Abstract
In agriculture, the use of fructans has gained relevance due to their ability to improve plant immunity and resistance to pathogens. However, many studies use high-purity fructans, which makes their application more expensive. In this work, the efficacy of two agave fructans, one food grade from Agave tequilana Weber var. Azul (FT) and the other obtained by semi-craft extraction from A. cupreata (FC) were evaluated in comparison with reagent-grade inulin from dahlia tubers. The effectiveness of their defense response against Phytophthora capsici infection in tomato (Solanum lycopersicum L.) was analyzed by evaluating defense mechanisms, including lignin deposition, hydrogen peroxide (H2O2) accumulation, and β-1,3-glucanase and peroxidase activity. The results indicated that foliar application of both fructans showed protection against infection, reducing disease incidence and severity. FT fructans at lower concentration (0.5 g/L) showed the highest protection, followed by FC, while dahlia inulin showed lower effectiveness. An early and progressive accumulation of H2O2 was observed in fructan-treated plants, in contrast to the late increase in untreated infected plants. Also, peroxidase activity was higher in the fructan treatments, suggesting a more efficient defense response. Although lignin deposition was not directly correlated with protection against P. capsici, fructans showed potential as resistance inducers. Given their low cost, easy extraction, and zero environmental impact, agave fructans represent a viable alternative for crop protection in sustainable agricultural systems. This study opens the door to their validation in the field and their application in other economically important crops, contributing to biological control strategies with less dependence on agrochemicals.
1. Introduction
Plant pathogens pose a challenge to food production because they cause enormous crop losses, which are aggravated by climate change. Plant diseases affect all components of the agricultural production chain, including production, distribution, availability, quality, and nutritional value [1,2,3], thereby putting food security at risk.
Phytophthora is an oomycete with more than 120 described species, exhibiting a worldwide distribution and causing destructive effects on a wide range of plants important to agriculture and natural ecosystems [4,5,6]. For example, Phytophthora capsici Leonian is a destructive soil-borne pathogen with a wide host range, including solanaceous, cucurbit, and fabaceous crops [7,8]. In tomato (Solanum lycopersicum L.), one of the main crops in the world, this pathogen causes root, crown, and fruit rot [9,10]. In Mexico, production is estimated to average 3.681 million tons per year, making it the eighth largest tomato producer and the leading exporter worldwide [11]. Due to its importance, its production methods are highly demanding, as it is a crop susceptible to a large number of pests and diseases. Among these diseases, the control of P. capsici is difficult and economically costly, in addition to the environmental impact caused by the excessive use of fungicides and chemical products. This is without neglecting integrated management, which includes cultural, genetic, chemical, and biological control [8,12].
There are new strategies for plant disease control that are not classified as chemical or biological control, known as biorationals. These are chemical, polymeric, or peptide compounds that facilitate recognition between plants and pathogens, acting as signaling molecules, phytohormones, or biostimulants. They function as a kind of “vaccine”, activating the plant’s defense system and triggering effective responses against infections or signaling processes, which generate a resistance response. These molecules are referred to as plant defense inducers, resistance inducers, or elicitors [13,14].
This alternative approach to disease management involves stimulating the plant by using compounds of various origins, such as inorganic salts, microorganisms, or biomolecules like fructans, which can induce plant defense through systemic acquired resistance or systemic induced resistance, thereby ensuring an efficient and rapid response to pathogen attack. Defense inducers offer different benefits; by acting on the plant, they can protect against different pathosystems without generating a significant environmental impact or inducing resistance in the pathogen [15].
Fructans are complex molecules composed of fructose chains linked together, exhibiting various shapes and structures that influence their biological activity. It has been reported that the application of agave fructans (Agave tequilana Weber var. Azul) with varying degrees of polymerization and concentrations in chili bell pepper crops reduces the incidence and severity of damage caused by P. capsici infection [16].
The use of inulin has had positive consequences in stimulating defense systems in plants. It has been demonstrated that the application of inulin from dahlia tubers to the roots of chili bell pepper plants provides protection against infection caused by P. capsici [17]. Additionally, it has been reported that the protective effect of dahlia inulin against P. capsici is achieved through the induction of an effective defense response in chili bell pepper [18]. López-Velázquez et al. [18] evaluated the protective effect of dahlia inulin against P. capsici on chili bell pepper plants, analyzing four different concentrations. It was found that inulin at 200 μM induced an effective defense response, increasing the activity of β-1,3-glucanases and peroxidases, which favored seedling resistance. This protection was manifested both locally and systemically, differing from untreated infected plants. However, the use of high-purity inulin increased application costs, suggesting the possibility of using other fructans of lower cost and purity with similar effects.
Fructans, which are long chains of fructose, are present in plant tissues as energy stores that can be easily isolated and recovered, allowing their effects on the plant to be analyzed. For example, the extraction of agave fructans from different species and collection sites has shown that their structural characteristics offer distinct benefits in their use and application [19]. Agave fructans are commercialized in Mexico and in the world under the trade name of agave inulin, mainly extracted from A. tequilana; although, fructans from A. salmiana can also be found, and their extraction and use as stimulants of the human immune system has been studied [20,21]. Commercial fructans of A. tequilana have been extensively characterized [22], and new studies are being conducted to characterize fructans of agave from other species, including semi-craft extraction methods that small producers can carry out [19]. With this premise, the evaluation and validation of fructans, obtained with more economical procedures, as plant defense inducers means a substantial advance for their application at a practical and commercial level.
In this work, the effectiveness of two types of agave fructans is evaluated for their potential in protecting tomato plants against Phytophthora capsici. The first fructan was a commercially available food-grade extract from Agave tequilana Weber var. Azul, while the second was a fructan derived from Agave cupreata using a semi-craft extraction method [19]. These agave fructans were compared to inulin extracted from dahlia tubers [18] to assess their ability to induce a biochemical defense response in tomato plants. The evaluation involved measuring the enzymatic activity of β-1,3-glucanases and peroxidases, determining the percentage of disease incidence in the plants, and conducting histochemical analyses to detect lignin and peroxide deposits in the tomato leaves.
2. Materials and Methods
2.1. Plant Material
Seeds of the tomato (Solanum lycopersicum L.), specifically the Rio Grande variety (Semillas Fax de Occidente S.A. de C.V., of USA origin), were used. The seeds were immersed for 3 min in distilled water and then placed in trays with a sterile commercial substrate composed of a 7:3 (v/v) ratio of peat moss and vermiculite for germination.
2.2. Biological Material
The Phytophthora capsici strain used was from the CIATEJ strain collection, specifically the P. capsici strain (CH11), which was provided and isolated in 2011 by Dr. Sylvia Fernández Pavía from the Instituto de Investigaciones Agropecuarias Forestales of the Universidad Michoacana San Nicolás de Hidalgo [23]. Culture and inoculum generation conditions were the same as described by López-Velázquez et al. [17].
