Polysaccharides from Agro-Industrial Waste and By-Products: An Overview on Green Synthesis of Metallic Nanoparticles—An Ecofriendly Approach
Abstract
1. Introduction
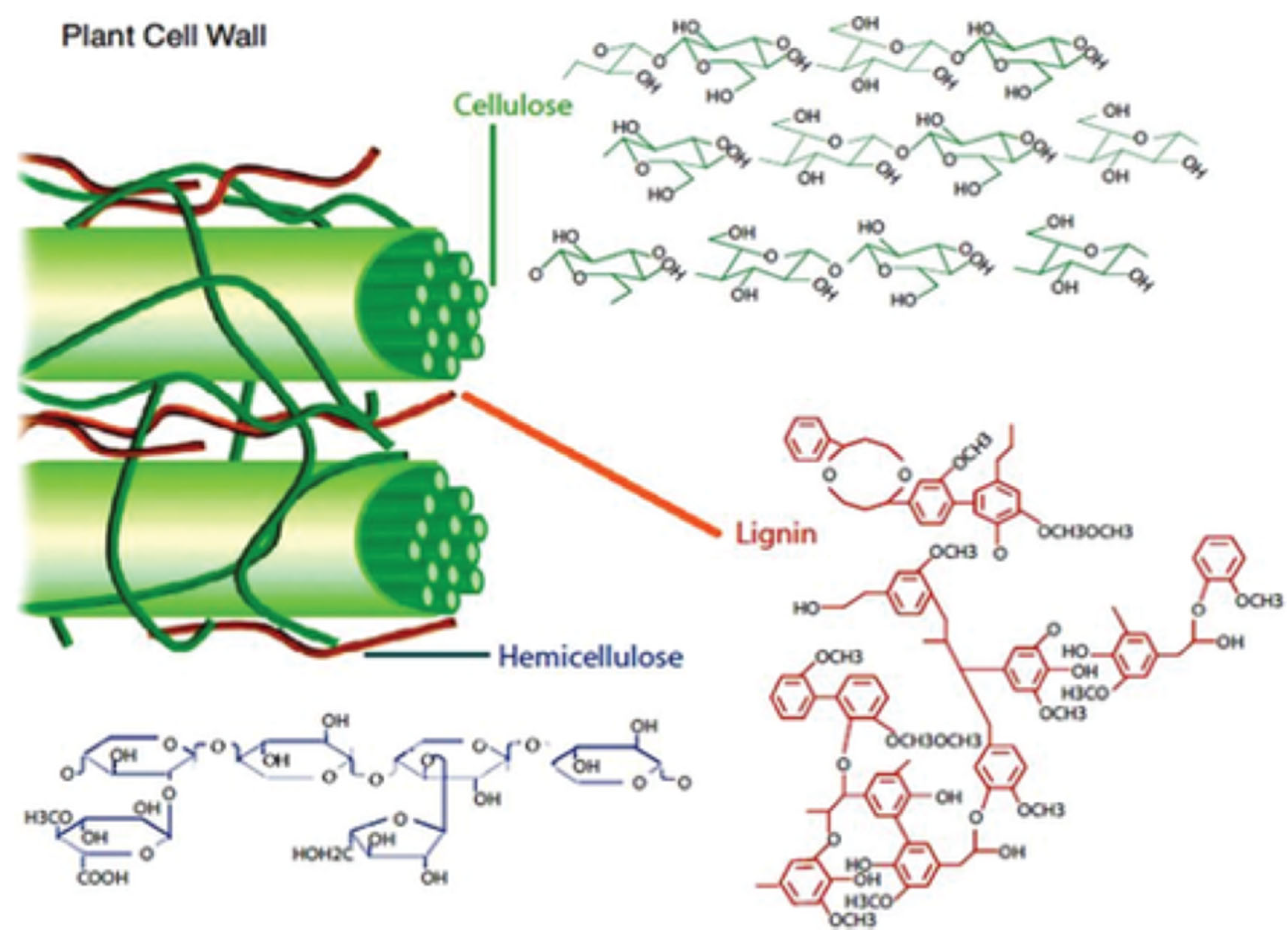
2. Description and Physicochemical Properties of Polysaccharides
2.1. Cellulose
2.2. Hemicellulose
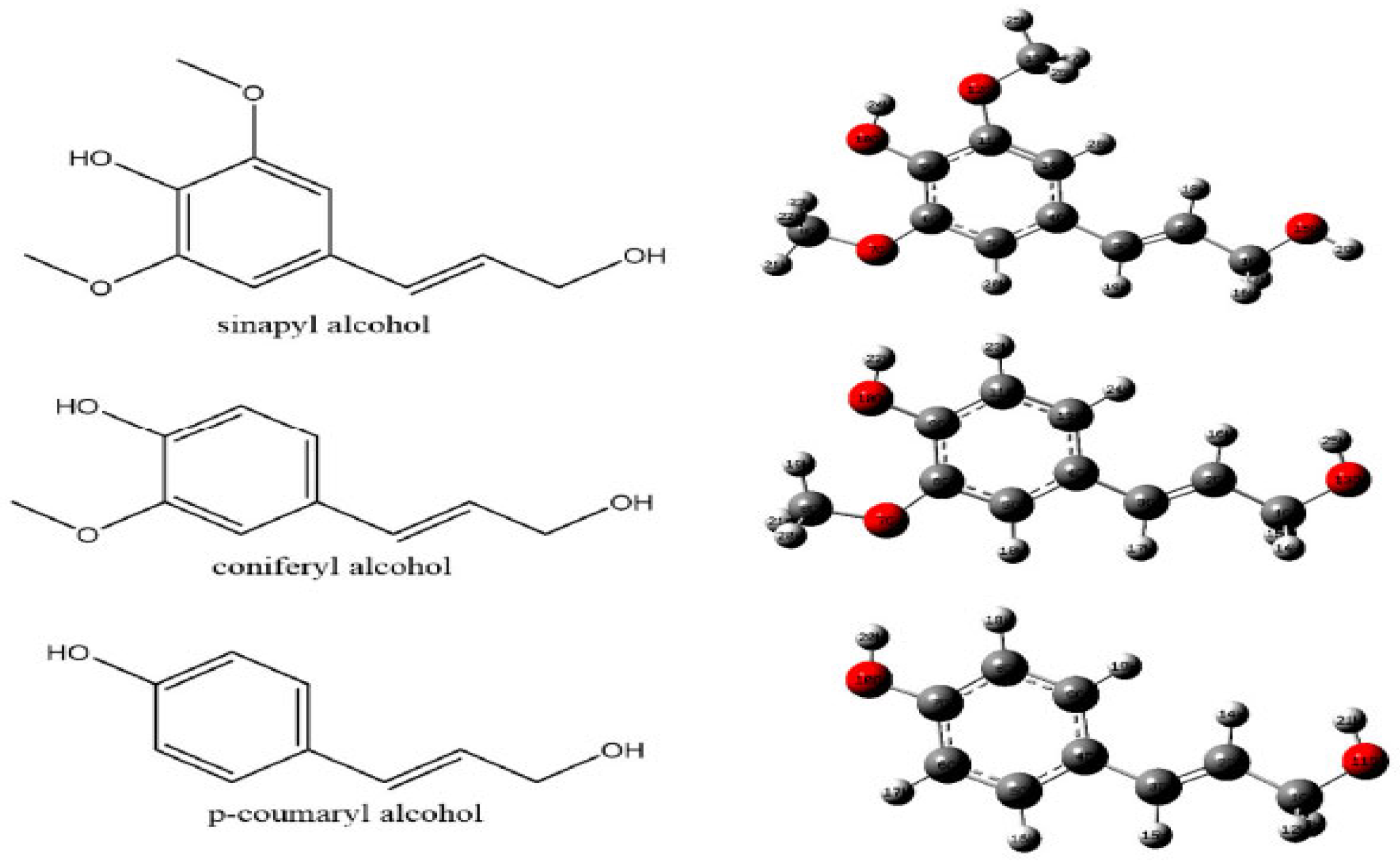
2.3. Lignin
- They are vegetable polymers built on the basis of phenylpropanoid units.
- They are characterized by the predominance of methoxyl groups contained in the wood.
- They are resistant to acid hydrolysis, easily oxidizable, soluble in bisulfite or hot alkalis, and easily condensable with phenols or thiols.
- When reacting with nitrobenzene in a hot alkaline solution, they primarily produce vanillin, syringaldehyde, and p-hydroxybenzaldehyde, depending on their origin.
- When boiled in an ethanolic solution of hydrochloric acid, lignins form monomers of the Hibbert ketone type (aromatic ketones resulting from the breaking of the main ether bonds (β–O–4) between lignin units) [21].
