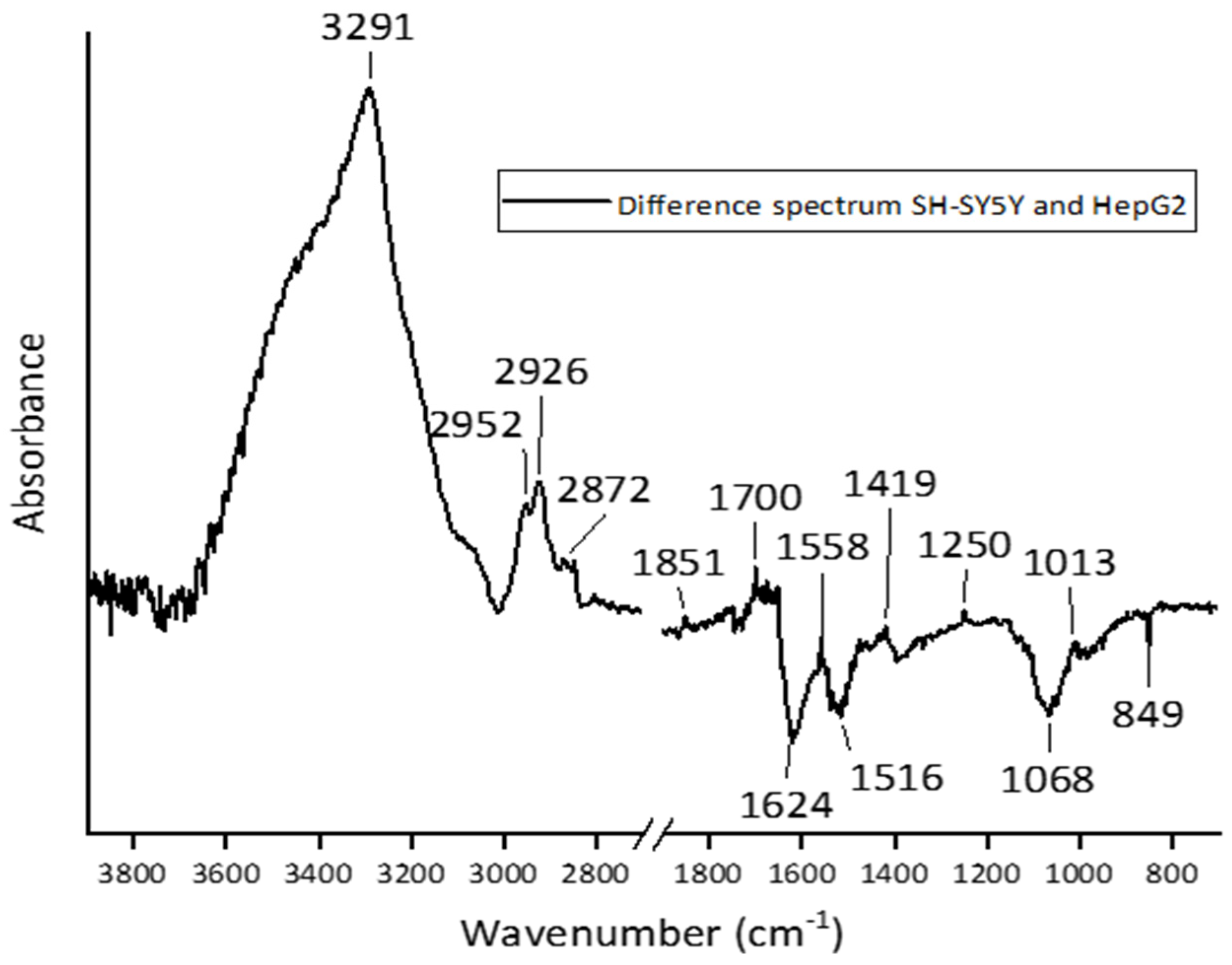
3.2. Comparative Analysis of Cell Lines
3.3. Analysis of Difference Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lasalvia, M.; Capozzi, V.; Perna, G. Classification of healthy and cancerous colon cells by Fourier transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 321, 124683. [Google Scholar] [CrossRef] [PubMed]
- Elshemey, W.M.; Ismail, A.M.; Elbialy, N.S. Molecular-level characterization of normal, benign, and malignant breast tissues using FTIR spectroscopy. J. Med. Biol. Eng. 2016, 36, 369–378. [Google Scholar] [CrossRef]
- Zendehdel, R.; Shirazi, F.H. Discrimination of human cell lines by infrared spectroscopy and mathematical modeling. Iran. J. Pharm. Res. IJPR 2015, 14, 803. [Google Scholar] [PubMed]
- Wang, Y.; Dai, W.; Liu, Z.; Liu, J.; Cheng, J.; Li, Y.; Li, X.; Hu, J.; Lü, J. Single-cell infrared microspectroscopy quantifies dynamic heterogeneity of mesenchymal stem cells during adipogenic differentiation. Anal. Chem. 2020, 93, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-R.; Hu, L.