2.3. Clarified V8 Culture Medium
V8 juice (Campbell’s) was utilized as the base for the culture medium. To eliminate pulp residues, the juice was clarified by filtration using 22 mm filter paper. Subsequently, 50 mL of the clarified juice was combined with 0.5 g of calcium carbonate (Sigma-Aldrich, St. Louis, MO, USA) and 15 g of agar (Sigma-Aldrich, St. Louis, MO, USA) in distilled water. The mixture was thoroughly agitated to ensure homogeneity and brought to a final volume of 1 L with distilled water. The prepared medium was then sterilized by autoclaving at 121 °C for 20 min under a pressure of 14 psi [24].
2.4. Dahlia Inulin Solution
Reagent-grade dahlia tuber inulin solutions (Sigma-Aldrich, St. Louis, MO, USA) were prepared at a concentration of 0.05 g/L [18] in distilled water. Subsequently, it was filtered through a 0.2 microns membrane for sterilization (Table 1).

Table 1.
Physicochemical properties of inulin and agave fructans.
2.5. Agave Fructan Solutions
A. cupreata fructans from Chilapa, Guerrero, were extracted, as reported by Camacho-Ruíz et al. [19]. Fructans of A. tequilana Weber var. Azul of food and commercial grade has the denomination “Organic Agave Inulin” (NB FOODS S. de R.L. de C.V., Zapopan, Jalisco, Mexico). Solutions of agave fructans were prepared at two concentrations: 1 and 0.5 g/L. Subsequently, the solutions were filtered through a 0.2 micron membrane for sterilization (Table 1).
2.6. Degree of Deacetylation by Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of solid samples of dahlia inulin and agave fructans were obtained using a GX System FTIR spectrophotometer (Perkin Elmer, Shelton, CT, USA) coupled to a DuraSamplIR II ATR accessory. All spectra were obtained between 4000 and 400 cm−1 with a resolution of 0.5 cm−1. For ATR spectrum acquisition, each sample was placed on the ATR crystal. The glass was cleaned with acetone after each spectrum was obtained. The spectra were obtained in triplicate with 24 scans and a resolution of 4 cm−1 and were normalized with an ordinate limit of up to 1.0 absorbance [25].
2.7. Assessment of Protection Against P. capsici Infection in Tomato
Ten 60-day-old tomato seedlings per treatment were transplanted into 275 mL polystyrene cups with sterile commercial substrate composed of peat moss and vermiculite in a 7:3 (v/v) ratio. After transplanting, 8 mL of each treatment was applied by spraying on the leaves. After 5 days from the first application of the treatments, a second application was made, and immediately thereafter, the root seedlings were inoculated with 8 mL of a P. capsici zoospore suspension (1 × 104 zoospores/mL) [26].
The experiment ended one week after inoculation, and a photographic record was taken. The severity of the symptoms was then determined at that time. The level of protection against the disease was determined considering the following disease severity scale, which was proposed based on the symptoms observed throughout the experiment: (1) healthy seedling, without evident disease symptoms; (2) Seedlings with few leaves affected with small lesions and partial chlorosis; (3) seedling with chlorosis, darkening of the stem and roots, and wilting of the leaves; (4) seedling with leaf damage, necrosis of the stem and roots, short roots, severe defoliation, and smaller size than the control; and (5) dead seedling. A randomized block experimental design with 10 replications each was used. Controls consisted of seedlings sprayed with sterile distilled water, serving as the negative control, and seedlings inoculated only with P. capsici, serving as the positive control. The experiment was performed in duplicate, simultaneously, and in separate spaces.
2.8. Histochemical Evaluation of Defense Induction in Tomato Plants
Leaf samples were taken at the time of inoculation (0 h) and after 1, 3, 5, 6, and 9 days after inoculation with the pathogen (daip). The leaves sampled were placed in a fixative solution in 25 mL conical tube.
2.8.1. Determination of Cell Wall Lignification
The leaf was immersed for 2 h in 2% (w/v) phloroglucinol diluted in ethanol (96% v/v). Subsequently, the leaf was placed on a slide, and 2 to 3 drops of 35% (v/v) HCl were added until the tissue was covered. Immediately afterward, the sample was flamed until a change in the color of the tissue to reddish-purple was observed, and observation was made under an optical microscope (Olympus, model BH2-RFCA, Lake Success, NY, USA) [27].
2.8.2. Determination of Hydrogen Peroxide Accumulation
The leaf was immersed for 6 h in a solution of 3,3-diaminobenzidine (DAB) at pH 3.8, 25 °C, and under light conditions. Subsequently, the DAB was removed, and 96% (v/v) ethanol was added, followed by boiling for 10 min. Subsequently, the ethanol was discarded, and 96% ethanol was added again, leaving the samples to stand for 4 h. Finally, the samples were observed under the optical microscope [28].
2.9. Biochemical Assessment of Defense Induction in Plants
Leaves were taken from the plants at the time of induction, on the day of inoculation with the pathogen (considered as day 0), and at days 1, 3, 6, and 9 daip. The enzymatic activities of β-1,3-glucanases and peroxidases, as well as pathogenesis-related proteins (PR), were determined. Samples were macerated with liquid nitrogen and re-suspended in 0.1 M sodium phosphate buffer pH 7.0.
2.9.1. Determination of the Activity of β-1,3-Glucanases
It was performed using the colorimetric method for the detection of reducing sugars at 515 nm as a product of enzymatic hydrolysis and reduction in DNS (3,5-dinitrosalicylic acid, Sigma-Aldrich, St. Louis, MO, USA). Briefly, 100 µL of enzyme extract was mixed with 100 µL of 0.5% laminarin (Sigma Life Science, Merck KGaA, Darmstadt, Germany) in 1 mL Eppendorf tubes. The mixture was incubated in a water bath at 55 °C for 5 min and immediately cooled on ice. Subsequently, 300 µL of DNS reagent was added, and the solution was heated again at 55 °C for 12 min. After cooling on ice, 500 µL of distilled water was added, and the solution was vortexed to ensure homogenization. After a 15 min rest at room temperature, 200 µL of the reaction mixture was transferred to a 96-well microplate for absorbance measurement at 515 nm. Quantification was performed using a standard curve of glucose. Specific enzyme activity was expressed in nanokatals (nkat) per gram of total protein (nkat/g TP), where one nanokatal (1 nkat) corresponds to the enzymatic release of 1 nmol of D-glucose per second from laminarin under the assay conditions [29].
2.9.2. Determination of Peroxidase Activity (POX)
Peroxidase activity was determined according to the method described by Oliveira et al. [29]. In a 96-well microplate, 10 µL of leaf extract was mixed with 200 µL of guaiacol (Sigma-Aldrich, St. Louis, MO, USA), 12.5 µL of distilled water, and 25 µL of hydrogen peroxide (Sigma-Aldrich, St. Louis, MO, USA). The absorbance was recorded immediately after the addition of hydrogen peroxide and again after three minutes, using a wavelength of 490 nm. Enzyme activity was expressed in activity units (AU), defined as the change in one absorbance unit per minute, and normalized to total protein content (AU/g TP).
2.10. Statistical Analysis
A single-factor analysis of variance (ANOVA) was performed using Minitab Statistical Software (version 22.1.0, Pennsylvania State University, USA), which allowed for the observation of statistical differences using the Fisher test and LSD Fisher test with a significance level of α = 0.05.