2.4. Pectin
2.5. Others
2.5.1. Starch
2.5.2. Gums
3. Green Synthesis of Metallic Nanoparticles
3.1. Fundamental
3.2. Characteristics
3.3. Advantages and Disadvantages Metallic Nanoparticles
3.3.1. Advantages
High Surface Area-to-Volume Ratio
Unique Optical Properties
Enhanced Mechanical Properties
Antimicrobial Properties
Catalytic Efficiency
3.3.2. Disadvantages
Potential Toxicity
Aggregation
Stability Issues
High Production Costs
Environmental Impact
4. Polysaccharides from Waste and By-Products
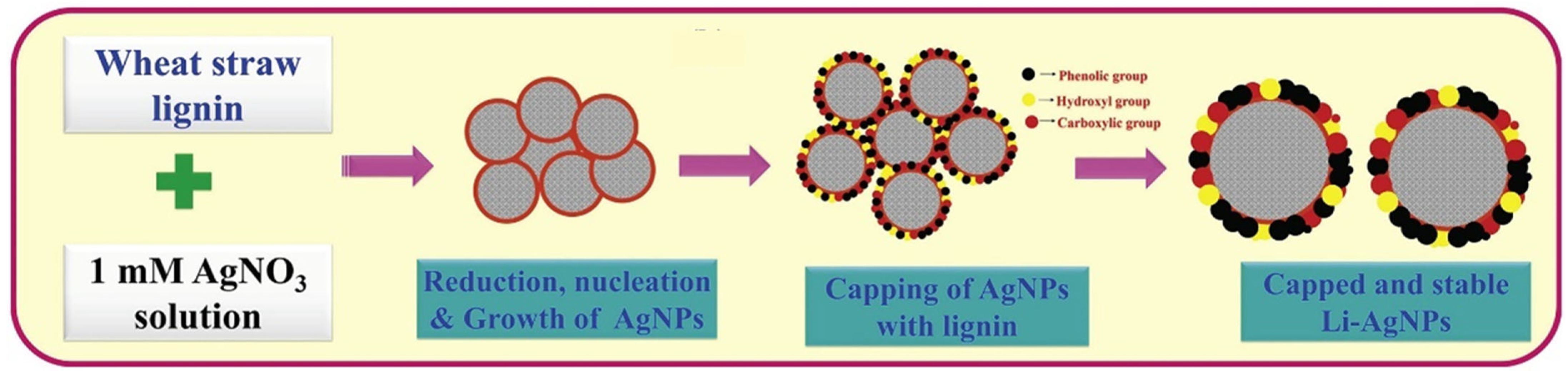
4.1. Wheat Straw
4.2. Lignocellulosic Material
4.2.1. Sodium Carboxylmetyl Cellulose
4.2.2. Cellulose Nanocrystal/Zinc Oxide
4.2.3. Silver–Lignin Nanoparticles
4.2.4. Shiitake (Lentinus edodes)
4.3. Fruits and Vegetables
4.3.1. Guava (Psidium guajava L.)
4.3.2. Okra (Abelmoschus esculentus)
4.3.3. Apple (Malus domestica)
4.3.4. Durian (Durio zibethinus)
4.3.5. Mango (Mangifera indica L.)
4.3.6. Banana (Musa paradisiaca)
4.3.7. Mangrove Fruit (Ceriops decandra)
4.3.8. Plant Waste
4.4. Agro-Waste
4.5. Advantages of Using Polysaccharides from Agro-Industrial By-Products for Nanoparticle Synthesis
5. Types of Metallic Nanoparticles That Can Be Synthesized from Polysaccharides
5.1. Monometallic Nanoparticles
5.1.1. Silver Nanoparticles
5.1.2. Gold Nanoparticles
5.1.3. Platinum Nanoparticles and Palladium Nanoparticles
5.2. Bimetallic Nanoparticles
5.2.1. Iron–Copper Nanoparticles
5.2.2. Iron–Zinc Nanoparticles
5.2.3. Silver–Gold
5.3. Metallic Oxide Nanoparticles
5.3.1. Zinc Oxide
5.3.2. Iron Oxide
5.3.3. Copper Oxide, Titanium Dioxide, and Manganese Oxide
6. Mechanistic Aspects of Metallic Nanoparticles from Polysaccharides
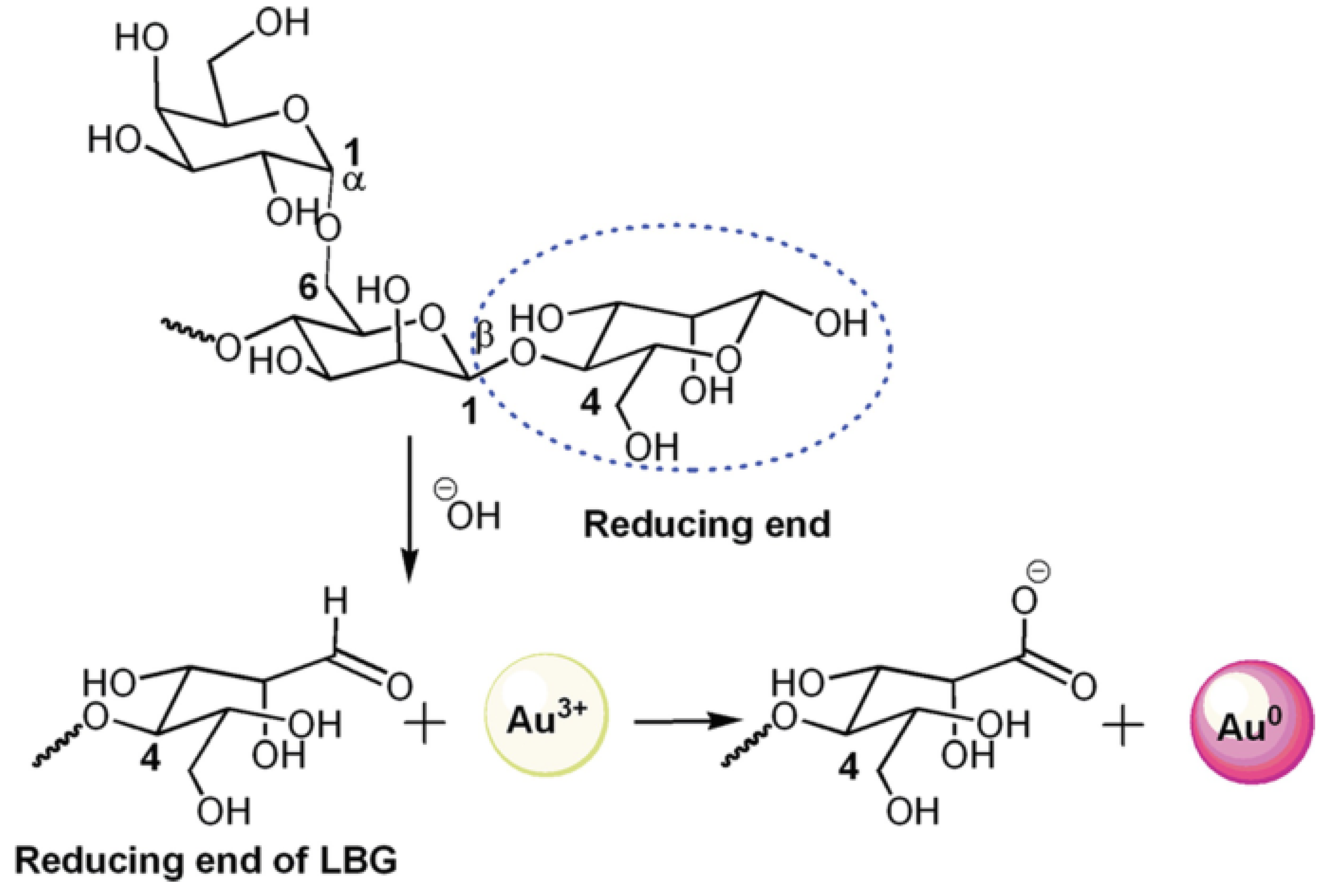
6.1. Mechanistic Aspects Using Lignocellulosic Material
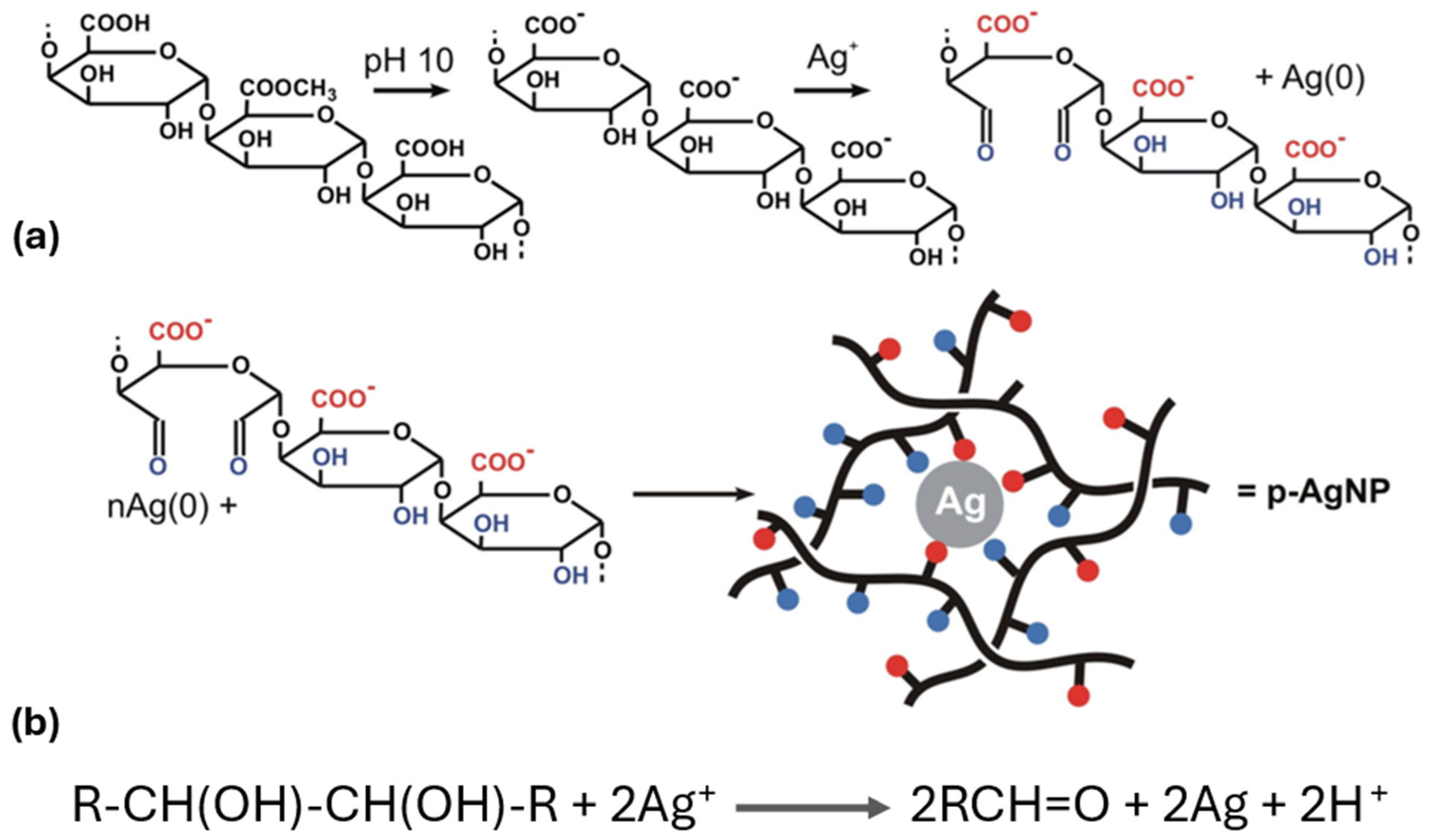
6.2. Mechanistic Aspects Using Pectin
6.3. Mechanistic Aspects Using Starch
6.4. Mechanistic Aspects Using Gums
7. Applications of Nanoparticles Synthesized from Polysaccharides
7.1. Food
7.2. Health
7.3. Agriculture
7.4. Environmental
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargas Corredor, Y.A.; Peréz Pérez, L.I. Aprovechamiento de residuos agroindustriales en el mejoramiento de la calidad del ambiente. Rev. Fac. Cienc. Básicas 2018, 1, 59–72. [Google Scholar] [CrossRef]
- Cury, R.K.; Aguas, M.Y.; Martinez, M.A.; Olivero, V.R.; Chams, C.L. Residuos agroindustriales su impacto, manejo y aprovechamiento. Rev. Colomb. Cienc. Anim.-RECIA 2017, 9 (Suppl. S1), 122–132. [Google Scholar] [CrossRef]
- Yusuf, M. Agro-Industrial Waste Materials and their Recycled Value-Added Applications: Review. In Handbook of Ecomaterials, 1st ed.; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Allwyn Sundarraj, A.; Vasudevan Ranganathan, T. A review on cellulose and its utilization from agro-industrial waste. Drug Invent. Today 2018, 10, 89–94. [Google Scholar]
- Mada, B.D.; Mpoy, K.P.; Ngombe, K.N.; Ndyanabo, M.T. Nécessité d’une gestion des résidus agricoles et agro-industriels à Kinshasa. Int. J. Biol. Chem. Sci. 2015, 9, 2234–2248. [Google Scholar] [CrossRef]
- Mejías-Brizuela, N.; Orozco-Guillen, E.; Galáan-Hernández, N. Aprovechamiento de los residuos agroindustriales y su contribución al desarrollo sostenible de México. Rev. Cienc. Ambient. Recur. Nat. 2016, 2, 27–41. [Google Scholar]
- Vera, G.; Farías, L.; Castañeda, A. Síntesis de Nanopartículas Metálicas por Rutas Verdes. Rev. Científica La Univ. Autónoma Coahuila 2017, 9, 15–20. [Google Scholar]
- Aranda Ferreyra, G.N.; Villalba, G.F.; Paéz, P.L.; Martínez, I.M.A.; Piccioni, M.N.; Guerrero, P.A.; Nazareno, M.A.; Dalmasso, P.R. Síntesis Green y Caracterización Biológica de AgNPs Empleando Extracto de Alcaparras. In I Congreso Internacional de Ingeniería Aplicada a la Innovación y Educación y Asamblea General de ISTEC 2019. 2019, pp. 307–316. Available online: https://sedici.unlp.edu.ar/handle/10915/97825 (accessed on 10 February 2025).
