Functional and Pharmaceutical Properties of Physically and Chemically Modified Rice Bean (Vigna umbellata) Starches
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Proximate Analysis of Rice Bean Seed Powder
2.3. Starch Extraction
2.4. Starch Modification
2.4.1. Pregelatinization
2.4.2. Phosphorylation
2.4.3. Carboxymethylation
2.5. Physicochemical Property Evaluation
2.5.1. Amylose Content
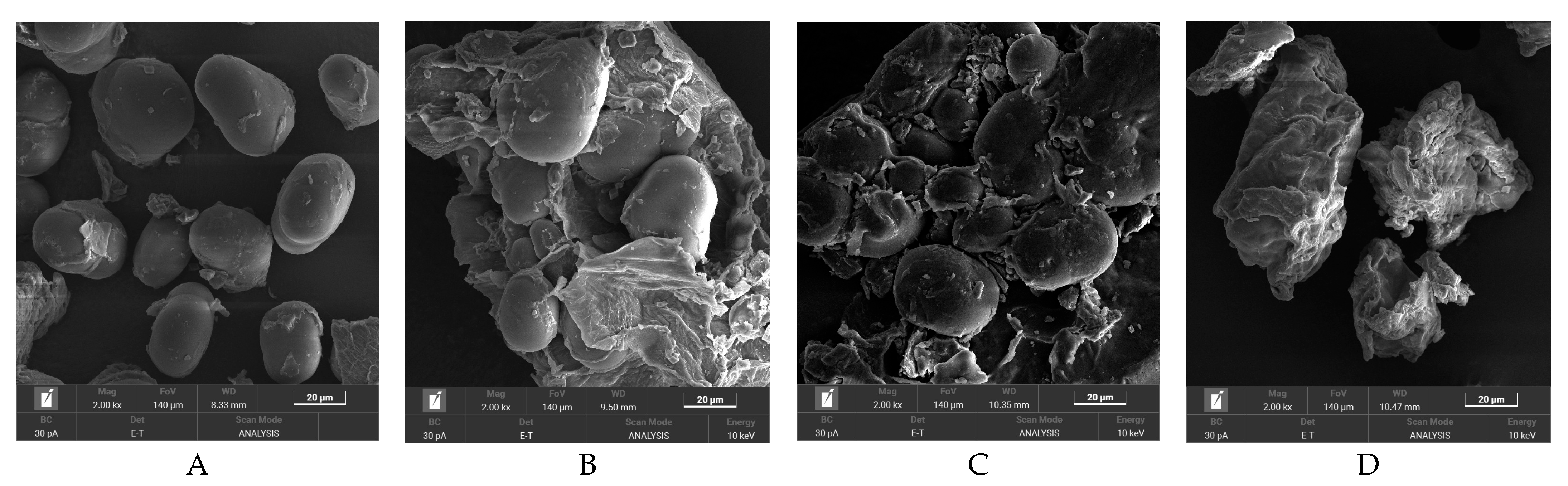
2.5.2. Scanning Electron Microscopic (SEM)
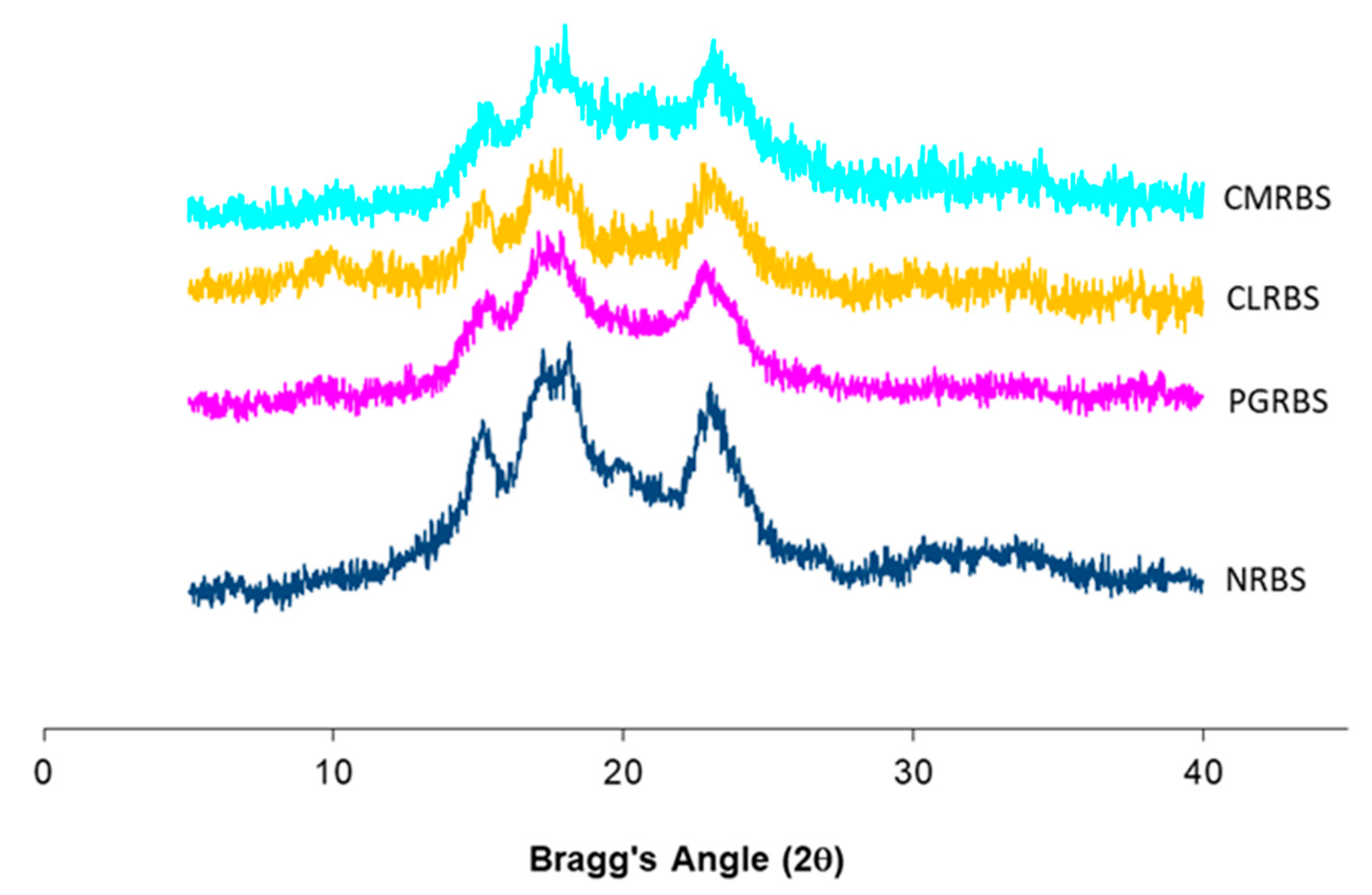
2.5.3. X-Ray Diffraction
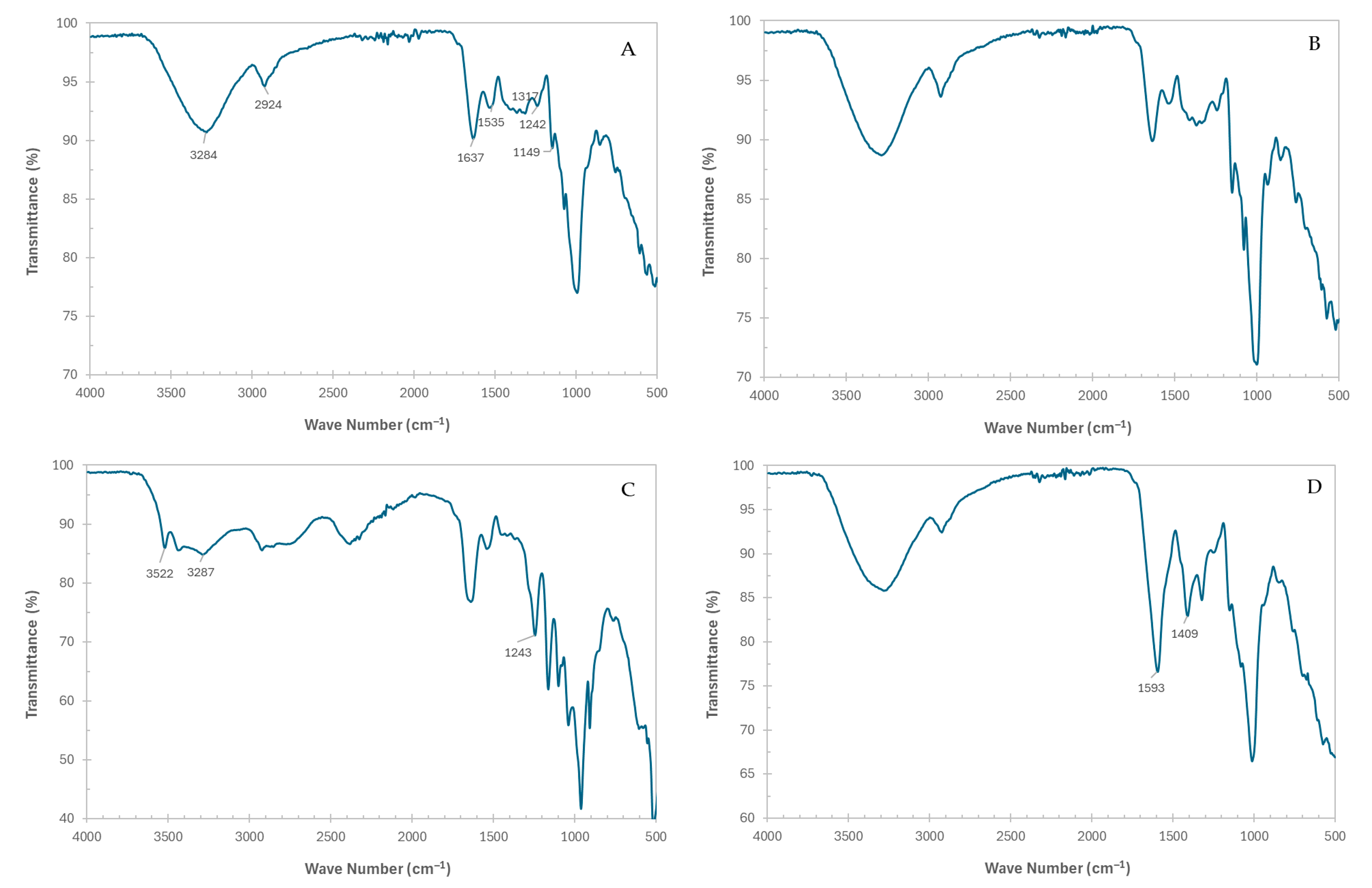
2.5.4. FT-IR
2.5.5. Moisture Content
2.5.6. Water Solubility (WS) and Swelling Power (SP) at 70 °C
2.5.7. Free-Swelling Capacity at 37 °C
2.5.8. Oil Absorption Capacity (OAC)
2.5.9. Starch Digestibility
2.6. Pharmaceutical Functionality and Evaluation as Potential Excipients
2.6.1. Density and Flow
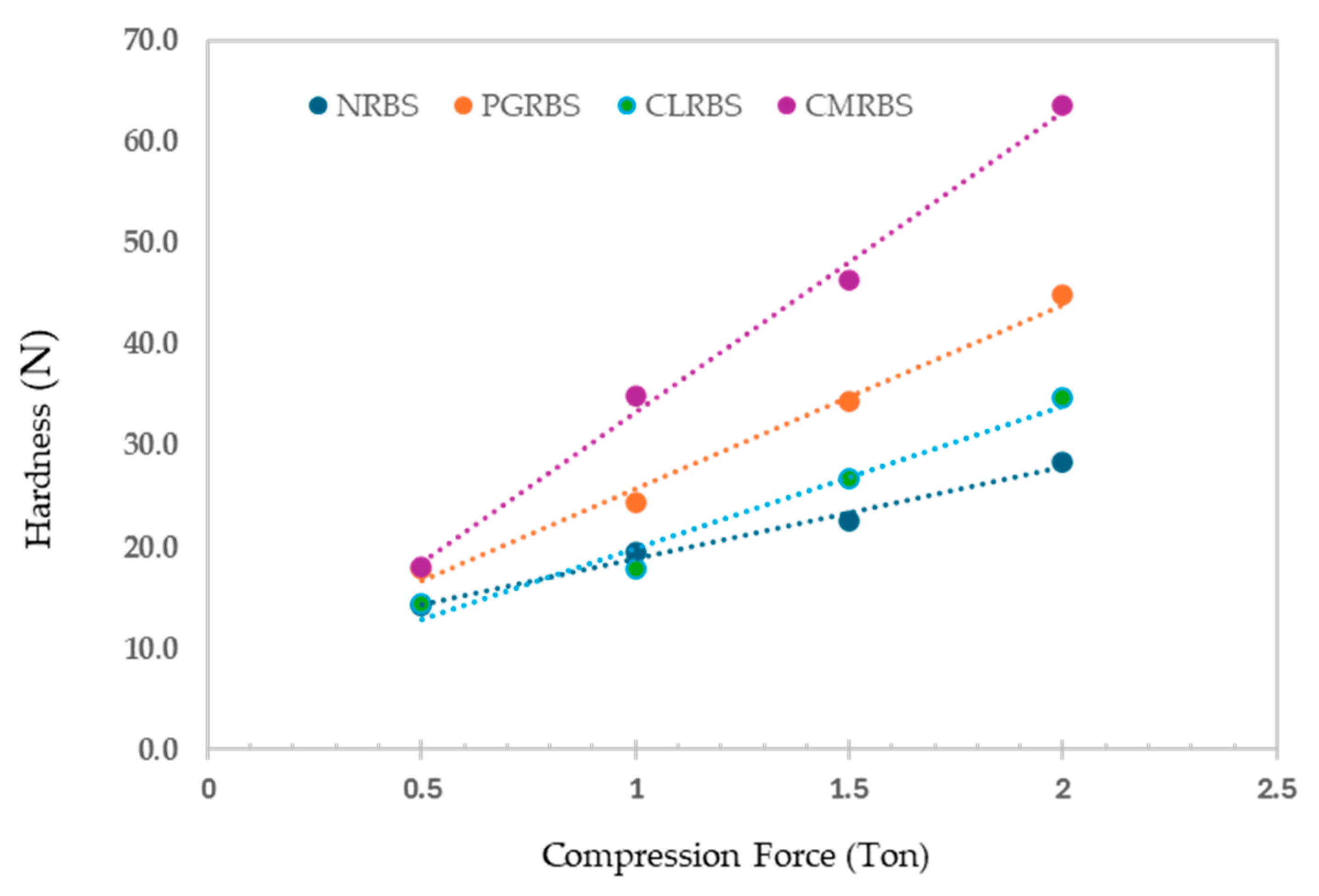
2.6.2. Compactibility
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis and Amylose Content
3.2. SEM
3.3. X-Ray Diffraction
3.4. FT-IR
3.5. Moisture Content (MC)
3.6. Swelling Power and Solubility
3.7. Free-Swelling Capacity
3.8. Oil Absorption Capacity (OAC)
3.9. In Vitro Digestibility
3.10. Pharmaceutical Functionality
3.10.1. Density and Powder Flow
3.10.2. Powder Compactibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pattanayak, A.; Roy, S.; Sood, S.; Iangrai, B.; Banerjee, A.; Gupta, S.; Joshi, D.C. Rice bean: A Lesser Known Pulse with Well-Recognized Potential. Planta 2019, 250, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Katoch, R.; Sanadya, S.K.; Pathania, K.; Chaudhary, H.K. Nutritional and Nutraceutical Potential of rice bean (Vigna umbellata)–A Legume with Hidden Potential. Front. Nutr. 2023, 10, 1126544. [Google Scholar] [CrossRef]