3. Results
3.1. Degree of Deacetylation by Fourier Transform Infrared Spectroscopy (FTIR)
FTI-R spectroscopy was performed to analyze the spectra of dahlia inulin and fructans from Agave tequilana Wever var. Azul and A. cupreata to compare their structural composition, where we observed a significant similarity between the fructan from A. tequilana and A. cupreata. The spectrum of dahlia inulin reveals the presence of O–H groups and the configuration of C–O, C–C, and C–H bonds, characteristic of this compound [30], which differs from the structure of fructans (Figure 1).
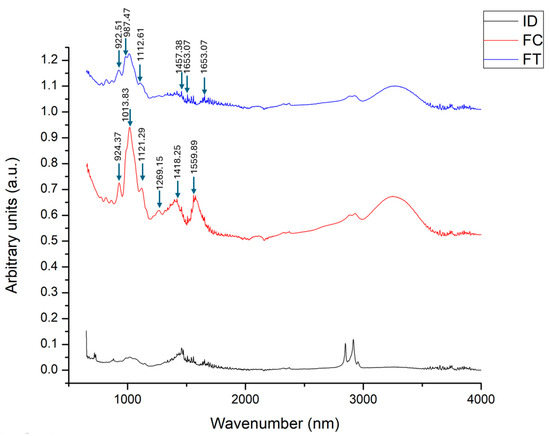
Figure 1.
FTIR spectra of inulin and agave fructans. ID: Dahlia inulin, FC: Agave cupreata fructans, FT: Agave tequilana fructans.
The bands present in the fructans of Agave cupreata (FC) for the region 1559.89, where it differs from the fructans of A. tequilana (FT), are associated with the symmetrical stretching of the N–C–O atomic bond system belonging to the type II amide and with N–H bending. In the 1000 cm−1 region, where a coincidence in the peak of both fructans is observed, it can be assigned to C–O stretching, carbohydrates with bending in the O–H plane, and bending in the C–H plane. The band corresponding to 1013.83 cm−1 is related to the C–O–C bonds of the intra- and intermonosaccharide rings. The vibration band at 1269.15 cm−1, shown by FC, corresponds to the vibrations of the C–H and C–O bonds [25,31]. The band at 1559.89 may refer to the presence of C=O. The differences between agaves can be attributed to the degree of polymerization and the agave species.
3.2. Application of Dahlia and Agave Fructans to Tomato Plants as Protection Against P. capsici
To determine the degree of protection in tomato plants against P. capsici infection by applying agave fructans and dahlia tubers, a biological effectiveness test was conducted through the reduction in disease symptoms and incidence. Additionally, to validate that this protection was associated with the induction of an effective defense response, histochemical tests related to Phytophthora resistance, H2O2 accumulation, and lignin deposition in leaves were performed. The evaluation of the biochemical resistance mechanism determined the enzymatic activity of β-1,3-glucanases and peroxidases.
The results obtained in the biological effectiveness test (Figure 2B) showed that the plants inoculated with the pathogen (P) began to exhibit disease symptoms from day 3 after pathogen inoculation (daip), with a disease severity level of 2 and 3, with an incidence of 62.5%. These levels increased to 100% incidence after 6 daip, with disease severity levels 4 and 5. capsici (ID+P) showed symptoms from 3 daip and an incidence of 75%, with a disease severity of levels 2 and 3. However, this incidence was maintained at 6 daip, and 100% incidence was reached at 9 daip, although disease severity was lower (levels 3 and 4) than that observed in diseased plants. On the other hand, plants treated with FC fructans exhibited a protective effect, which varied depending on the concentration used. Plants treated with a concentration of 0.5 g/L (FC5+P) showed symptoms from 3 daip and an incidence of 75%, with disease severity of levels 2 and 3. This incidence was maintained at day 6 daip and reached 100% incidence until 9 daip, although at a disease severity lower than that observed in diseased plants with a lower percentage of plants with levels 2, 3, and 4. Plants treated with the highest concentration of 1 g/L (FC1+P) also showed symptoms from 3 daip and an incidence of 75% with a disease severity of levels 2 and 3. 100% incidence was reached at 9 daip, with disease severity of levels 2, 3, and 4, a similar response to plants treated with ID. The highest protection was observed with the lowest dose (0.5 g/L).
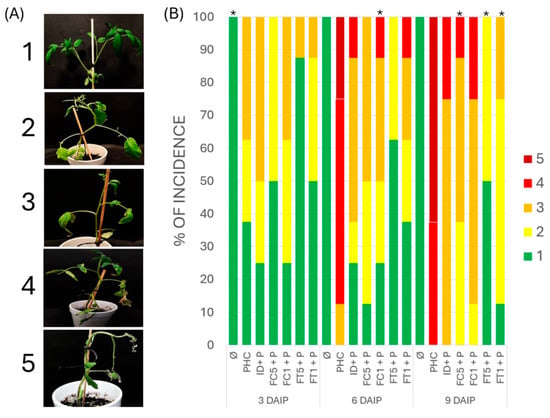
Figure 2.
Evaluation of the biological effectiveness of dahlia and agave fructans in protecting tomato plants against P. capsici infection. (A) Disease severity scale in tomato plants inoculated with P. capsici. (1) Healthy seedling, without evident disease symptoms; (2) Seedlings with few leaves affected with small lesions and partial chlorosis; (3) seedling with chlorosis, darkening of the stem and roots, and wilting of the leaves; (4) seedling with leaf damage, necrosis of the stem and roots, short roots, severe defoliation, and smaller size than the control; and (5) dead seedling. (B) Evaluation of protection against P. capsici infection in tomato. C: Control plants, P: plants inoculated with P. capsici, ID+P: plants inoculated with P. capsici and treated with dahlia inulin (0.05 g/L), FC5+P: plants inoculated with P. capsici and treated with Agave cupreata fructans (0.5 g/L), FC1+P: plants inoculated with P. capsici and treated with A. cupreata fructans (1 g/L), FT5+P: plants inoculated with P. capsici and treated with A. tequilana fructans (0.5 g/L), FT1+P: plants inoculated with P. capsici and treated with A. tequilana fructans (1 g/L). * Indicate significant differences with respect to P using the FISHER test with a significance level of α = 0.05.
Plants treated with FT fructans also showed a protective effect, depending on the concentration used, which was greater than that observed with FC fructans. Plants treated with the concentration of 0.5 g/L (FT5+P) showed symptoms from 3 daip, with 10% incidence and disease severity of level 3. This incidence increased to 40% at 6 daip, with a disease severity of level 2. Plants treated with the highest concentration of 1 g/L (FT1+P) showed symptoms from 3 daip, an incidence of 50%, and a disease severity of levels 2 and 3. This incidence increased by 60% at 6 daip and at disease severity levels 2, 3, and 4. The 0.5 g/L concentration of FT fructan was the treatment that showed the highest protection against P. capsici infection in the biological effectiveness test.