-S.; Li, G.-Y. SH-SY5Y human neuroblastoma cell line: In vitrocell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar] [PubMed]
- López-Terrada, D.; Cheung, S.W.; Finegold, M.J.; Knowles, B.B. Hep G2 is a hepatoblastoma-derived cell line. Hum. Pathol. 2009, 40, 1512. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Silva, S.; Duarte, D.; Crista, D.M.; da Silva, L.P.; da Silva, J.C.E. Normal breast epithelial MCF-10A cells to evaluate the safety of carbon dots. RSC Med. Chem. 2021, 12, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lasch, P. Spectral pre-processing for biomedical vibrational spectroscopy and microspectroscopic imaging. Chemom. Intell. Lab. Syst. 2012, 117, 100–114. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Ricciardi, V.; Portaccio, M.; Piccolella, S.; Manti, L.; Pacifico, S.; Lepore, M. Study of SH-SY5Y cancer cell response to treatment with polyphenol extracts using FT-IR spectroscopy. Biosensors 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Gasper, R.; Dewelle, J.; Kiss, R.; Mijatovic, T.; Goormaghtigh, E. IR spectroscopy as a new tool for evidencing antitumor drug signatures. Biochim. Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Delfino, I.; Ricciardi, V.; Manti, L.; Lasalvia, M.; Lepore, M. Multivariate analysis of difference raman spectra of the irradiated nucleus and cytoplasm region of SH-SY5Y human neuroblastoma cells. Sensors 2019, 19, 3971. [Google Scholar] [CrossRef] [PubMed]
- Lorenz-Fonfria, V.A. Infrared difference spectroscopy of proteins: From bands to bonds. Chem. Rev. 2020, 120, 3466–3576. [Google Scholar] [CrossRef] [PubMed]
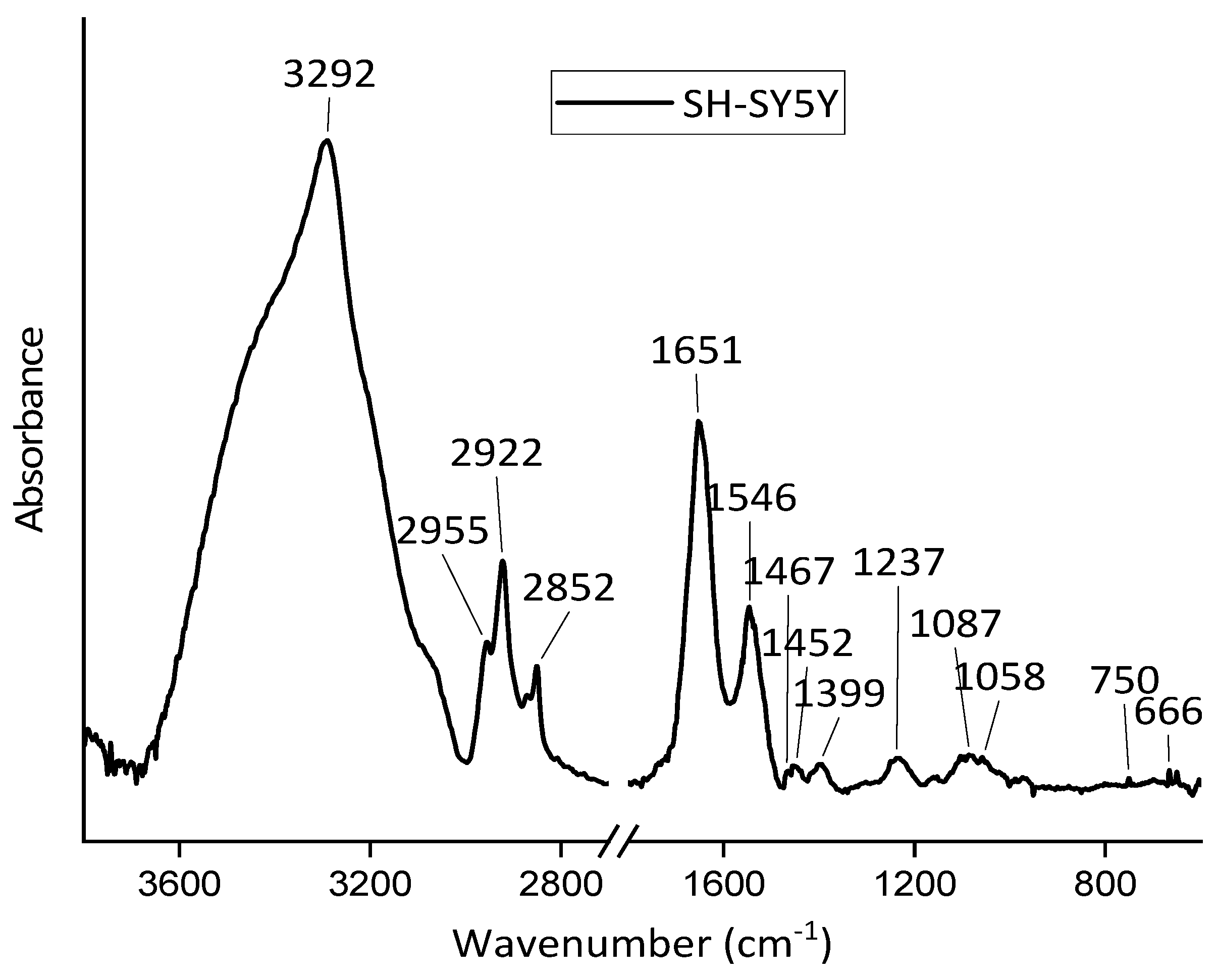
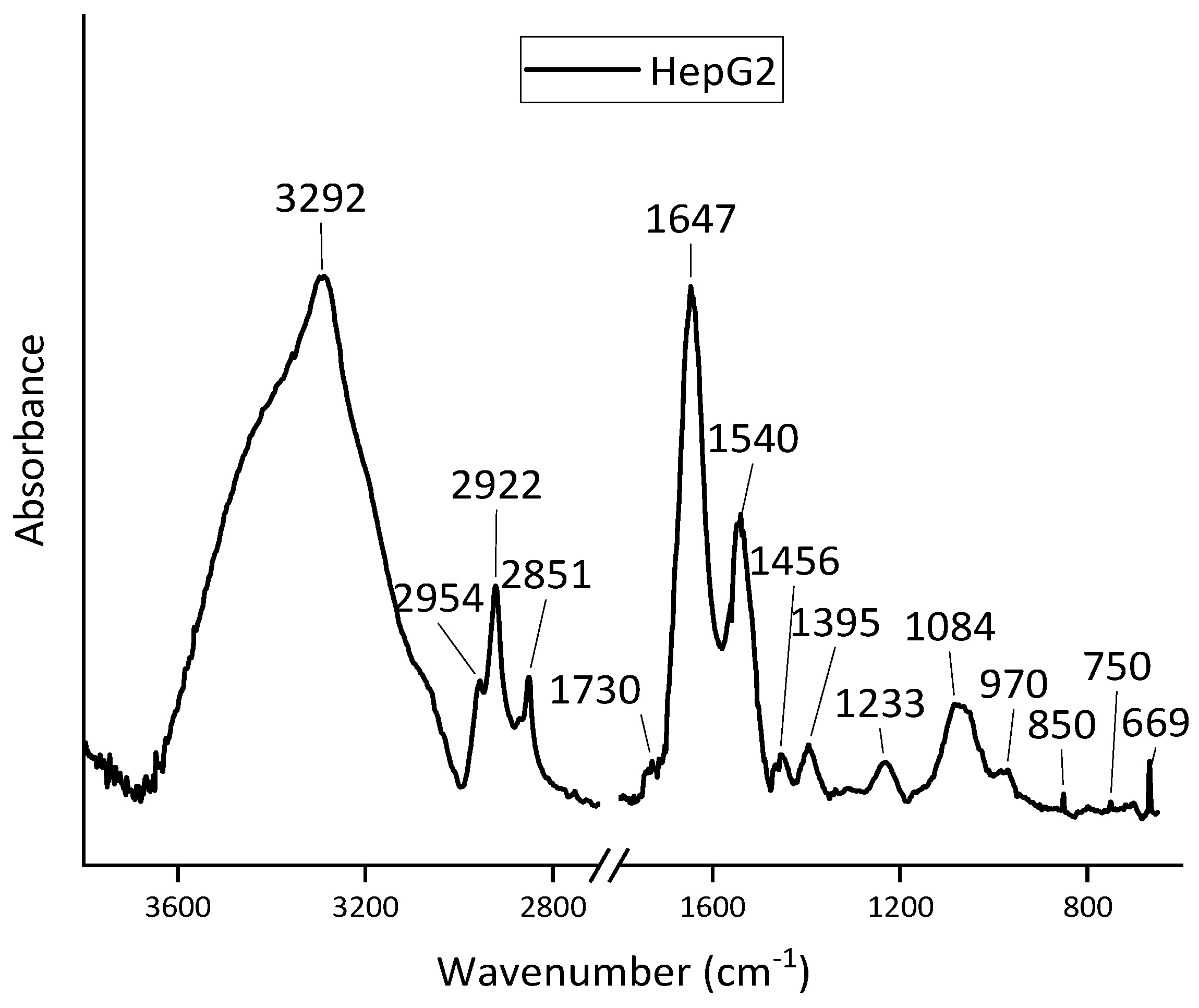
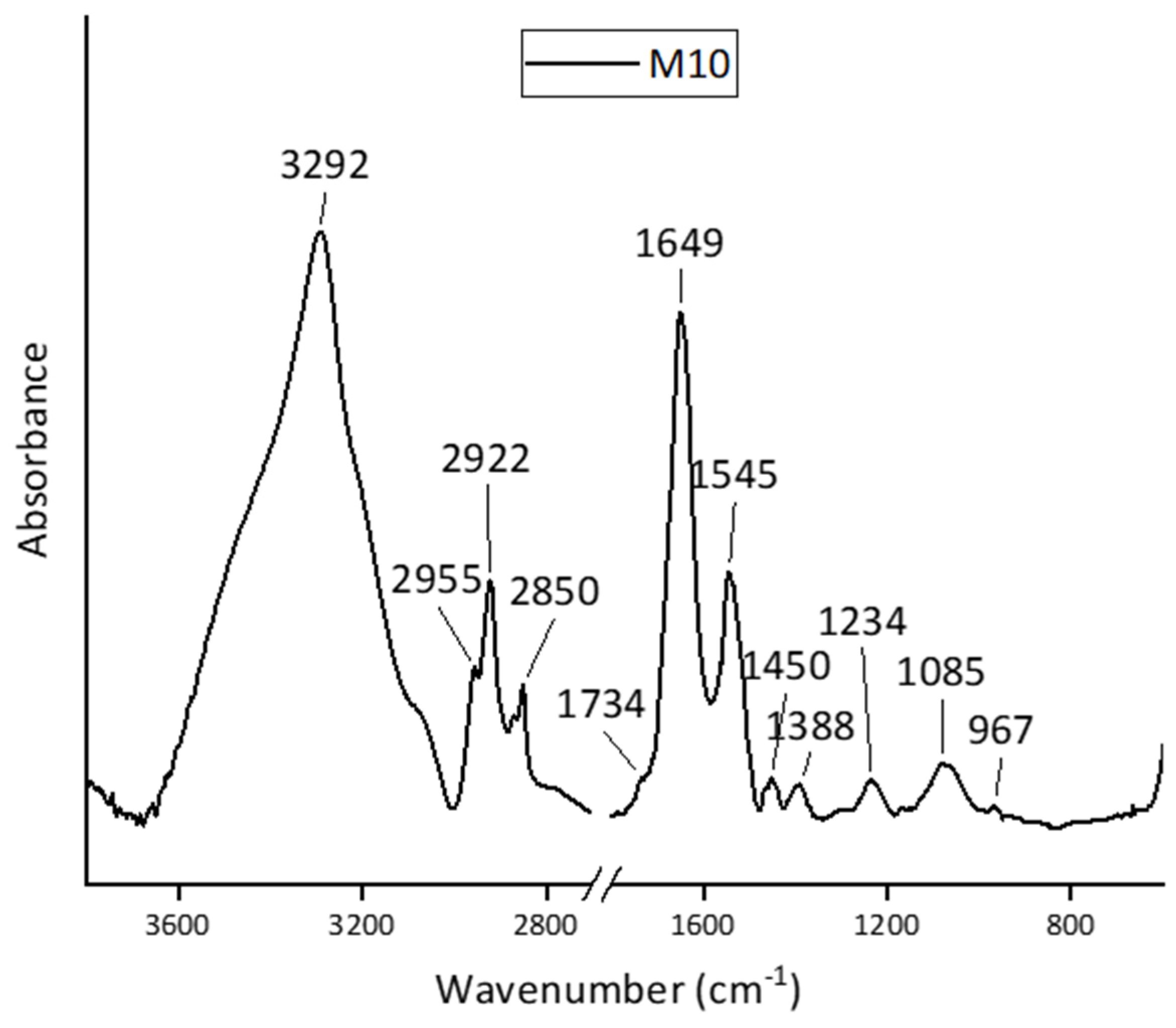