- Ahmed, S.; Jamil, S. rice bean (Vigna umbellata) the Forgotten Gold: Unraveling the Commercial, Nutritional and Medicinal Value. J. Pharmacogn. Phytochem. 2024, 13, 34–36. [Google Scholar] [CrossRef]
- Rahman, U.; Younas, Z.; Ahmad, I.; Yousaf, T.; Latif, R.; Rubab, U.; Hassan, H.; Shafi, U.; Mashwani, Z.-u.-R. Enhancing Health and Therapeutic Potential: Innovations in the Medicinal and Pharmaceutical Properties of Soy Bioactive Compounds. Front. Pharmacol. 2024, 15, 1397872. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.; de Freitas Barbosa, M.; da Silva, P.B.; de Oliveira, J.P.; da Silva, T.L.; Teixeira Junior, D.L.; de Moura Rocha, M. Health Benefits and Industrial Applications of Functional Cowpea Seed Proteins. In Grain and Seed Proteins Functionality; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Dhull, S.B.; Rani, J.; Rohilla, S.; Rose, P.K.; Chawla, P.; Kidwai, M.K.; Chandak, A.; Bamel, P.; Kumar, M.; Kirrolia, A.S. Moth Bean (Vigna aconitifolia) Starch: Properties, Modifications and Applications—A Review. Legume Sci. 2023, 5, e237. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, W.; Yao, Y.; Chen, C.; Li, X.; Yin, B.; Li, H.; Zhang, Y. Analysis and Research on Starch Content and Its Processing, Structure and Quality of 12 Adzuki Bean Varieties. Foods 2022, 11, 3381. [Google Scholar] [CrossRef] [PubMed]
- Maphosa, Y.; Jideani, V.A.; Ikhu-Omoregbe, D.I. Vigna subterranea (L.) Verdc Starch-Soluble Dietary Fibre Potential Nanocomposite: Thermal Behaviour, Morphology and Crystallinity. Processes 2022, 10, 299. [Google Scholar] [CrossRef]
- Chavan, U.D.; Momin, A.; Chavan, J.K.; Amarowicz, R. Characteristics of Starch from Rice Bean (Vigna umbellata L.) Seeds—A Short Report. Pol. J. Food Nutr. Sci. 2009, 59, 25–28. [Google Scholar]