3.3. Mechanism of Defense Induction in Tomato Plants by the Application of Dahlia and Agave Fructans
Two defense mechanisms associated with resistance against P. capsici were evaluated through histochemical tests: lignin deposition [27] and H2O2 accumulation in tomato leaves [28]. Lignin deposits were observed as reddish-purple spots along the cell wall (Figure 3A). Sampling was performed on different days; however, differences in lignin accumulation were observed on 9 daip. Plants treated with A. cupreata fructans at a concentration of 0.5 g/L (FC5+P) presented the maximum lignin accumulation. Similar results were observed in plants treated with 0.5 g/L of A. tequilana fructans (FT5+P) compared to the controls (C and P). Treatments with higher cell lignification showed positive effects in the biological effectiveness test. In particular, plants treated with FT5+P obtained the best protection, also being one of the treatments with the highest degree of lignification. Plants treated with dahlia inulin showed an increase and thickening of cell walls but did not show the same protection effects.
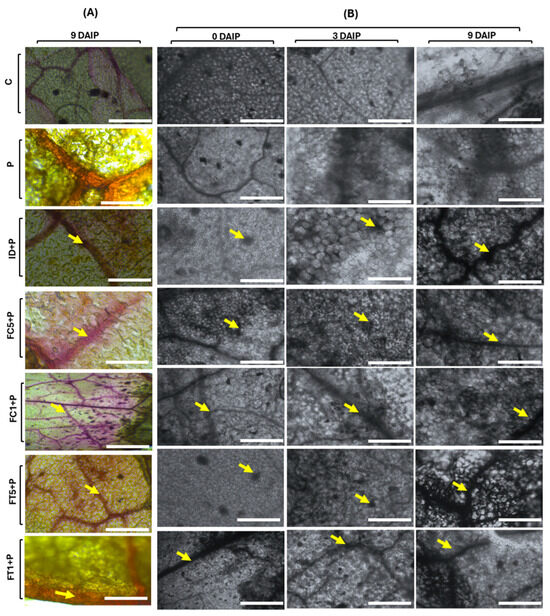
Figure 3.
Histochemical evaluation of defense induction in tomato plants by the application of dahlia and agave fructans. (A) Lignin deposition in tomato leaves treated with inulin and fructans. (B) Accumulation of hydrogen peroxide in tomato leaves treated with inulin and fructans. C: Control plants, P: plants inoculated with P. capsici, ID+P: plants inoculated with P. capsici and treated with dahlia inulin (0.05 g/L), FC5+P: plants inoculated with P. capsici and treated with Agave cupreata fructans (0.5 g/L), FC1+P: plants inoculated with P. capsici and treated with A. cupreata fructans (1 g/L), FT5+P: plants inoculated with P. capsici and treated with A. tequilana fructans (0.5 g/L), FT1+P: Plants inoculated with P. capsici and treated with A. tequilana fructans (1 g/L). Arrows indicate the accumulation of lignin or hydrogen peroxide. Scale is 25 µm.
For H2O2 accumulation in plants infected with P. capsici (P) (Figure 3B), a slight increase in H2O2 accumulation was observed starting at 6 daip and continued increase up to 9 daip. For plants treated with dahlia inulin (ID+P), a slight accumulation of H2O2 was observed starting at 3 daip, increasing by 6 daip, and a large accumulation by 9 daip. For plants treated with A. cupreata fructans (FC+P) at both concentrations, a progressive accumulation of H2O2 was observed starting at 3 daip. However, the concentration lower than 0.5 g/L (FC5+P) showed the highest accumulation of H2O2. For plants treated with A. tequilana fructans (FT+P) at both concentrations, a progressive accumulation of H2O2 was observed after 3 daip. In general, the application of fructans resulted in an increase in H2O2 accumulation in tomato leaves, occurring more rapidly than in infected plants.
3.4. Enzyme Activity in Tomato Plants by Application of Dahlia and Agave Fructans
For the biochemical defense response, the activity of β-1,3-glucanases and peroxidases, enzymes associated with inducing defense against Phytophthora in tomato [29], was evaluated.
The β-1,3-glucanase activity of tomato seedling leaves infected with P. capsici increased up to 9 daip with respect to the control (Figure 4). For their part, in dahlia inulin-treated seedlings infected with P. capsici (ID+P), there were no significant differences with respect to diseased plants (P) until 9 daip, when a decrease in activity was observed with respect to P. As for plants treated with FC5+P fructans, there was a decrease in activity with respect to infected plants (P) at 1 daip. This activity increased to the same level of the pathogen at 3 daip and was maintained until 6 daip to increase the activity to 9 daip finally, although significantly lower than infected plants. For plants treated with FC1+P fructans, activity increased significantly on the day of pathogen inoculation and decreased at 1 daip; from this time on, activity behaved similarly to the FC5+P treatment. As for the plants treated with FT5+P fructans, there was a decrease in activity with respect to the infected plants at 6 daip, which was maintained until 9 daip. In plants treated with FC1+P fructans, the activity was lower with respect to plants infected at 9 daip. The activity of β-1,3-glucanases in tomato leaves in response to fructan application differed from that in plants infected with P. capsici.
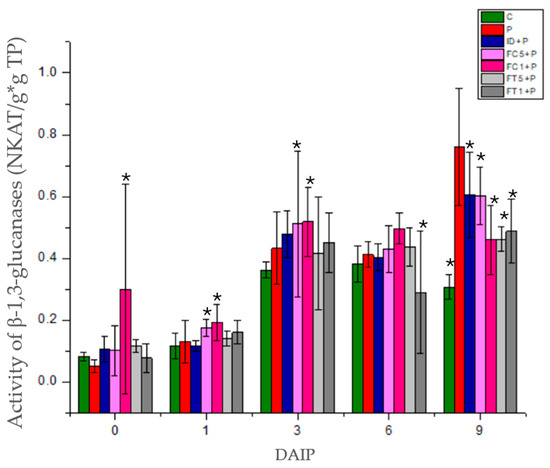
Figure 4.
Evaluation of β-1,3-glucanase activity in tomato seedlings inoculated with P. capsici and treated with dahlia or agave fructans. C: Control plants, P: plants inoculated with P. capsici, ID+P: plants inoculated with P. capsici and treated with dahlia inulin (0.05 g/L), FC5+P: plants inoculated with P. capsici and treated with Agave cupreata fructans (0.5 g/L), FC1+P: plants inoculated with P. capsici and treated with A. cupreata fructans (1 g/L), FT5+P: plants inoculated with P. capsici and treated with A. tequilana fructans (0.5 g/L), FT1+P: plants inoculated with P. capsici and treated with A. tequilana fructans (1 g/L). * Indicate significant differences with respect to P using the LSD FISHER test with a significance level of α = 0.05.