- Deokar, G.K.; Ingale, A.G. Green synthesis of gold nanoparticles (Elixir of Life) from banana fruit waste extract-an efficient multifunctional agent. RSC Adv. 2016, 6, 74620–74629. [Google Scholar] [CrossRef]
- Maity, G.N.; Sarkar, J.; Khatua, S.; Mondal, S.; Acharya, K. Green Synthesis of Silver Nanoparticles Using Mangrove Fruit Polysaccharide for Bacterial Growth Inhibition. Asian J. Pharm. Clin. Res. 2019, 12, 179–183. [Google Scholar] [CrossRef]
- Miranda-Valdez, I.Y.; Camarillo-Hernández, C.A.; Reyes-Melo, M.E.; Puente-Córdova, J.G.; López-Walle, B. Aspectos estructurales, reológicos y dieléctricos de la etil celulosa. Ingenierías 2019, 22, 40–53. [Google Scholar]
- Deaquiz Oyala, Y.A.; Moreno Medina, B.L. Producción y Biosíntesis de Fibras Vegetales. Una revisión. Conex. Agropecu. 2016, 6, 29–42. [Google Scholar]
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An update on overview of cellulose, its structure and applications. Cellulose 2019, 13, 59–79. [Google Scholar] [CrossRef]
- Gañán, P.; Zuluaga, R.; Castro, C.; Restrepo-Osorio, A.; Velásquez Cock, J.; Osorio, M.; Montoya, U.; Vélez, L.; Álvarez, C.; Correa, C.; et al. Celulosa: Un polímero de siempre con mucho futuro. Rev. Colomb. Mater. 2017, 11, 1–4. [Google Scholar]
- Goswami, M.; Baruah, D.; Das, A.M. Green synthesis of silver nanoparticles supported on cellulose and their catalytic application in the scavenging of organic dyes. New J. Chem. 2018, 42, 10868–10878. [Google Scholar] [CrossRef]
- Farhat, W.; Venditti, R.; Quick, A.; Taha, M.; Mignard, N.; Becquart, F.; Ayoub, A. Hemicellulose extraction and characterization for applications in paper coatings and adhesives. Ind. Crops Prod. 2017, 107, 370–377. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A critical review on hemicellulose pyrolysis. Energy Technol. 2017, 5, 52–79. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.; Peng, W.; Zhang, J.; Ruan, R. Green Synthesis and Stability Evaluation of Ag Nanoparticles Using Bamboo Hemicellulose. BioResources 2016, 11, 385–399. [Google Scholar]
- Barhoum, A.; Jeevanandam, J.; Rastogi, A.; Samyn, P.; Boluk, Y.; Dufresne, A.; Danquahh, M.K.; Bechelany, M. Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale 2020, 12, 22845–22890. [Google Scholar] [CrossRef]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Lignina: Composición, síntesis y evolución. Madera Y Bosques 2021, 27, e2722137. [Google Scholar] [CrossRef]
- Chávez-Sifontes, M.; Domine, M.E. Lignina, Estructura Y Aplicaciones: Métodos De Despolimerización Para La Obtención De Derivados Aromáticos De Interés Industrial. Av. Cienc. Ing. 2013, 4, 15–45. [Google Scholar]
- Aadil, K.R.; Pandey, N.; Mussatto, S.I.; Jha, H. Green synthesis of silver nanoparticles using acacia lignin, their cytotoxicity, catalytic, metal ion sensing capability and antibacterial activity. J. Environ. Chem. Eng. 2019, 7, 103296. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Akman, F.A. Density functional theory study based on monolignols: Molecular structure, homo-lumo analysis, molecular electrostatic potential. Cellul. Chem. Technol. 2019, 53, 243–250. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.M.; Pagliaro, M. Pectin production and global market. Agro Food Ind. Hi Tech. 2016, 27, 17–20. [Google Scholar]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Funakawa, H.; Miwa, K. Synthesis of borate cross-linked rhamnogalacturonan II. Front. Plant Sci. 2015, 6, 223. [Google Scholar] [CrossRef]
- Jiménez-Maldonado, M.I. Análisis de Fragmentos de Ramnogalacturonano i Como Inductores del Mecanismo de Defensa del Tomate; Centro de Investigación en Alimentación y Desarrollo, A.C.: Hermosillo, Mexico, 2016. [Google Scholar]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Yang, Z.; Chaib, S.; Gu, Q.; Hemar, Y. Impact of pressure on physicochemical properties of starch dispersions. Food Hydrocoll. 2017, 68, 164–177. [Google Scholar] [CrossRef]
- Zhu, F. Impact of ultrasound on structure, physicochemical properties, modifications, and applications of starch. Trends Food Sci. Technol. 2015, 43, 1–17. [Google Scholar] [CrossRef]
- Yu, S.; Ma, Y.; Menager, L.; Sun, D.W. Physicochemical Properties of Starch and Flour from Different Rice Cultivars. Food Bioprocess Technol. 2012, 5, 626–637. [Google Scholar] [CrossRef]
- Berruezo, M.; Ludueña, L.N.; Rodriguez, E.; Alvarez, V.A. Preparation and characterization of polystyrene/starch blends for packaging applications. J. Plast. Film Sheeting 2014, 30, 141–161. [Google Scholar] [CrossRef]
- Pasquel, A. Gomas: Una aproximación a la industria de alimentos. Mundo Aliment. 2010, 1, 6–12. [Google Scholar]
- Mirhosseini, H.; Amid, B.T. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res. Int. 2012, 46, 387–398. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Castillo-Martínez, W.E.; Cachay-Santillán, K.M.; del Milagro Bances-Majuan, K.; Siche, R. Substitution of xanthan gum (xanthomonas campestris), with vegetable gums in Greek yogurt: Rheological and sensory properties. In Proceedings of the LACCEI International Multi-Conference for Engineering, Education and Technology, Virtual, 27–31 July 2020; pp. 27–31. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef]
- Kurhade, P.; Kodape, S.; Choudhury, R. Overview on Green Synthesis of Metallic Nanoparticles; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 75. [Google Scholar] [CrossRef]
- Dhand, V.; Kumari, S.C.; Padma, P.N. Green synthesis of metallic nanoparticles: A review. Nanomaterials 2021, 259–281. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Wang, C.; Gao, X.; Chen, Z.; Chen, Y.; Chen, H. Preparation, characterization and application of polysaccharide-based metallic nanoparticles: A review. Polymers 2017, 9, 689. [Google Scholar] [CrossRef]
- Shavandi, A.; Saeedi, P.; Ali, M.A.; Jalalvandi, E. Green Synthesis of Polysaccharide-Based Inorganic Nanoparticles and Biomedical Aspects; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-edge advances in tailoring size, shape, and functionality of nanoparticles and nanostructures: A review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.; Arshad, M.M.; Poopalan, P.; Perumal, V. Nanoparticle synthetic methods: Strength and limitations. Nanoparticles Anal. Med. Devices 2021, 31–43. [Google Scholar] [CrossRef]
- Barui, A.K.; Das, S.; Patra, C.R. Biomedical Applications of Green-Synthesized Metal Nanoparticles Using Polysaccharides; Elsevier Ltd.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Banerjee, A.; Halder, U.; Bandopadhyay, R. Preparations and Applications of Polysaccharide Based Green Synthesized Metal Nanoparticles: A State-of-the-Art. J. Clust. Sci. 2017, 28, 1803–1813. [Google Scholar] [CrossRef]
- Adwoa, O. Impact of pH on the Catalytic Activity of Metal Nanoparticles in Organic Reactions in Ghana. J. Chem. 2024, 3, 12–23. [Google Scholar] [CrossRef]