- Kaur, A.; Kaur, P.; Singh, N.; Virdi, A.S.; Singh, P.; Rana, J.C. Grains, Starch and Protein Characteristics of Rice Bean (Vigna umbellata) Grown in Indian Himalaya Regions. Food Res. Int. 2013, 54, 102–110. [Google Scholar] [CrossRef]
- Li, H.-T.; Zhang, W.; Zhu, H.; Chao, C.; Guo, Q. Unlocking the Potential of High-Amylose Starch for Gut Health: Not All Function the Same. Fermentation 2023, 9, 134. [Google Scholar] [CrossRef]
- Munjal, S.D.; Dhankhar, J.; Sharma, A.; Guleria, P. Studies on Physicochemical Properties of Rice Bean (Vigna umbellata) Starch: An Underutilized Legume. Curr. Res. Nutr. Food Sci. J. 2024, 12, 408–422. [Google Scholar] [CrossRef]
- Sharma, P.; Goudar, G.; Chandragiri, A.K.; Ananthan, R.; Subhash, K.; Chauhan, A.; Longvah, T.; Singh, M.; Bhardwaj, R.; Parida, S.K.; et al. Assessment of Diversity in Anti-Nutrient Profile, Resistant Starch, Minerals and Carbohydrate Components in Different Ricebean (Vigna umbellata) Accessions. Food Chem. 2023, 405, 134835. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M. Chemical Composition of Ricebean (Vigna umbellata): Effect of Domestic Processing. Indian J. Appl. Res. 2015, 5, 311–313. [Google Scholar]
- Bepary, R.H.; Wadikar, D.D.; Neog, S.B.; Patki, P.E. Studies on Physico-Chemical and Cooking Characteristics of rice bean Varieties Grown in NE Region of India. J. Food Sci. Technol. 2017, 54, 973–986. [Google Scholar] [CrossRef]
- Thakur, Y.; Thory, R.; Sandhu, K.S.; Kaur, M.; Sinhmar, A.; Pathera, A.K. Effect of Selected Physical and Chemical Modifications on Physicochemical, Pasting, and Morphological Properties of Underutilized Starch from rice bean (Vigna umbellata). J. Food Sci. Technol. 2021, 58, 4785–4794. [Google Scholar] [CrossRef]