Peroxidase activity was statistically different (p < 0.05) among treatments with respect to plants infected with P. capsici (P) (Figure 5). From 3 daip onwards, peroxidase activity increased significantly in plants infected with P. capsici compared to the control, whereas at 6 daip, the activity returned to the level of the control, which was maintained until 9 daip. On the other hand, the activity of peroxidases in seedlings treated with ID+P decreased significantly at 3 daip with respect to diseased plants; then, it increased to the level of infected plants at 6 daip; finally, the activity had a greater increase at 9 daip. As for FC5+P-treated plants, no significant difference was observed with respect to infected plants at 1, 3, and 6 daip. However, up to 9 daip, the activity increased significantly. For FC1+P-treated plants, activity decreased at 3 daip with respect to diseased plants; subsequently, activity increased to the level of infected plants at 6 daip and was maintained at 9 daip. As for FT5+P-treated plants, activity was significantly reduced at 3 daip compared to diseased plants; however, activity increased at the level of infected plants at 6 daip, ultimately elevating its activity with respect to infected plants at 9 daip. Activity in FC1+P-treated plants decreased at 3 daip compared to diseased plants; then, it increased to the level of infected plants at 6 daip and further increased with respect to infected plants at 9 daip, a behavior similar to that observed with the lowest concentration. The response of tomato plants to fructan application, in terms of leaf peroxidase activity, differed from that observed in plants infected with P. capsici.
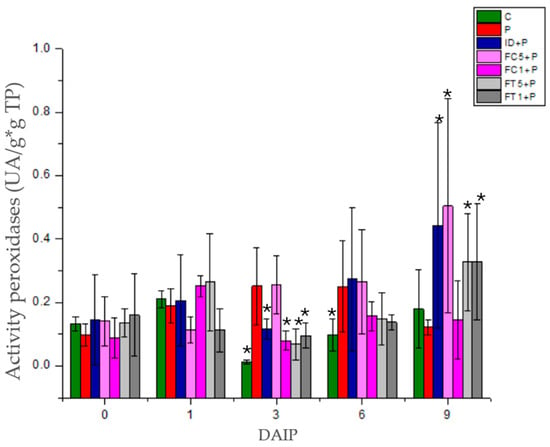
Figure 5.
Peroxidase activity in tomato seedlings inoculated with P. capsici and treated with dahlia inulin or agave fructans. C: Control plants, P: plants inoculated with P. capsici, ID+P: plants inoculated with P. capsici and treated with dahlia inulin (0.05 g/L), FC5+P: Plants inoculated with P. capsici and treated with Agave cupreata fructans (0.5 g/L), FC1+P: plants inoculated with P. capsici and treated with A. cupreata fructans (1 g/L), FT5+P: plants inoculated with P. capsici and treated with A. tequilana fructans (0.5 g/L), FT1+P: plants inoculated with P. capsici and treated with A. tequilana fructans (1 g/L). * Indicate significant differences with respect to P using the LSD FISHER test with a significance level of α = 0.05.
4. Discussion
The application of fructans in agriculture for disease control has gained significant attention due to their role in enhancing plant immunity and resistance against diseases caused by pathogens. Fructans such as inulin, levan, and agavins have been shown to increase plant resistance against various pathogens [16,18,32].
However, most of these studies use high purity fructans in order to associate their physicochemical characteristics with a level of induction for plant protection against phytopathogens. It has been reported that a relationship exists between the priming effect on plants and the chemical structure of fructans, which is influenced by the composition of fructans, particularly their degree of polymerization (PD) and glycosidic bonds. These structural characteristics determine the interaction of fructans with the improving the plant immune system and enhancing their resistance to biotic stress factors. The purity and PD of fructans significantly influence functional applications and their price, mainly due to the cost of extraction and purification of these molecules. Therefore, the use of fructans with specific PD, high purity, and high probiotic or food value complicates their agricultural use. For this reason, this study chooses to evaluate fructans of a low cost, with a simplified extraction process, easy access, or with characteristics that can facilitate their incorporation into agriculture [19,22].
The tomato was chosen because it is a crop of great importance worldwide and is susceptible to different diseases, including infection by Phytophthora sp., such as late blight and early blight [33]. López-Velázquez et al. [17,18] reported that the application of inulin from reagent-grade dahlia tubers at the base of the plant protected against P. capsici infection in chili, decreasing the incidence and reducing disease symptoms in the plant. The use of inulin as a resistance elicitor involved plants having a more effective response in the interaction against P. capsici, resulting in a faster and more efficient response that involved different physiological and biochemical processes. In this study, the protection effect of foliar application of inulin on tomato plants is lower than that reported by López-Velázquez et al. [18] on serrano chili plants. Inulin provided the lowest degree of protection of fructans at the described concentration (0.05 g/L) in the tests carried out for blight on tomato.
Regarding food-grade and simplified or semi-craft extraction of agave fructans, protection against P. capsici infection was observed in both cases, with a decrease in the incidence and severity of the disease at the lowest concentration (0.5 g/L) (Figure 2). The greatest protection was observed with A. tequilana food grade fructans at the lowest concentration (FT5), resulting in a significant 50% decrease in incidence compared to the other treatments. These results show the potential of these fructans to be used as resistance elicitors in agriculture; because of their physicochemical characteristics, they could be ideal in terms of cost and accessibility [34]. The molecular weight (Mv), degree of polymerization (PD), and the ratio between polymerized fructans (PF, PD > 10) and fructooligosaccharides (FOS, with PD of 3–10) of both fructan extracts are very similar (Table 1) [19]. By having a lower proportion of FOS with respect to FP, their application and use in other industries would decrease, and not taking advantage of their potential as probiotics and biological activity would increase the likelihood of their use in agriculture. It has been reported that FOS are beneficial for digestive health; in particular, they could help with obesity problems by decreasing weight gain in mice fed high-fat diets [35]. Agaves with high FOS content have been proposed for use as prebiotics in various formulations or as high-fiber ingredients with applications in digestive health. On the other hand, fructans high in long-chain fructans can be employed as thickeners in food formulations and as plant disease protectants, among other uses [19]. This agrees with the results of Navarro-López et al. [16], who found that foliar application of high PD fructans from A. tequilana var. Cenizo had greater protection than the application of intermediate- and low-PD fructans. However, evaluating fructan extracts from other agave species, which have different PF:FOS ratios, and their effects on protection against P. capsici infection, is also important.
Fructans have emerged as important players in plant immunity, particularly in their role as pathogen-associated molecular patterns (PAMPs) [36]. This concept is based on the ability of fructans to elicit immune responses in plants, similar to how traditional PAMPs function by activating pattern recognition receptors (PRRs) in host plants [37]. Regarding the induction of an effective plant defense response through the evaluated defense mechanisms, it was observed that lignin accumulation increased after fructan application (Figure 2). However, it was not possible to associate this accumulation with greater or lesser protection since some treatments that had greater accumulation were not those that presented greater protection. The same was the case for β-1,3-glucanase activity. No significant differences were found in the β-1,3-glucanase activities of tomato plants treated with fructans in a manner consistent with lower or higher protection. High activity could be observed on the day of pathogen inoculation, being significant only in A. cupreata fructans at the highest concentration (FC1) but not in the other treatments. In the treatments with A. cupreata fructans, a significant increase was observed at 24 h. The greatest difference was observed up to 9 days after inoculation with the pathogen (daip), with a significant decrease compared to infected plants. Sun et al. [38] described an increase in β-1,3-glucanase activity and protection against Botrytis cinerea infection with postharvest application of Burdock fructooligosaccharides (BFO) in Kyoho grapes (Vitis vinifera ‘Kyoho’). The activity increased in the first few days after daip (1, 2, and 3 daip) and then decreased by 4 and 5 days post-inoculation. In this study, this response is different, showing no significant differences in the first days after pathogen inoculation. The response was similar to what was observed in chili bell pepper plants when dahlia inulin was applied [18], where an increase was observed from 2 daip and a decrease until 9 daip. In this study, there is an increase in activity at 3 daip in some treatments (FC5 and FC1), although the same response was for all treatments with agave fructans at 9 daip.