- Kumar, C.G.; Mamidyala, S.K.; Reddy, M.N.; Reddy, B.V.S. Silver glyconanoparticles functionalized with sugars of sweet sorghum syrup as an antimicrobial agent. Process Biochem. 2012, 47, 1488–1495. [Google Scholar] [CrossRef]
- Carles, R.; Farcau, C.; Bonafos, C.; Benassayag, G.; Bayle, M.; Benzo, P.; Groenen, J.; Zwickal, A. Three Dimensional Design of Silver Nanoparticle Assemblies Embedded in Dielectrics for Raman Spectroscopy Enhancement and Dark-Field Imaging. ACS Nano 2011, 5, 8774–8782. [Google Scholar] [CrossRef] [PubMed]
- Myroshnychenko, V.; Nelayah, J.; Adamo, G.; Geuquet, N.; Rodríguez-Fernández, J.; Pastoriza-Santos, I.; MacDonald, K.F.; Henrard, L.; Liz-Marzán, L.M.; Zheludev, N.I.; et al. Plasmon Spectroscopy and Imaging of Individual Gold Nanodecahedra: A Combined Optical Microscopy, Cathodoluminescence, and Electron Energy-Loss Spectroscopy Study. Nano Lett. 2012, 12, 4172–4180. [Google Scholar] [CrossRef]
- Lertvachirapaiboon, C.; Maruyama, T.; Baba, A.; Ekgasit, S.; Shinbo, K.; Kato, K. Optical Sensing Platform for the Colorimetric Determination of Silver Nanoprisms and Its Application for Hydrogen Peroxide and Glucose Detections Using a Mobile Device Camera. Anal. Sci. 2019, 35, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Jafari, S.M. Impact of metal nanoparticles on the mechanical, barrier, optical and thermal properties of biodegradable food packaging materials. Crit. Rev. Food Sci. Nutr. 2021, 61, 2640–2658. [Google Scholar] [CrossRef]
- Guerrero-Almonacid, M.; Pariguana, M.; Gonzalez, G.; Durán-Lara, E.; López-Cabaña, Z. Antibacterial Activity of Metal Nanoparticles Against Bacteria of Clinical Origin. Acta Microsc. 2023, 32, 42–55. [Google Scholar]
- Sadiq, S.; Khan, I.; Shen, Z.; Wang, M.; Xu, T.; Khan, S.; Zhou, X.; Bahadur, A.; Rafiq, M.; Sohail, S.; et al. Recent updates on multifunctional nanomaterials as antipathogens in humans and livestock: Classification, application, mode of action, and challenges. Molecules 2023, 28, 7674. [Google Scholar] [CrossRef]
- Manikandan, V.; Min, S.C. Roles of polysaccharides-based nanomaterials in food preservation and extension of shelf-life of food products: A review. Int. J. Biol. Macromol. 2023, 252, 126381. [Google Scholar] [CrossRef]
- Wang, J.; Cheon, W.S.; Lee, J.Y.; Yan, W.; Jung, S.; Jang, H.W.; Shokouhimehr, M. Magnetic boron nitride adorned with Pd nanoparticles: An efficient catalyst for the reduction of nitroarenes in aqueous media. Dalton Trans. 2023, 52, 3567–3574. [Google Scholar] [CrossRef]
- Shahrezaei, M.; Hejazi, S.H.; Kmentova, H.; Sedajova, V.; Zboril, R.; Naldoni, A.; Kment, S. Ultrasound-driven defect engineering in TiO2−x nanotubes─toward highly efficient platinum single atom-enhanced photocatalytic water splitting. ACS Appl. Mater. Interfaces 2023, 5, 37976–37985. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.K.; Nguyen, M.K.; Lin, C.; Hoang, T.D.; Nguyen, T.C.; Lone, A.M.; Khedulkar, A.P.; Gaballah, M.S.; Singh, J.; Chung, W.J.; et al. Review on fate, transport, toxicity and health risk of nanoparticles in natural ecosystems: Emerging challenges in the modern age and solutions toward a sustainable environment. Sci. Total Environ. 2023, 912, 169331. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Salman, A.; Khan, Z.; Khan, S.; Krishnaraj, C.; Yun, S.I. Metallic nanoparticles: A promising arsenal against antimicrobial resistance—Unraveling mechanisms and enhancing medication efficacy. Int. J. Mol. Sci. 2023, 24, 14897. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, S.; Wadhawan, D.; Jain, A.; Mehta, S.K. Toxic implication of nanoparticles: A review of fac-tors, mechanism, exposure and control strategies. Int. J. Environ. Sci. Technol. 2025, 22, 1203–1224. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. The use of capping agents in the stabilization and functionalization of metallic nanoparticles for biomedical applications. Part. Part. Syst. Charact. 2023, 40, 2200146. [Google Scholar] [CrossRef]
- Rodriguez-Loya, J.; Lerma, M.; Gardea-Torresdey, J.L. Dynamic light scattering and its application to control nanoparticle aggregation in colloidal systems: A review. Micromachines 2023, 15, 24. [Google Scholar] [CrossRef]
- Khoramian, R.; Issakhov, M.; Pourafshary, P.; Gabdullin, M.; Sharipova, A. Surface modification of nanoparticles for enhanced applicability of nanofluids in harsh reservoir conditions: A comprehensive review for improved oil recovery. Adv. Colloid Interface Sci. 2024, 333, 103296. [Google Scholar] [CrossRef]
- Shi, P.; Sun, X.; Yuan, H.; Chen, K.; Bi, S.; Zhang, S. Nanoscale Metal–Organic Frameworks Combined with Metal Nanoparticles and Metal Oxide/Peroxide to Relieve Tumor Hypoxia for Enhanced Photodynamic Therapy. ACS Biomater. Sci. Eng. 2023, 9, 5441–5456. [Google Scholar] [CrossRef]
- Jin, H.; Cai, M.; Deng, F. Antioxidation effect of graphene oxide on silver nanoparticles and its use in antibacterial applications. Polymers 2023, 15, 3045. [Google Scholar] [CrossRef]
- Tukova, A.; Nie, Y.; Tavakkoli Yaraki, M.; Tran, N.T.; Wang, J.; Rodger, A.; Gu, Y.; Wang, Y. Shape dependent protein-induced stabilization of gold nanoparticles: From a protein corona perspective: Special Collection: Distinguished Australian Researchers. Aggregate 2023, 4, e323. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Zhang, J.M.; Junaid, M.; Saleem, S.; Ali, A.; Ullah, A.; Khan, S. Metal-based nanoparticles: Basics, types, fabrications and their electronic applications. Z. Phys. Chemie 2024, 238, 965–995. [Google Scholar] [CrossRef]
- Huang, X.; Auffan, M.; Eckelman, M.J.; Elimelech, M.; Kim, J.H.; Rose, J.; Zuo, K.; Li, Q.; Alvarez, P.J. Trends, risks and opportunities in environmental nanotechnology. Nat. Rev. Earth Environ. 2024, 5, 572–587. [Google Scholar] [CrossRef]
- Samal, D.; Khandayataray, P.; Sravani, M.; Murthy, M.K. Silver nanoparticle ecotoxicity and phytoremediation: A critical review of current research and future prospects. Environ. Sci. Pollut. Res. 2024, 31, 8400–8428. [Google Scholar] [CrossRef]
- Isibor, P.O.; Oyegbade, S.A.; Oni, J.G.; Ahmed, W.W.; Abiodun, E.O.; Oyewole, O.A. Nanoparticles in Food Chains: Bioaccumulation and Trophic Transfer. In Environmental Nanotoxicology; Springer: Cham, Switzerland, 2024; pp. 203–233. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Huang, C.; Ling, Z.; Lai, C.; Yong, Q. Natural surfactant-aided dilute sulfuric acid pretreatment of waste wheat straw to enhance enzymatic hydrolysis efficiency. Bioresour. Technol. 2021, 324, 124651. [Google Scholar] [CrossRef] [PubMed]