- Compart, J.; Singh, A.; Fettke, J.; Apriyanto, A. Customizing Starch Properties: A Review of Starch Modifications and Their Applications. Polymers 2023, 15, 3491. [Google Scholar] [CrossRef]
- Kapoor, D.U.; Pareek, A.; Sharma, M.; Prajapati, B.G.; Suttiruengwong, S.; Sriamornsak, P. Exploring Starch-Based Excipients in Pharmaceutical Formulations: Versatile Applications and Future Perspectives. Eur. J. Pharm. Biopharm. 2025, 212, 114727. [Google Scholar] [CrossRef] [PubMed]
- Sewnarain, P.; Dwarka, D.; Mellem, J.J. Characterisation of Resistant Starch from Vigna unguiculata for the Development of an Oral-Colon Delivery Agent. Int. J. Food Sci. Technol. 2024, 59, 3642–3651. [Google Scholar] [CrossRef]
- Dhillon, P.K.; Tanwar, B. Rice Bean: A Healthy and Cost-Effective Alternative for Crop and Food Diversity. In Food Security: The Science, Sociology and Economics of Food Production and Access to Food; The International Society for Plant Pathology: Saint Paul, MN, USA; Springer: Cham, Switzerland, 2018; Volume 10, pp. 525–535. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Singh, N.; Malhi, N.S. Physicochemical and Thermal Properties of Starches Separated from Corn Produced from Crosses of Two Germ Pools. Food Chem. 2005, 89, 541–548. [Google Scholar] [CrossRef]
- Jubril, I.; Muazu, J.; Muhammed, G.T. Effects of Phosphate Modified and Pregelatinized Sweet Potato Starches on Disintegrant Property of Paracetamol Tablet Formulation. J. Appl. Pharm. Sci. 2012, 2, 32–36. [Google Scholar]
- Adeyanju, O.; Olademehin, O.P.; Hussaini, Y.; Nwanta, U.C.; Adejoh, A.I.; Plavec, J. Synthesis and Characterization of Carboxymethyl Plectranthus esculentus Starch: A Potential Disintegrant. J. Pharm. Appl. Chem. 2016, 2, 189–195. [Google Scholar] [CrossRef]