A significant difference in defense response, consistent with fructan application, was observed in H2O2 accumulation (Figure 3) and peroxidase enzyme activity (Figure 5). In infected (P) tomato plants, H2O2 accumulation begins at 6 ddip and increases slightly at 9 daip. Whereas, in fructan-treated plants, accumulation occurs early at 3 daip and increases progressively up to 9 daip. There were differences between the treatments, with the plants treated with dahlia inulin and the lower concentration of A. tequilana fructans (FT5) exhibiting the highest accumulation [29,36]. Regarding peroxidase activity, in infected plants, there were no significant differences with the control at 0 and 1 daip. A significant increase in peroxidase activity was observed at 3 daip, compared to the control maintained at 6 and 9 daip. In fructan-treated plants, a decrease in peroxidase activity was observed at 3 daip with respect to diseased plants and a significant increase at 9 daip in most treatments. These values may be related to the induction of an effective defense response [39]. P. capsici is susceptible to the accumulation of H2O2 and other ROS, which may also be related to molecules triggering programmed cell death or signaling for other defense mechanisms [28]. In this study, it is observed that in infected plants, P. capsici increased peroxidase activity early after treatment, which could prevent ROS accumulation and thus facilitate its infection process [40,41]. In contrast, in fructan-treated plants, a decrease in peroxidase activity was observed early, which was subsequently reflected in the early accumulation of ROS. This progressive accumulation was controlled until 9 daip with a significant increase in peroxidase activity. One of the primary mechanisms by which fructans contribute to plant defense is the modulation of ROS dynamics. Rensburg et al. [36] reported that Arabidopsis plants treated with fructans exhibit increased resistance to the necrotrophic pathogen Botrytis cinerea, mainly through the activation of NADPH-oxidase, which is responsible for generating ROS during pathogen invasion. The application of fructans leads to an increased accumulation of H2O2, which is crucial for activating defense mechanisms in plants [36,42]. This burst of ROS was more pronounced in fructan-treated plants compared to untreated controls. Similarly, when barley was treated with the fructan levan, both H2O2 content and peroxidase activity were observed to change significantly when exposed to pathogens such as Fusarium graminearum and Rhizoctonia solani [43]. When inoculated with F. graminearum, the H2O2, proline, and jasmonic acid contents, as well as the peroxidase and ascorbate peroxidase activities, in levan-treated barley were higher than in the control. In contrast, when inoculated with R. solani, H2O2 content and peroxidase activity in levan-treated leaves showed faster induction than the control with early priming, and proline and methyl salicylate content were higher than that of the control.
A relevant finding of this study is that fructans, despite sharing largely similar physicochemical properties (Table 1), though not entirely identical (Figure 1), exhibited notable differences in their biological effectiveness. This variability suggests the possible presence of impurities that may influence the observed activity. However, the scope of the present study does not permit a definitive conclusion on this matter. One plausible explanation involves the extraction and purification methods, which can introduce variability in the biological activity of polysaccharides derived from identical or closely related sources [44]. Emphasized that extraction procedures affect both the yield and structural integrity of these compounds, potentially altering their bioactivity. For instance, ultrasound-assisted extraction has been shown to modify polysaccharide structures, diminishing their efficacy at higher intensities due to polymer chain degradation [45]. This sensitivity to processing conditions could lead to structural modifications and the presence of impurities in fructans, which may account for the differences in biological performance observed in this study. It is important to note that the fructans used here were characterized solely based on molecular weight (Mv) and degree of polymerization (PD). While impurities may play a role in modulating their protective effects, further studies are needed to substantiate this hypothesis. Future research should consider evaluating a broader range of food-grade agave fructans from various sources, along with comprehensive analysis of their compositional profiles and potential impurities, to investigate this premise further.
In this study, foliar-applied fructans induce protection against P. capsici infection in tomato. Considering that the agave fructans used are of commercial food grade or with a semi-craft extraction method [19], their accessibility could be guaranteed to large and small producers of important crops managed in rural areas and close to population centers, where the use of agrochemicals with a certain degree of toxicity or risk in their home storage represents a latent danger. Fructans are harmless and do not pose a risk to health or the environment and can be stored at home without any risk to humans or animals. Additionally, their preparation for agricultural applications is very easy and accessible, as they are water-soluble and have small particle sizes, eliminating the need for complex supplements. Therefore, knowing that its use is not subject to high purity levels and complex proportional formulations brings its potential use in agriculture closer, leaving the way for validation tests in the field and in other crops of agricultural importance.
5. Conclusions
In this study, foliar application of fructans induces effective protection against P. capsici on tomatoes, reducing disease incidence and severity. It is observed that A. tequilana fructans at a concentration of 0.5 g/L provided the highest protection, followed by A. cupreata, while dahlia inulin was less effective.
Associated defense mechanisms included early accumulation of H2O2 and increased peroxidase activity, suggesting a modulation in reactive oxygen species (ROS) dynamics. Although lignin deposition was not directly correlated with protection, the results indicate that fructans may act as resistance elicitors in plants. Given their low cost, easy extraction, and zero environmental impact, agave fructans represent a viable alternative for crop protection in sustainable agricultural systems. This opens the possibility of their validation in the field and their application in other economically important crops, promoting biological control strategies and reducing dependence on agrochemicals.
Regarding the defense mechanisms involved, it was observed that the accumulation of lignin increased after the application of fructans. However, it was not possible to associate this accumulation with greater or lesser protection. No significant differences were found in the β-1,3-glucanase activities of fructan-treated tomato plants, consistent with lower or higher protection. A significant difference in defense response consistent with fructan application was observed in H2O2 accumulation and peroxidase enzyme activity, an indication of modulation of ROS dynamics. Fructan-treated plants accumulated H2O2 early and increased progressively. The highest accumulation occurred in plants treated with dahlia inulin, and the lowest concentration was found in A. tequilana fructans (FT5). While in plants treated with fructans, peroxidase activity decreased in the first daip, and at the end of the experiment, there was a significant increase, behavior contrary to that observed in infected plants. Considering that the agave fructans used are of commercial or semi-craft food grade, their accessibility could be guaranteed to large and small producers of crops of importance and managed in rural areas and near population centers, where the use of agrochemicals with a certain degree of toxicity or risk in their home storage represents a latent danger.