- Tufail, T.; Saeed, F.; Afzaal, M.; Ain, H.B.U.; Gilani, S.A.; Hussain, M.; Anjum, F.M. Wheat straw: A natural remedy against different maladies. Food Sci. Nutr. 2021, 9, 2335–2344. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kumar, B.; Rhim, J.W. Green and facile synthesis of carboxymethylcellulose/ZnO nanocomposite hydrogels crosslinked with Zn2+ ions. Int. J. Biol. Macromol. 2020, 162, 229–235. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, H.Y.; Abdalkarim, S.Y.H.; Wang, C.; Tang, F.; Marek, J.; Chen, W.L.; Militky, J.; Yao, Y.M. Green one-step synthesis of ZnO/cellulose nanocrystal hybrids with modulated morphologies and superfast absorption of cationic dyes. Int. J. Biol. Macromol. 2019, 132, 51–62. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ghodake, G.; Cho, S.K.; Kadam, A.; Kumar, A.; Jeon, B.H.; Pant, D.; Bhatnagar, A.; Shin, H.S. Wheat straw extracted lignin in silver nanoparticles synthesis: Expanding its prophecy towards antineoplastic potency and hydrogen peroxide sensing ability. Int. J. Biol. Macromol. 2019, 128, 391–400. [Google Scholar] [CrossRef]
- Dong, L.; Li, R.; Wang, L.; Lan, X.; Sun, H.; Zhao, Y.; Wang, L. Green synthesis of platinum nanoclusters using lentinan for sensitively colorimetric detection of glucose. Int. J. Biol. Macromol. 2021, 172, 289–298. [Google Scholar] [CrossRef]
- Sánchez-Zúñiga, K.; Castro-Piedra, S.; Moreira-González, I.; Arnáez-Serrano, E.; Navarro-Hoyos, M.; Vargas-Huertas, F. Evaluación de las propiedades citotóxicas de un extracto de frutos de guayaba (Psidium guajava Var. Tai-Kuo-Bar). Rev. Tecnol. Marcha 2017, 30, 150. [Google Scholar] [CrossRef]
- Wang, L.; Xie, J.; Huang, T.; Ma, Y.; Wu, Z. Characterization of silver nanoparticles biosynthesized using crude polysaccharides of Psidium guajava L. leaf and their bioactivities. Mater. Lett. 2017, 208, 126–129. [Google Scholar] [CrossRef]
- Al-Shawi, A.A.A.; Hameed, M.F.; Hussein, K.A.; Thawini, H.K. Review on the “Biological Applications of Okra Polysaccharides and Prospective Research”. Futur. J. Pharm. Sci. 2021, 7, 102. [Google Scholar] [CrossRef]
- Sangode, C.M.; Mahant, S.A.; Tidke, P.C.; Umekar, M.J.; Lohiya, R.T. Green synthesized of novel iron nanoparticles as promising antimicrobial agent: A review. GSC Biol. Pharm. Sci. 2021, 15, 117–127. [Google Scholar] [CrossRef]
- Ravichandran, V.; Sumitha, S.; Ning, C.Y.; Xian, O.Y.; Yu, U.K.; Paliwal, N.; Shah, S.A.A.; Tripathy, M. Durian waste mediated green synthesis of zinc oxide nanoparticles and evaluation of their antibacterial, antioxidant, cytotoxicity and photocatalytic activity. Green Chem. Lett. Rev. 2020, 13, 102–116. [Google Scholar] [CrossRef]
- Sapin, A.B.; Alaon, M.K.N.; Tambalo, F.M.Z.; Perez, R.H.; Gaylon, A. Evaluation of the bioactivities of natural phenolics from mango (Mangifera indica linn) leaves for cosmetic industry applications. Philipp. J. Sci. 2021, 150, 397–406. [Google Scholar] [CrossRef]
- Rowida, Y.E.; Elsebaie, E.M. Immobilization of Synthesized Silver Nanoparticles Using Mango Peel Extract on Low Density Polyethylene Surface and its Application as Biologically Active Packages. Alex. J. Food Sci. Technol. 2016, 13, 31–38. [Google Scholar] [CrossRef]
- Begum, Y.A.; Deka, S.C. Green synthesis of pectin mediated hydroxyapatite nanoparticles from culinary banana bract and its characterization. Acta Aliment. 2017, 46, 428–438. [Google Scholar] [CrossRef]
- Di Donato, P.; Poli, A.; Taurisano, V.; Nicolaus, B.; Di Donato, P.; Poli, A.; Taurisano, V.; Nicolaus, B. Polysaccharides from bioagro-waste for new biomolecules. In Polysaccharides: Bioactivity and Biotechnology; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 603–637. [Google Scholar]
- Rivera-Rangel, R.D.; González-Muñoz, M.P.; Avila-Rodriguez, M.; Razo-Lazcano, T.A.; Solans, C. Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 60–67. [Google Scholar] [CrossRef]
- Vasquez, R.D.; Apostol, J.G.; de Leon, J.D.; Mariano, J.D.; Mirhan, C.M.C.; Pangan, S.S.; Reyes, A.G.M.; Zamora, E.T. Polysaccharide-mediated green synthesis of silver nanoparticles from Sargassum siliquosum J.G. Agardh: Assessment of toxicity and hepatoprotective activity. OpenNano 2016, 1, 16–24. [Google Scholar] [CrossRef]
- Kesharwani, J.; Yoon, K.Y.; Hwang, J.; Rai, M. Phytofabrication of silver nanoparticles by leaf extract of Datura metel: Hypothetical mechanism involved in synthesis. J. Bionanosci. 2009, 3, 39–44. [Google Scholar] [CrossRef]
- Wang, C.; Mathiyalagan, R.; Kim, Y.J.; Castro-Aceituno, V.; Singh, P.; Ahn, S.; Wang, D.; Yang, D.C. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. Int. J. Nanomed. 2016, 11, 3691–3701. [Google Scholar] [CrossRef]
- Wang, B.; Yang, G.; Chen, J.; Fang, G. Green synthesis and characterization of gold nanoparticles using lignin nanoparticles. Nanomaterials 2020, 10, 1869. [Google Scholar] [CrossRef]
- Waiba, P.S.; Sherab, J.; Hingmang, A.R. An empirical study on quality of education: A case study in primary schools in wangduephodrang. Int. J. Eng. Appl. Sci. Technol. 2021, 6, 36–44. [Google Scholar]
- Cheng, J.; Lin, X.; Wu, X.; Liu, Q.; Wan, S.; Zhang, Y. Preparation of a multifunctional silver nanoparticles polylactic acid food packaging film using mango peel extract. Int. J. Biol. Macromol. 2021, 188, 678–688. [Google Scholar] [CrossRef]
- Saha, P.; Mahiuddin, M.; Islam, A.B.M.N.; Ochiai, B. Biogenic Synthesis and Catalytic Efficacy of Silver Nanoparticles Based on Peel Extracts of Citrus macroptera Fruit. ACS Omega 2021, 6, 18260–18268. [Google Scholar] [CrossRef]
- Lomelí-Rosales, D.A.; Zamudio-Ojeda, A.; Reyes-Maldonado, O.K.; López-Reyes, M.E.; Basulto-Padilla, G.C.; Lopez-Naranjo, E.J.; Zuñiga-Mayo, V.M.; Velázquez-Juárez, G. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Capsicum chinense Plant. Molecules 2022, 27, 1692. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. Fabrication and characterization of novel lignocellulosic biomass tailored Fe3O4 nanocomposites: Influence of annealing temperature and chlorazol black e sequestration. RSC Adv. 2015, 5, 107466–107473. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; García-Simarro, M.P.; Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. A review on the encapsulation of “eco-friendly” compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 2024, 280, 136030. [Google Scholar] [CrossRef]
- Flores-Contreras, E.A.; González-González, R.B.; Pablo Pizaña-Aranda, J.J.; Parra-Arroyo, L.; Rodríguez-Aguayo, A.A.; Iñiguez-Moreno, M.; González-Meza, G.M.; Araújo, R.G.; Ramírez-Gamboa, P.; Parra-Saldivar, R.; et al. Agricultural waste as a sustainable source for nanoparticle synthesis and their antimicrobial properties for food preservation. Front. Nanotechnol. 2024, 6, 1346069. [Google Scholar] [CrossRef]
- Nath, S.; Haque, M.; Sinha, S.N. A Sustainable Approach to Biosynthesis of Nanoparticles from Agro-waste. In Agro-Waste to Microbe Assisted Value Added Product: Challenges and Future Prospects: Recent Developments in Agro-Waste Valorization Research; Springer Nature: Cham, Switzerland, 2024; pp. 405–412. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Shin, H.S.; Jacob, J.M.; Pugazhendhi, A.; Bhaisare, M.; Kumar, G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 2018, 25, 10164–10183. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Benelli, G.; Kumar, G.; Pugazhendhi, A.; Kim, D.S.; Shin, H.S. Anti-diabetic Potential of Silver Nanoparticles Synthesized with Argyreia nervosa Leaf Extract High Synergistic Antibacterial Activity with Standard Antibiotics Against Foodborne Bacteria. J. Clust. Sci. 2017, 28, 1709–1727. [Google Scholar] [CrossRef]