- Gibson, T.S.; Solah, V.A.; McCleary, B.V. A procedure to measure amylose in cereal starches and flours with concanavalin A. J. Cereal Sci. 1997, 25, 111–119. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, W.; Li, T.; Wang, L. Understanding the Relationship between the Molecular Structure and Physicochemical Properties of Soft Rice Starch. Foods 2023, 12, 3611. [Google Scholar] [CrossRef] [PubMed]
- Heß, C.; Hartmann, B.; Lechner, M.D.; Nierling, W.; Seidel, C.; Kulicke, W.M. Influence of soluble polymer residues in crosslinked carboxymethyl starch on some physical properties of its hydrogels. Starch-Stärke 2007, 59, 425–429. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohyd. Polym. 2006, 66, 521–527. [Google Scholar] [CrossRef]
- Standardized Tapped Density Testing for Pharmaceutical Powders. Available online: https://www.news-medical.net/whitepaper/20211208/Standardized-Tapped-Density-Testing-for-Pharmaceutical-Powders.aspx (accessed on 29 April 2025).
- Shweta; Katoch, R.; Kumari, M. Proximate and Anti-Nutritional Composition of Underutilized and Common Vigna Species of Himachal Pradesh. Bull. Env. Pharmacol. Life Sci. 2017, 6, 24–31. [Google Scholar]
- Xie, Z.; Guan, J.; Chen, L.; Jin, Z.; Tian, Y. Effect of Drying Processes on the Fine Structure of A-, B-, and C-Type Starches. Starch/Stärke 2017, 69, 1700218. [Google Scholar] [CrossRef]
- Fonseca, A.M.; El Halal, S.L.M.; Dias, A.R.G.; Zavareze, E.R. Physical Modification of Starch by Heat-Moisture Treatment and Annealing and Their Applications: A Review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef]
- Agvaandorj, A.; Li, Y.; No, J. Effects of Structural Changes in Cross-Linked Mung Bean Starch on Freeze–Thaw Properties and In Vitro Digestibility. Foods 2025, 14, 689. [Google Scholar] [CrossRef]