Author Contributions
Conceptualization, J.A.Q.-Z. and E.S.-J.; methodology, E.S.-J. and K.A.H.-L.; formal analysis, E.S.-J. and J.C.L.-V.; investigation, E.S.-J. and K.A.H.-L.; writing—original draft-review and editing, E.S.-J., J.A.Q.-Z., M.I.M.-C. and S.G.-M.; project administration, J.A.Q.-Z. and M.I.M.-C.; funding acquisition, J.A.Q.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the project “Estrategias multidisciplinarias para incrementar el valor agregado de las cadenas productivas de café, frijol, mango, agave mezcalero y productos acuícolas (tilapia) en la región pacífico sur a través de la ciencia, tecnología e innovación” grant number FORDECYT 292474 from Secretaría de Ciencia, Humanidades, Tecnología e Innovación.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Savary, S.; Bregaglio, S.; Willocquet, L. Crop health and its global impacts on the components of food security. Food Sec. 2017, 9, 311–327. [Google Scholar] [CrossRef]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M.; et al. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118. [Google Scholar] [CrossRef]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security and paths forward. Nat. Rev. Microbiol. 2023, 10, 640–656. [Google Scholar] [CrossRef]
- Érsek, T.; Ribeiro, O. Mini review article: An annotated list of new Phytophthora species described post 1996. Acta Phytopathol. Et Entomol. Hung. 2010, 45, 251–266. [Google Scholar] [CrossRef]
- Lamour, K.H.; Hu, J. Diversity and Phytophthora: A threat to forests, crops and traditional laboratory research-mini review. CAB Rev. 2013, 8, 038. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.J.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Govers, F. The top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2014, 4, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Quesada-Ocampo, L.M.; Parada-Rojas, C.H.; Hansen, Z.; Vogel, G.; Smart, C.; Hausbeck, M.K.; Carmo, R.M.; Huitema, E.; Naegele, R.P.; Kousik, C.S.; et al. Phytophthora capsici: Recent Progress on Fundamental Biology and Disease Management 100 Years After Its Description. Annu. Rev. Phytopathol. 2023, 61, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, W.A.; Bodine, E.W.; Durrell, L.W. Cucurbit diseases and rot of tomato fruit caused by Phytophthora capsici. Phytopathology 1940, 30, 972–976. [Google Scholar]
- Satour, M.M.; Butler, E.E. A root and crown rot of tomato caused by Phytophthora capsici and Phytophthora parasitica. Phytopathology 1967, 57, 510–515. [Google Scholar]
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Available online: https://www.gob.mx/agricultura/dgsiap/acciones-y-programas/produccion-agricola-404122 (accessed on 5 March 2025).
- Moreira-Morrillo, A.; Monteros, A.A.; Reis, A.; Garcés, F.F.R. Phytophthora capsici on Capsicum Plants: A Destructive Pathogen in Chili and Pepper Crops. In Capsicum Current Trends and Perspectives; Baylen Yllano, O., Ed.; IntechOpen: London, UK, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- Gowthami, L. Role of elicitors in plant defense mechanism. J. Pharmacog. Phytochem. 2018, 7, 2806–2812. [Google Scholar]
- Qui-Zapata, J.A.; Montero-Cortés, M.I.; García-Morales, S.; Espinosa-Andrews, H.; Uc-Varguez, A.; Cano-Sosa, J.; Enríquez-Vara, J.N.; Ramos-Díaz, A.L.; López-Velázquez, J.C.; Leal-García, I.; et al. Biorational strategies for the control of coffee rust (Hemileia vastatrix). In Semillas de Cambio en la Región Pacífico Sur: Ciencia, Tecnología y Sociedad para el Desarrollo Local en las Cadenas Productivas Principales en Guerrero, Oaxaca y Chiapas; Center for Research and Assistance in Technology and Design of the State of Jalisco: Guadalajara, Mexico, 2024; pp. 307–343. [Google Scholar] [CrossRef]
- Bellini, A.; Pugliese, M.; Guarnaccia, V.; Meloni, G.R.; Gullino, L.M. Calcium oxide, potassium phosphite and a Trichoderma enriched compost water suspension protect Capsicum annuum against Phytophthora capsici by priming the immune system. Pest Manag. Sci. 2021, 77, 3484–3490. [Google Scholar] [CrossRef]
- Navarro-López, D.; López-Velázquez, J.C.; Saavedra-Loera, D.; García-Gamboa, R.; González-Ávila, M.; Ortiz-Basurto, R.; Qui-Zapata, J.; García-Morales, S. Effect of the polymerization degree of agave fructans for the control of Phytophthora capsici. In Sustainable and Integrated Use of Agave; Gutiérrez, M.A., Ed.; Center for Research and Assistance in Technology and Design of the State of Jalisco: Zapopan, Mexico, 2018; pp. 107–112. Available online: https://ciatej.mx/el-ciatej/comunicacion/proyectos-de-divulgacion (accessed on 1 March 2025).
- López-Velázquez, J.C.; Navarro-López, D.E.; Qui-Zapata, J.A.; León-Morales, J.M.; Saavedra-Loera, D.I.; García-Morales, S. Effect of selenite and inulin on Capsicum annuum L.-Phytophthora capsici interaction in greenhouse. Plant Biotechnol. 2019, 19, 25–34. [Google Scholar]
- López-Velázquez, J.C.; García-Morales, S.; Qui-Zapata, J.A.; García-Carvajal, Z.Y.; Navarro-López, D.E.; García-Varela, R. Induction of defense response mediated by inulin from dahlia tubers (Dahlia sp.) in Capsicum annuum. Mex. J. Phytopathol. 2024, 42, 9. [Google Scholar] [CrossRef]
- Camacho-Ruíz, R.M.; Estarrón, E.M.; Arrizón, G.J.P.; Gschaedler, M.A.C. Southern agaves and their fructans. Horiz. Transdiciplinarios 2023, 1, 13–21. [Google Scholar]
- Moreno-Vilet, L.; García, H.M.H.; Delgado, P.R.E.; Corral, F.N.E.; Cortez, E.N.; Ruíz, C.M.A.; Portales, P.D.P. In vitro assessment of Agave fructans (Agave salmiana) as prebiotics and immune system activators. Int. J. Biol. Macromol. 2014, 63, 181–187. [Google Scholar] [CrossRef]
- Plascencia, A.; Gutiérrez, M.A.; Rodríguez, D.J.M.; Castañeda-Nava, J.J.; Gallardo-Valdez, J.; Shimada, H.; Camacho-Ruíz, R.M. Molecular weight distribution of fructans extracted from Agave salmiana leaves. Bot. Sci. 2022, 100, 657–666. [Google Scholar] [CrossRef]
- Alvarado, C.; Camacho, R.M.; Cejas, R.; Rodríguez, J.A. Profiling of commmercial agave fructooligosaccharides using ultrafiltration and high performance thin layer chromatography. Rev. Mex. Ing. Quim. 2014, 13, 417–427. Available online: https://www.redalyc.org/pdf/620/62031508006.pdf (accessed on 25 February 2025).