| SH-SY5Y | HepG2 | MCF-A10 | Assignments | |||
|---|---|---|---|---|---|---|
| Peaks (cm−1) | Peaks (cm−1) | Peaks (cm−1) | DNA/RNA | Protein | Lipids | Carbohydrate |
| 3292 | 3292 | 3292 | Amide A () | (O– v) | ||
| 2955 | 2954 | 2955 | CH3 as. v | CH3 as. v | ||
| 2922 | 2922 | 2922 | CH2 as. v | |||
| 2852 | 2851 | 2850 | CH2 s. v | |||
| - | 1730 | 1734 | () | () | ||
| 1651 | 1647 | 1649 | Amide I () | |||
| 1546 | 1540 | 1545 | Amide II | |||
| 1467 | - | - | CH2 | CH2 | ||
| - | 1456 | - | CH3 as. | CH3 as. | ||
| 1452 | - | 1450 | CH3 as. | |||
| 1399 | - | - | CH3 s. | CH3 s. | ||
| - | 1395 | - | CH3 s. | |||
| - | - | 1388 | COO− s. v | COO− s. v | ||
| 1237 | 1233 | 1234 | as. v | Amide III | ||
| 1170 | - | - | CO as. v | |||
| 1087 | 1084 | 1085 | s. v | |||
| 1058 | - | - | CO v | |||
| - | 970 | 967 | CO s. v, s. v | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faramarzi, B.; Portaccio, M.; Manti, L.; Falco, M.D.; Iezzi, M.; Lepore, M. Discrimination of Different Human Cell Lines by Using FT-IR Spectra Spectroscopy. Eng. Proc. 2024, 82, 54. https://doi.org/10.3390/ecsa-11-20499
Faramarzi B, Portaccio M, Manti L, Falco MD, Iezzi M, Lepore M. Discrimination of Different Human Cell Lines by Using FT-IR Spectra Spectroscopy. Engineering Proceedings. 2024; 82(1):54. https://doi.org/10.3390/ecsa-11-20499
Chicago/Turabian StyleFaramarzi, Bahar, Marianna Portaccio, Lorenzo Manti, Maria Daniela Falco, Manuela Iezzi, and Maria Lepore. 2024. "Discrimination of Different Human Cell Lines by Using FT-IR Spectra Spectroscopy" Engineering Proceedings 82, no. 1: 54. https://doi.org/10.3390/ecsa-11-20499
APA StyleFaramarzi, B., Portaccio, M., Manti, L., Falco, M. D., Iezzi, M., & Lepore, M. (2024). Discrimination of Different Human Cell Lines by Using FT-IR Spectra Spectroscopy. Engineering Proceedings, 82(1), 54. https://doi.org/10.3390/ecsa-11-20499