- Malik, M.K.; Kumar, V.; Singh, J.; Bhatt, P.; Dixit, R.; Kumar, S. Phosphorylation of Alkali Extracted Mandua Starch by STPP/STMP for Improving Digestion Resistibility. ACS Omega 2023, 8, 11750–11767. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Cui, C.; Gao, L.; Qin, Y.; Ji, N.; Dai, L.; Wang, Y.; Xiong, L.; Shi, R.; Sun, Q. A Review of Starch Swelling Behavior: Its Mechanism, Determination Methods, Influencing Factors, and Influence on Food Quality. Carbohydr. Polym. 2023, 321, 121260. [Google Scholar] [CrossRef]
- Benevides, C.M.J.; Trindade, B.A.; Lopes, M.V. Potentialities of Legumes in the Pharmaceutical Industry. J. Anal. Pharm. Res. 2018, 7, 369–373. [Google Scholar] [CrossRef][Green Version]
- Wang, N.; Maximiuk, L.; Fenn, D.; Nickerson, M.T.; Hou, A. Development of a Method for Determining Oil Absorption Capacity in Pulse Flours and Protein Materials. Cereal Chem. 2020, 97, 1111–1117. [Google Scholar] [CrossRef]
- Baptista, N.T.; Dessalles, R.; Illner, A.-K.; Ville, P.; Ribet, L.; Anton, P.M.; Durand-Dubief, M. Harnessing the Power of Resistant Starch: A Narrative Review of Its Health Impact and Processing Challenges. Front. Nutr. 2024, 11, 1369950. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Sample | MC (%) | Swelling Power at 80 °C (g/g) | Solubility at 80 °C (%) | Free Swelling Capacity at 37 °C (g/g) | Oil Absorbance Capacity (g/g) |
|---|---|---|---|---|---|
| NRBS | 10.47 ± 0.62 b | 12.25 ± 0.50 c | 19.48 ± 0.85 c | 1.99 ± 0.04 c | 1.08 ± 0.04 c |
| PGRBS | 9.89 ± 0.83 b | 16.34 ± 0.78 b | 21.63 ± 1.24 b | 2.11 ± 0.09 b | 1.02 ± 0.03 d |
| CLRBS | 8.45 ± 0.73 c | 4.11 ± 0.23 d | 2.28 ± 0.12 d | 1.63 ± 0.08 d | 1.22 ± 0.08 b |
| CMRBS | 12.19 ± 1.14 a | 18.91 ± 1.25 a | 53.12 ± 8.29 a | 6.12 ± 0.23 a | 1.67 ± 0.11 a |
| Sample | RDS | SDS | RS |
|---|---|---|---|
| NRBS | 23.54 ± 3.62 ab | 63.70 ± 5.29 a | 11.31 ± 0.67 d |