- Shen, Z.; Han, G.; Liu, C.; Wang, X.; Sun, R. Green synthesis of silver nanoparticles with bagasse for colorimetric detection of cysteine in serum samples. J. Alloys Compd. 2016, 686, 82–89. [Google Scholar] [CrossRef]
- Yang, N.; Wei, X.F.; Li, W.H. Sunlight irradiation induced green synthesis of silver nanoparticles using peach gum polysaccharide and colorimetric sensing of H2O2. Mater. Lett. 2015, 154, 21–24. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 368, 58–63. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Harish, B.S.; Uppuluri, K.B.; Anbazhagan, V. Synthesis of fibrinolytic active silver nanoparticle using wheat bran xylan as a reducing and stabilizing agent. Carbohydr. Polym. 2015, 132, 104–110. [Google Scholar] [CrossRef]
- Velmurugan, P.; Park, J.H.; Lee, S.M.; Jang, J.S.; Yi, Y.J.; Han, S.S.; Lee, S.H.; Cho, K.M.; Cho, M.; Oh, B.T. Reduction of silver (I) using defatted cashew nut shell starch and its structural comparison with commercial product. Carbohydr. Polym. 2015, 133, 39–45. [Google Scholar] [CrossRef]
- Yang, N.; Li, W.H. Mango peel extract mediated novel route for synthesis of silver nanoparticles and antibacterial application of silver nanoparticles loaded onto non-woven fabrics. Ind. Crops Prod. 2013, 48, 81–88. [Google Scholar] [CrossRef]
- Chutrakulwong, F.; Thamaphat, K.; Limsuwan, P. Photo-irradiation induced green synthesis of highly stable silver nanoparticles using durian rind biomass: Effects of light intensity, exposure time and ph on silver nanoparticles formation. J. Phys. Commun. 2020, 4, 095015. [Google Scholar] [CrossRef]
- Pechyen, C.; Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N. Waste fruit peel—Mediated green synthesis of biocompatible gold nanoparticles. J. Mater. Res. Technol. 2021, 14, 2982–2991. [Google Scholar] [CrossRef]
- Lopes, L.C.; Lima, D.; Hacke, A.C.M.; Schveigert, B.S.; Calaça, G.N.; Simas, F.F.; Pereira, R.P.; Iacomini, M.; Viana, A.G.; Pessôa, C.A. Gold nanoparticles capped with polysaccharides extracted from pineapple gum: Evaluation of their hemocompatibility and electrochemical sensing properties. Talanta 2021, 223, 121634. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.P.; Ng, S.H.; Huang, Y.; Li, S.F.Y. Green synthesis of gold nanoparticles using palm oil mill effluent (POME): A low-cost and eco-friendly viable approach. Bioresour. Technol. 2012, 113, 132–135. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf. B Biointerfaces 2010, 80, 45–50. [Google Scholar] [CrossRef]
- Suganya, K.S.U.; Govindaraju, K.; Kumar, V.G.; Karthick, V.; Parthasarathy, K. Pectin mediated gold nanoparticles induces apoptosis in mammary adenocarcinoma cell lines. Int. J. Biol. Macromol. 2016, 93, 1030–1040. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Palakshi Reddy, B.; Sarada, N.C.; Chidambaram, K.; Khadeer Pasha, S. Watermelon rind-mediated green synthesis of noble palladium nanoparticles: Catalytic application. Appl. Nanosci. 2015, 5, 223–228. [Google Scholar] [CrossRef]
- Ishak, N.A.I.M.; Kamarudin, S.K.; Timmiati, S.N.; Basri, S.; Karim, N.A. Exploration of biogenic Pt nanoparticles by using agricultural waste (Saccharum officinarum L. Bagasse Extract) as nanocatalyst for the electrocatalytic oxidation of methanol. Mater. Today Proc. 2019, 42, 138–147. [Google Scholar] [CrossRef]
- Ishak, N.A.I.M.; Kamarudin, S.K.; Timmiati, S.N.; Karim, N.; Basri, S. Enhanced performance of methanol oxidation reaction via green synthesis of platinum electro-catalyst from sugar cane bagasse. Int. J. Energy Res. 2021, 45, 7380–7403. [Google Scholar] [CrossRef]
- Li, J.; Tian, B.; Li, T.; Dai, S.; Weng, Y.; Lu, J.; Xu, X.; Jin, Y.; Pang, R.; Hua, Y. Biosynthesis of Au, Ag and Au–Ag bimetallic nanoparticles using protein extracts of deinococcus radiodurans and evaluation of their cytotoxicity. Int. J. Nanomed. 2018, 13, 1411–1424. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Gómez-López, P.; Espro, C.; Rodríguez-Padrón, D.; Balu, A.M.; Ivars-Barceló, F.; Moreda, O.I.; Alvarado-Beltrán, C.G.; Luque, R. Mechanochemical preparation of magnetically separable fe and cu-based bimetallic nanocatalysts for vanillin production. Nanomaterials 2021, 11, 1050. [Google Scholar] [CrossRef]
- Oruç, Z.; Ergüt, M.; Uzunoǧlu, D.; Özer, A. Green synthesis of biomass-derived activated carbon/Fe-Zn bimetallic nanoparticles from lemon (Citrus limon (L.) Burm. f.) wastes for heterogeneous Fenton-like decolorization of Reactive Red 2. J. Environ. Chem. Eng. 2019, 7, 103231. [Google Scholar] [CrossRef]
- Kang, C.W.; Kolya, H. Green synthesis of ag-au bimetallic nanocomposites using waste tea leaves extract for degradation congo red and 4-nitrophenol. Sustainability 2021, 13, 3318. [Google Scholar] [CrossRef]
- Chandna, S.; Thakur, N.S.; Reddy, Y.N.; Kaur, R.; Bhaumik, J. Engineering Lignin Stabilized Bimetallic Nanocomplexes: Structure, Mechanistic Elucidation, Antioxidant, and Antimicrobial Potential. ACS Biomater. Sci. Eng. 2019, 5, 3212–3227. [Google Scholar] [CrossRef]
- Oskam, G. Metal oxide nanoparticles: Synthesis, characterization and application. J. Sol-Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Annu; Ali, A.; Ahmed, S. Green Synthesis of Metal, Metal Oxide Nanoparticles, and Their Various Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef]
- López-Téllez, G.; Balderas-Hernández, P.; Barrera-Díaz, C.E.; Vilchis-Nestor, A.R.; Roa-Morales, G.; Bilyeu, B. Green method to form iron oxide nanorods in orange peels for chromium(VI) reduction. J. Nanosci. Nanotechnol. 2013, 13, 2354–2361. [Google Scholar] [CrossRef]
- Sebastian, A.; Nangia, A.; Prasad, M.N.V. A green synthetic route to phenolics fabricated magnetite nanoparticles from coconut husk extract: Implications to treat metal contaminated water and heavy metal stress in Oryza sativa L. J. Clean. Prod. 2018, 174, 355–366. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Li, Y.; Tang, J.; Wang, Z.; Tang, J.; Li, X.; Hu, K. Facile synthesis of tea waste/Fe3O4 nanoparticle composite for hexavalent chromium removal from aqueous solution. RSC Adv. 2017, 7, 7576–7590. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Li, J.; Mei, H.; Yu, Y.; Hu, S.; Chen, X.; Li, X.; Guo, G. Agro-waste mediated biopolymer for production of biogenic nano iron oxide with superparamagnetic power and antioxidant strength. J. Clean. Prod. 2021, 311, 127512. [Google Scholar] [CrossRef]
- Prasad, C.; Gangadhara, S.; Venkateswarlu, P. Bio-inspired green synthesis of Fe3O4 magnetic nanoparticles using watermelon rinds and their catalytic activity. Appl. Nanosci. 2016, 6, 797–802. [Google Scholar] [CrossRef]
- Stan, M.; Lung, I.; Soran, M.L.; Leostean, C.; Popa, A.; Stefan, M.; Lazar, M.D.; Opris, O.; Silipas, T.D.; Porav, A.S. Removal of antibiotics from aqueous solutions by green synthesized magnetite nanoparticles with selected agro-waste extracts. Process Saf. Environ. Prot. 2017, 107, 357–372. [Google Scholar] [CrossRef]