- Reyes-Tena, A.; Castro-Rocha, A.; Rodríguez-Alvarado, G.; Vázquez-Marrufo, G.; Pedraza-Santos, M.E.; Lamour, K.; Larsen, J.; Fernández-Pavía, S.P. Virulence phenotypes on chili pepper for Phytophthora capsici isolates from Michoacán, Mexico. HortScience 2019, 54, 1526–1531. [Google Scholar] [CrossRef]
- Trinidad-Cruz, J.R.; Rincón-Enríquez, G.; Evangelista-Martínez, Z.; Quiñones-Aguilar, E.E. Biorational control of Phytophthora capsici in pepper plants using Streptomyces spp. Rev. Chapingo Ser. Hortic. 2021, 27, 85–99. [Google Scholar] [CrossRef]
- Vázquez-Vuelvas, O.F.; Chávez-Camacho, F.A.; Meza-Velázquez, J.A.; Mendez-Merino, E.; Ríos-Licea, M.M.; Contreras-Esquivel, J.C. A comparative FTIR study for supplemented agavin as functional food. Food Hydrocoll. 2020, 103, 105642. [Google Scholar] [CrossRef]
- Wang, J.E.; Li, D.W.; Zhang, Y.L.; Zhao, Q.; He, Y.M.; Gong, Z.H. Defence responses of pepper (Capsicum annuum L.) infected with incompatible and compatible strains of Phytophthora capsici. Eur. J. Plant Pathol. 2013, 136, 625–638. [Google Scholar] [CrossRef]
- Pye, M.F.; Hakuno, F.; MacDonald, J.D.; Bostock, R.M. Induced resistance in tomato by sar activators during predisposing salinity stress. Front. Plant Sci. 2013, 4, 116. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Offler, C.E.; Ruan, Y.-L. A simple, rapid and reliable protocol to localize hydrogen peroxide in large plant organs by DAB-mediated tissue printing. Front. Plant Sci. 2014, 5, 745. [Google Scholar] [CrossRef]
- Oliveira, J.T.A.; Barreto, A.L.H.; Vasconcelos, I.M.; Eloy, Y.R.G.; Gondim, D.M.F.; Fernades, C.D.F.; Freire-Filho, F.R. Role of antioxidant enzymes, hydrogen peroxide and PR proteins in the compatible and incompatible interactions of cowpea (Vigna unguiculata) genotypes with the fungus Colletotrichum gloeosporioides. J. Plant Physiol. Pathol. 2014, 2, 1000131. [Google Scholar] [CrossRef]
- Melanie, H.; Susilowati, A.; Iskandar, Y.M.; Lotulung, P.D.; Andayani, D.G.S. Characterization of Inulin from Local Red Dahlia (Dahlia sp. L) Tubers by Infrared Spectroscopy. Procedia Chem. 2015, 16, 78–84. [Google Scholar] [CrossRef]
- Díaz-Ramos, D.I.; Jiménez-Fernández, M.; García-Barradas, O.; Chacón-López, M.A.; Montalvo-González, E.; López-García, U.M.; Beristain-Guevara, C.I.; Ortiz-Basurto, R.I. Structural, thermal, and functional properties of Agave tequilana fructan fractions modified by acylation. Rev. Mex. De Ing. Química 2023, 22, 1–15. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Poel, B.V.D.; Höfte, M.; Ende, W.V.D. Sweet immunity: Inulin boosts resistance of lettuce (Lactuca sativa) against grey mold (Botrytis cinerea) in an ethylene-dependent manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Tang, Y.; Luo, Y.; Zhang, Z. Hydrogen peroxide regulated salicylic acid- and jasmonic acid-dependent systemic defenses in tomato seedlings. Food Sci. Technol. 2022, 42, e54920. [Google Scholar] [CrossRef]
- Thangaraj, R.; Anandamurugan, S.; Pandiyan, P.; Kaliappan, V.K. Artificial intelligence in tomato leaf disease detection: A comprehensive review and discussion. J. Plant Dis. Prot. 2022, 129, 469–488. [Google Scholar] [CrossRef]
- Márquez-Aguirre, A.L.; Camacho, R.R.M.; Arriaga, A.M.; Padilla, C.E.; Kirchmayr, M.R.; Blasco, J.L.; González, A.M. Effects of Agave tequilana fructans with different degree of polymerization profiles on the body weight, blood lipids and count of fecal Lactobacilli/Bifidobacteria in obese mice. Food Funct. 2013, 4, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Rensburg, H.C.C.J.V.; Takács, Z.; Freynschlag, F.; Öner, E.T.; Jonak, C.; Ende, W.V.D. Fructans prime ROS dynamics and Botrytis cinerea resistance in Arabidopsis. Antioxidants 2020, 9, 805. [Google Scholar] [CrossRef]
- Nguyen, T.N.H.; Leclerc, L.; Manzanares, D.M.; Gravot, A.; Vicré, M.; Morvan, B.A.; Prud’Homme, M. Fructan exohydrolases (FEHs) are upregulated by salicylic acid together with defense-related genes in non-fructan accumulating plants. Physiol. Plant. 2023, 175, e13975. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, P.; Guo, M.; Yu, W.; Chen, K. Burdock fructooligosaccharide induces fungal resistance in postharvest Kyoho grapes by activating the salicylic acid-dependent pathway and inhibiting browning. Food Chem. 2013, 138, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Q.; Li, L.; Li, P.; Yin, M.; Xu, S.; Bi, W. Chemical composition and antifungal activity of Zanthoxylum armatum fruit essential oil against Phytophthora capsici. Molecules 2022, 27, 8636. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Yang, Y.; Li, T.; Lu, W.; Du, Y.; Qiang, X.; Shan, W. A Phytophthora capsici rxlr effector targets and inhibits a plant piase to suppress endoplasmic reticulum-mediated immunity. Mol. Plant 2018, 11, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bao, Y.; Zhang, M.; Du, D.; Rao, S.; Li, Y.; Dou, D. A Phytophthora capsici rxlr effector targets and inhibits the central immune kinases to suppress plant immunity. New Phytol. 2021, 232, 264–278. [Google Scholar] [CrossRef]
- Versluys, M.; Tarkowski, Ł.P.; Ende, W.V.d. Fructans as damps or mamps: Evolutionary prospects, cross-tolerance, and multistress resistance potential. Front. Plant Sci. 2017, 7, 2061. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, Z.; Chen, F.; Zhang, Z.; Gao, W. Optimization of ultrasonic-assisted extraction of polysaccharides from hemerocallis citrina and the antioxidant activity study. J. Food Sci. 2021, 86, 3082–3096. [Google Scholar] [CrossRef]
- Liu, X.; Wan, Z.; Shi, L.; Lü, X. Preparation and antiherpetic activities of chemically modified polysaccharides from polygonatum cyrtonema hua. Carbohydr. Polym. 2011, 83, 737–742. [Google Scholar] [CrossRef]
- Lian, D.; Shui, C.; Yang, L.; Cheng, Y.; Zheng, S.; Shen, H.; Liang, M. Levan differentially primes barley defense against infections by Fusarium graminearum, Rhizoctonia solani and Pyricularia oryzae. Plant Pathol. 2024, 73, 859–872. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).