| PGRBS | 29.42 ± 4.74 a | 62.87 ± 7.11 a | 5.49 ± 1.07 |
| CLRBS | 15.83 ± 2.89 c | 64.59 ± 4.89 a | 17.38 ± 2.17 c |
| CMRBS | 22.01 ± 1.59 b | 55.31 ± 4.37 b | 21.65 ± 1.59 b |
| HAC | 5.04 ± 0.88 d | 39.77 ± 2.73 c | 53.19 ± 6.77 a |
| Sample * | Density (g/cm3) | %CI | HR | AR | |
|---|---|---|---|---|---|
| Bulk | Tapped | ||||
| NRBS | 0.64 ± 0.01 c | 0.86 ± 0.00 b | 25.52 ± 0.75 b | 1.34 ± 0.01 b | 37.75 ± 3.58 a |
| PGRBS | 0.60 ± 0.01 d | 0.70 ± 0.01 d | 14.47 ± 1.06 c | 1.17 ± 0.02 c | 33.39 ± 3.08 b |
| CLRBS | 0.66 ± 0.01 b | 0.75 ± 0.00 c | 12.16 ± 0.70 d | 1.14 ± 0.01 d | 32.79 ± 2.29 b |
| CMRBS | 0.69 ± 0.01 a | 0.94 ± 0.01 a | 27.14± 1.03 a | 1.37 ± 0.02 a | 36.08 ± 4.49 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittipongpatana, O.S.; Trisopon, K.; Kittipongpatana, N. Functional and Pharmaceutical Properties of Physically and Chemically Modified Rice Bean (Vigna umbellata) Starches. Polysaccharides 2025, 6, 71. https://doi.org/10.3390/polysaccharides6030071
Kittipongpatana OS, Trisopon K, Kittipongpatana N. Functional and Pharmaceutical Properties of Physically and Chemically Modified Rice Bean (Vigna umbellata) Starches. Polysaccharides. 2025; 6(3):71. https://doi.org/10.3390/polysaccharides6030071
Chicago/Turabian StyleKittipongpatana, Ornanong S., Karnkamol Trisopon, and Nisit Kittipongpatana. 2025. "Functional and Pharmaceutical Properties of Physically and Chemically Modified Rice Bean (Vigna umbellata) Starches" Polysaccharides 6, no. 3: 71. https://doi.org/10.3390/polysaccharides6030071
APA StyleKittipongpatana, O. S., Trisopon, K., & Kittipongpatana, N. (2025). Functional and Pharmaceutical Properties of Physically and Chemically Modified Rice Bean (Vigna umbellata) Starches. Polysaccharides, 6(3), 71. https://doi.org/10.3390/polysaccharides6030071