- Yadav, S.; Chauhan, M.; Mathur, D.; Jain, A.; Malhotra, P. Sugarcane bagasse-facilitated benign synthesis of Cu2O nanoparticles and its role in photocatalytic degradation of toxic dyes: A trash to treasure approach. Environ. Dev. Sustain. 2021, 23, 2071–2091. [Google Scholar] [CrossRef]
- Xue, H.; Chen, Y.; Liu, X.; Qian, Q.; Luo, Y.; Cui, M.; Chen, Y.; Yang, D.P.; Chen, Q. Visible light-assisted efficient degradation of dye pollutants with biomass-supported TiO2 hybrids. Mater. Sci. Eng. C 2018, 82, 197–203. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, H.; Chen, L.; Zhu, G.; Wang, Z.; Xu, H.; Yu, A. Supercapacitive properties of Mn3O4 nanoparticles bio-synthesized from banana peel extract. RSC Adv. 2014, 4, 23649–23652. [Google Scholar] [CrossRef]
- Musa, A.; Ahmad, M.; Tian, C.Y.; Salisu, A.; Barde, M.I.; Olawale, L.A.; Usman, A. Synthesis and characterization of copper nanoparticles using different concentration of rice straw. ChemSearch J. 2019, 10, 64–70. [Google Scholar]
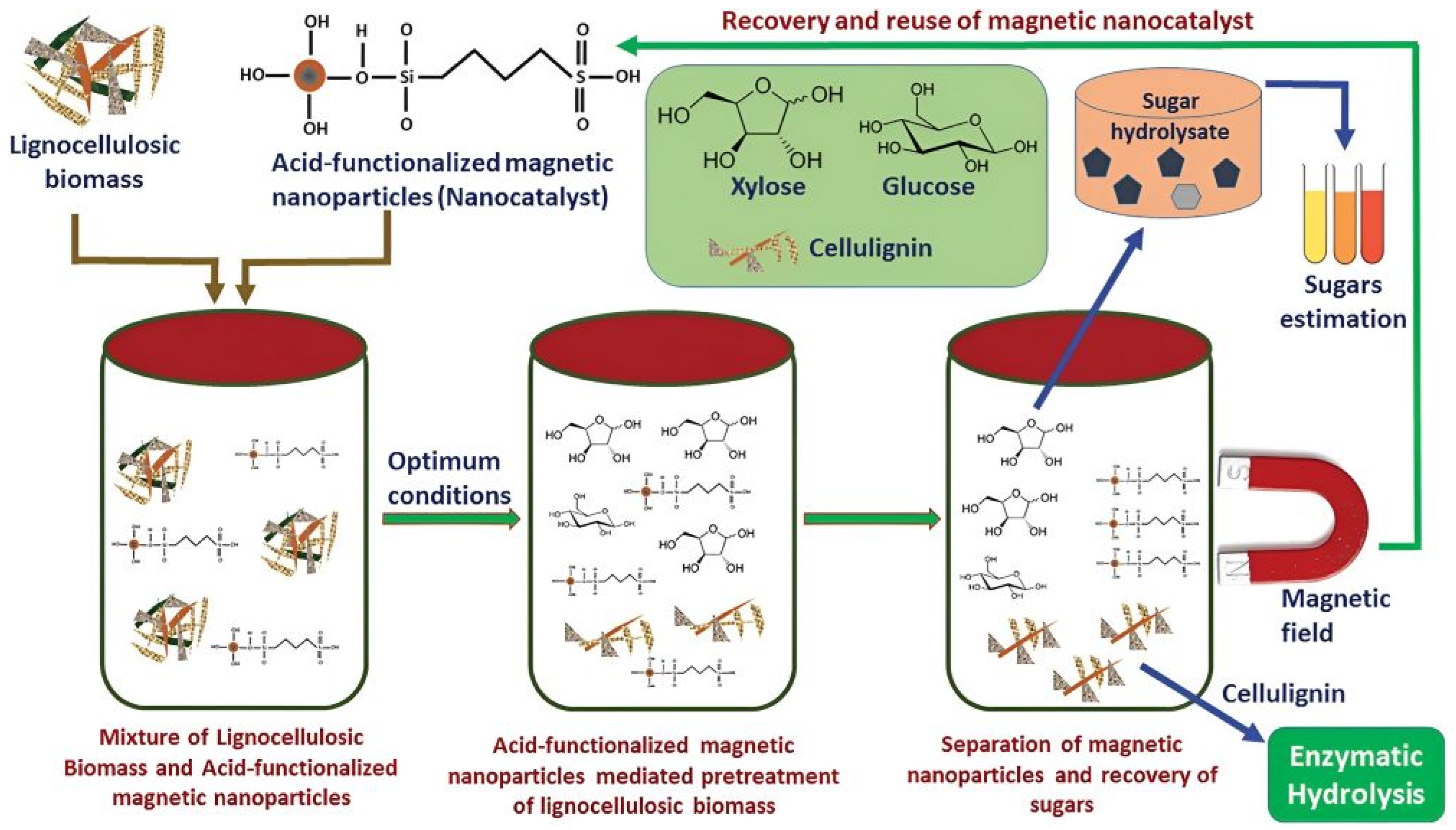
- Ingle, A.P.; Chandel, A.K.; Antunes, F.A.F.; Rai, M.; da Silva, S.S. New trends in application of nanotechnology for the pretreatment of lignocellulosic biomass. Biofuels Bioprod. Biorefining 2019, 13, 776–788. [Google Scholar] [CrossRef]
- Areeshi, M.Y. Rice straw mediated green synthesis and characterization of iron oxide nanoparticles and its application to improve thermal stability of endoglucanase enzyme. Int. J. Food Microbiol. 2022, 374, 109722. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.M.; Deng, L.; Guo, Q.X.; Fu, Y. Hydrolysis of biomass by magnetic solid acid. Energy Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Zhang, M. Hemicellulose and unlocking potential for sustainable applications in biomedical, packaging, and material sciences: A narrative review. Int. J. Biol. Macromol. 2024, 28, 135657. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Jung, S.; Yun, H.; Won, S.; Choi, I.G.; Kwak, H.W. Effect of hemicellulose hydrolysate addition on the dehydration and redispersion characteristic of cellulose nanofibrils. Carbohydr. Polym. 2024, 334, 122036. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.O.; Zandoná Filho, A.; de Freitas, R.A.; Noseda, M.D.; Szameitat, E.S.; Faulds, C.; Coutinho, P.; Bertrand, E.; et al. Lignin from oil palm empty fruit bunches: Characterization, biological activities and application in green synthesis of silver nanoparticles. Int. J. Biol. Macromol. 2021, 167, 1499–1507. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Ran, G.; Liu, Y.; Liu, S.; Zhou, B.; Wang, Z. Synthesis of lignin-modified silica nanoparticles from black liquor of rice straw pulping. Powder Technol. 2013, 246, 664–668. [Google Scholar] [CrossRef]
- Hu, S.; Hsieh, Y.L. Silver nanoparticle synthesis using lignin as reducing and capping agents: A kinetic and mechanistic study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef]
- Dmochowska, A.; Czajkowska, J.; Jędrzejewski, R.; Stawiński, W.; Migdał, P.; Fiedot-Toboła, M. Pectin based banana peel extract as a stabilizing agent in zinc oxide nanoparticles synthesis. Int. J. Biol. Macromol. 2020, 165, 1581–1592. [Google Scholar] [CrossRef]
- Wang, D.; Geng, W.; Li, Q.; Li, G.; Zhang, D.; Zhang, H. Ultrasonic green synthesis of silver nanoparticles mediated by Pectin: Characterization and evaluation of the cytotoxicity, antioxidant, and colorectal carcinoma properties. Arab. J. Chem. 2022, 15, 103500. [Google Scholar] [CrossRef]
- Pallavicini, P.; Arciola, C.R.; Bertoglio, F.; Curtosi, S.; Dacarro, G.; D’Agostino, A.; Ferrari, F.; Milanese, C.; Rossi, C.; Taglietti, A. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J. Colloid Interface Sci. 2017, 498, 271–281. [Google Scholar] [CrossRef]
- Khan, Z. Encapsulation of silver nanoparticles into the helix of water soluble starch and their sensing propierties. Int. J. Biol. Macromol. 2019, 136, 165–176. [Google Scholar] [CrossRef]
- Madhusudhan, A.; Reddy, G.B.; Krishana, I.M. Green Synthesis of Gold Nanoparticles by Using Natural Gums. In Nanomaterials and Plant Potential; Husen, A., Iqbal, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 111–134. [Google Scholar] [CrossRef]
- Taher, M.A.; Khojah, E.; Darwish, M.S.; Elsherbiny, E.A.; Elawady, A.A.; Dawood, D.H. Biosynthesis of Silver Nanoparticles by Polysaccharide of Leucaena leucocephala Seeds and Their Anticancer, Antifungal Properties and as Preservative of Composite Milk Sample. J. Nanomater. 2022, 2022, 7490221. [Google Scholar] [CrossRef]
- Tagad, C.K.; Rajdeo, K.S.; Kulkarni, A.; More, P.; Aiyer, R.C.; Sabharwal, S. Green synthesis of polysaccharide stabilized gold nanoparticles: Chemo catalytic and room temperature operable vapor sensing application. RSC Adv. 2014, 4, 24014–24019. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; López-Cota, A.G.; Moreno-Vásquez, M.J.; Graciano-Verdugo, A.Z.; Quintero-Reyes, I.E.; Del-Toro-Sánchez, C.L.; Tapia-Hernández, J.A. Sustainable-green synthesis of silver nanoparticles using safflower (Carthamus tinctorius L.) waste extract and its antibacterial activity. Heliyon 2021, 7, e06923. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Z.; Qin, Z.; El-kott, A.F.; AlShehri, M.A. Sustainable synthesis of Au nanoparticles templated over chitosan/pectin hydrogel for the treatment of gastric cancer. J. Mol. Struct. 2024, 1318, 139260. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Zhang, X.; Lin, J.; Wang, M.H. In situ, synthesis of chitosan fabricated tellurium nanoparticles for improved antimicrobial and anticancer applications. Int. J. Biol. Macromol. 2024, 258 Pt 1, 128778. [Google Scholar] [CrossRef]
- Park, H.; Kim, W.; Kim, M.; Lee, G.; Lee, W.; Park, J. Eco-friendly and enhanced colorimetric detection of aluminum ions using pectin-rich apple extract-based gold nanoparticles. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2021, 245, 118880. [Google Scholar] [CrossRef]
- Chrouda, A.; Ayed, D.; Zinoubi, K.; Majdoub, H.; Jaffrezic-Renault, N. Highly stable and ultra-sensitive amperometric aptasensor based on pectin stabilized gold nanoparticles on graphene oxide modified GCE for the detection of aflatoxin M1. Food Chem. Adv. 2022, 1, 100068. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Mosalam, F.M.; Ghorab, M.M.; Hanora, A.; Elbarbary, A.M. Antimicrobial, antioxidant and anticancer activities of zinc nanoparticles prepared by natural polysaccharides and gamma radiation. Int. J. Biol. Macromol. 2018, 107, 2298–2311. [Google Scholar] [CrossRef]
- Pal, N.; Agarwal, M.; Ghosh, A. Green synthesis of silver nanoparticles using polysaccharide-based guar gum. Mater. Today Proc. 2023, 76, 212–218. [Google Scholar] [CrossRef]
- Jagtap, R.R.; Garud, A.; Puranik, S.S.; Rudrapal, M.; Ansari, M.A.; Alomary, M.N.; Alshamrani, M.; Salawi, A.; Almoshari, Y.; Khan, J.; et al. Biofabrication of silver nanoparticles (AgNPs) using embelin for effective therapeutic management of lung cancer. Front. Nutr. 2022, 9, 960674. [Google Scholar] [CrossRef]
- Martínez-Torres, A.C.; Lorenzo-Anota, H.Y.; García-Juárez, M.G.; Zarate-Triviño, D.G.; Rodríguez-Padilla, C. Chitosan gold nanoparticles induce different ROS-dependent cell death modalities in leukemic cells. Int. J. Nanomed. 2019, 14, 7173–7190. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, J.H.; Choi, Y.J.; Oh, J.M.; Park, J. Hyaluronic acid-coated gold nanoparticles as a controlled drug delivery system for poorly water-soluble drugs. RSC Adv. 2023, 13, 5529–5537. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, N.M.; Abo-Eleneen, M.A.; Al-Madboly, L.A.; Sharaf, M.M.; Othman, S.S.; Ibrahim, O.M.; Mubarak, M.S. Biogenically synthesized polysaccharides-capped silver nanoparticles: Immunomodulatory and antibacterial potentialities against resistant Pseudomonas aeruginosa. Front. Bioeng. Biotechnol. 2020, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.D.; De Paula, L.B.; Melo, P.S.; Ferreira, I.R.; Almeida, A.B.A.; Torsoni, A.S.; Alves, O.L. In vivo evaluation of complex biogenic silver nanoparticle and enoxaparin in wound healing. J. Nanomater. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.; Lillard, J.W.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef]
- Khan, S.; Zahoor, M.; Khan, R.S.; Ikram, M.; Islam, N.U. The impact of silver nanoparticles on the growth of plants: The agriculture applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef]
- Prabha, M.; Malviya, T.; Kumar, A.; Singh, V. Effect of Gum acacia capped Cu-Ag bimetallic nanoparticles on germination and growth of gram seeds (Cicer arietinum L.). Mater. Today Proc. 2023, 106, 122–133. [Google Scholar] [CrossRef]
- Asanova, A.A.; Yashin, S.E.; Trofimova, T.V.; Polonskiy, V.I. Application of silver nanoparticles to improve wheat seedlings growth. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 052041. [Google Scholar] [CrossRef]
- Mani, V.; Devasenathipathy, R.; Chen, S.M.; Vasantha, V.S.; Ali, M.A.; Huang, S.T.; Al-Hemaid, F.M. A simple electrochemical platform based on pectin stabilized gold nanoparticles for picomolar detection of biologically toxic amitrole. Analyst 2015, 140, 5764–5771. [Google Scholar] [CrossRef]
- Flores-Gómez, J.; Mota-Macías, S.; Guerrero-Jiménez, J.P.; Romero-Arellano, V.H.; Morales-Rivera, J. Sol–Gel Synthesis of TiO2 with Pectin and Their Efficiency in Solar Cells Sensitized by Quantum Dots. Gels 2024, 10, 470. [Google Scholar] [CrossRef]
- Apriceno, A.; Silvestro, I.; Girelli, A.; Francolini, I.; Pietrelli, L.; Piozzi, A. Preparation and characterization of chitosan-coated manganese-ferrite nanoparticles conjugated with laccase for environmental bioremediation. Polymers 2021, 13, 1453. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Ayub, A.; Anam, A.; Aljuwayid, A.M.; Alwash, S.W.; Abbass, R.; Ruhaima, A.A.K.; Potrich, E.; Sillanpaa, M.; Gul, S.; et al. Fabrication of ZnO decorated porous chitosan beads for the sustainable bioremediation of Cr(VI) contaminated water. J. Environ. Chem. Eng. 2023, 11, 110445. [Google Scholar] [CrossRef]
- Aziz, A.; Ali, N.; Khan, A.; Bilal, M.; Malik, S.; Ali, N.; Khan, H. Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for azo dyes. Int. J. Biol. Macromol. 2020, 153, 502–512. [Google Scholar] [CrossRef] [PubMed]
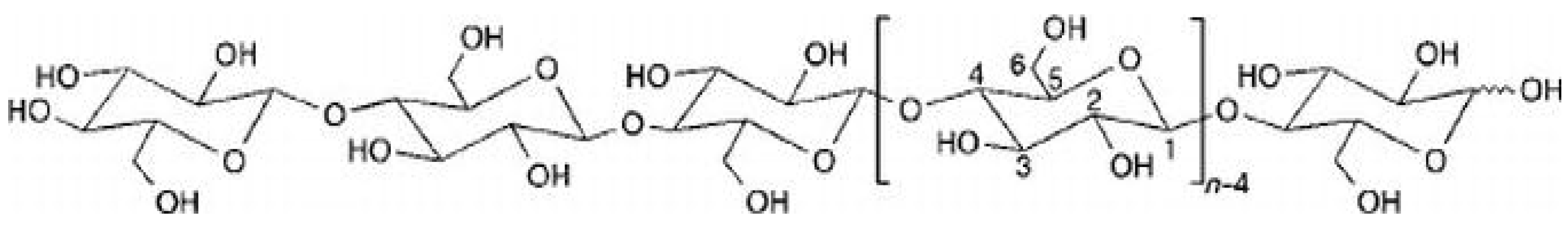
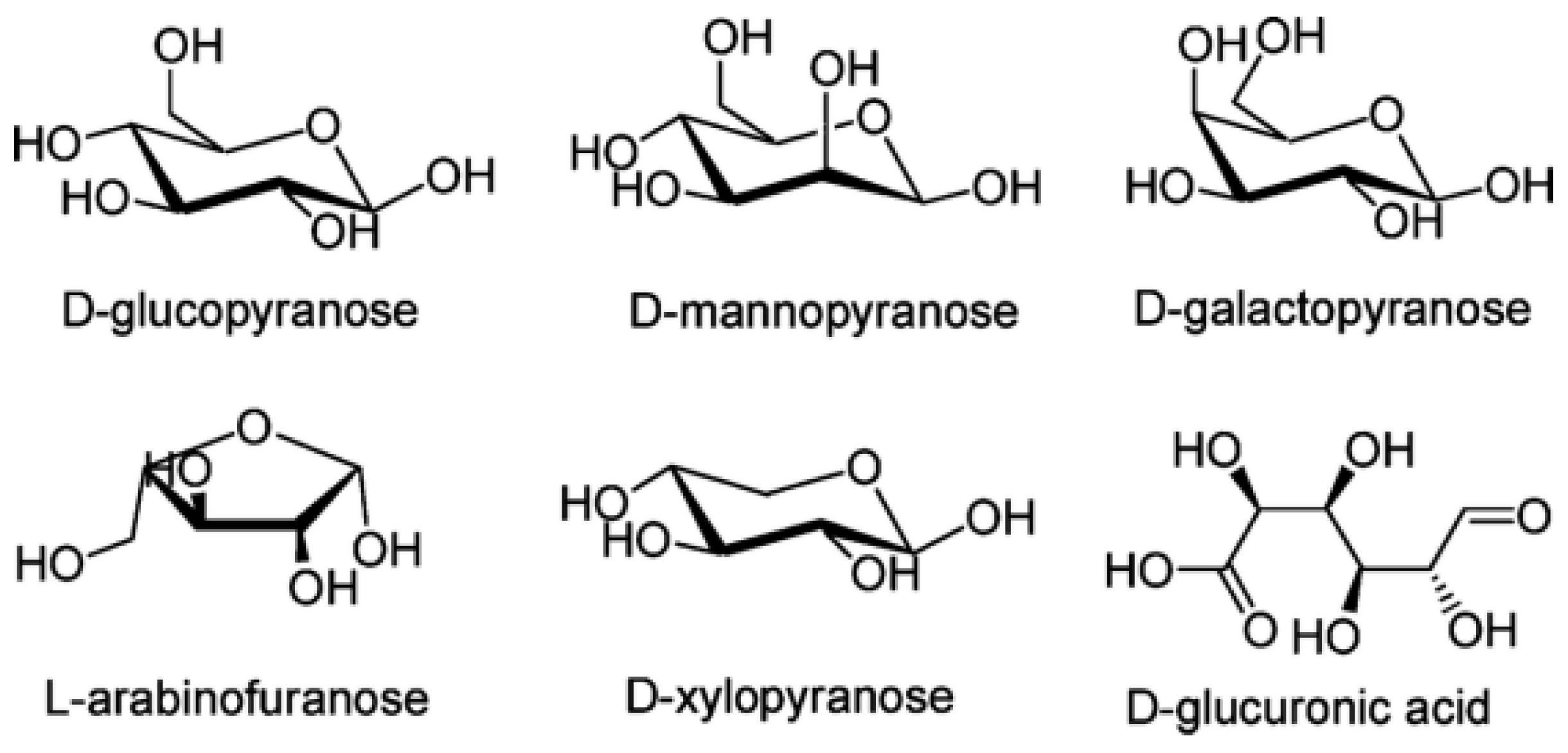



| Hemicellulose Groups | Characteristics |
|---|---|
| 1 | Xyloglucans that have a structure that is a glucose residue where xylose, fucose, or galactose residues are coupled. |
| 2 | Xylans include glucuronoxylans, which have a structure of xylose residues to which glucuronic acid residues are attached. |
| 3 | Mannans that have a structure of mannose residues and glucomannans. They have a backbone of mannose and glucose residues. |
| 4 | Mixed link glucans are unbranched chains of D-glucose residues joined by β-(1,3) or β-(1,4) bonds. |
| Agro-Waste or By-Product | Properties of the Obtained Nanoparticles | Shape and Size of Synthesized Nanoparticles | Reference |
|---|---|---|---|
| Orange peel | Cr (VI) removal | Nanoparticles clustered in nanorods in the range of 20 to 40 nm in diameter. | [131] |
| Tea waste | Cr (VI) removal | Form not specified. Size approximately 20 nm. | [133] |
| Coconut (Cocos nucifera L.) husk | Absorption of Ca and Cd | Not specified. | [132] |
| Papaya leaves | Dye sequestration | Nanoparticles with distorted spherical shape and diameter of 10.39 nm. | [98] |
| Paddy and wheat straw | Magnetic and antioxidant | Spherical nanoparticles with sizes 20–32 nm. | [134] |
| Watermelon (Citrullus lanatus) rinds | Catalyst in the synthesis of 2-oxo-1,2,3,4-tetrahydropyrimidine derivatives | Spherical nanoparticles with sizes 2–20 nm. | [135] |
| Peel extracts of C. limon, V. vinifera, and C. sativus | Removal of antibiotics | Spherical and polyhedral agglomerated nanoparticles. Diameters from 8 to 12 nm. | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Larez, F.L.; Vergel-Alfonso, A.A.; Ruiz-Velducea, H.A.; Ozuna-Valencia, K.H.; Urías-Torres, M.Á.; Rodríguez-Félix, D.E.; Moreno-Vásquez, M.J.; Barreras-Urbina, C.G.; Álvarez-Chávez, C.R.; López-Corona, B.E.; et al. Polysaccharides from Agro-Industrial Waste and By-Products: An Overview on Green Synthesis of Metallic Nanoparticles—An Ecofriendly Approach. Polysaccharides 2025, 6, 53. https://doi.org/10.3390/polysaccharides6020053
García-Larez FL, Vergel-Alfonso AA, Ruiz-Velducea HA, Ozuna-Valencia KH, Urías-Torres MÁ, Rodríguez-Félix DE, Moreno-Vásquez MJ, Barreras-Urbina CG, Álvarez-Chávez CR, López-Corona BE, et al. Polysaccharides from Agro-Industrial Waste and By-Products: An Overview on Green Synthesis of Metallic Nanoparticles—An Ecofriendly Approach. Polysaccharides. 2025; 6(2):53. https://doi.org/10.3390/polysaccharides6020053
Chicago/Turabian StyleGarcía-Larez, Frida Lourdes, Ariel Alain Vergel-Alfonso, Hylse Aurora Ruiz-Velducea, Karla Hazel Ozuna-Valencia, Miguel Ángel Urías-Torres, Dora Evelia Rodríguez-Félix, María Jesús Moreno-Vásquez, Carlos Gregorio Barreras-Urbina, Clara Rosalía Álvarez-Chávez, Betzabe Ebenhezer López-Corona, and et al. 2025. "Polysaccharides from Agro-Industrial Waste and By-Products: An Overview on Green Synthesis of Metallic Nanoparticles—An Ecofriendly Approach" Polysaccharides 6, no. 2: 53. https://doi.org/10.3390/polysaccharides6020053
APA StyleGarcía-Larez, F. L., Vergel-Alfonso, A. A., Ruiz-Velducea, H. A., Ozuna-Valencia, K. H., Urías-Torres, M. Á., Rodríguez-Félix, D. E., Moreno-Vásquez, M. J., Barreras-Urbina, C. G., Álvarez-Chávez, C. R., López-Corona, B. E., Quintero-Reyes, I. E., Rodríguez-Félix, F., & Tapia-Hernández, J. A. (2025). Polysaccharides from Agro-Industrial Waste and By-Products: An Overview on Green Synthesis of Metallic Nanoparticles—An Ecofriendly Approach. Polysaccharides, 6(2), 53. https://doi.org/10.3390/polysaccharides6020053








