Ionic Strength of Methylcellulose-Based Films: An Alternative for Modulating Mechanical Performance and Hydrophobicity for Potential Food Packaging Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Production of MC-Based Films and Active Films
2.3. Film Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Energy Dispersive Spectroscopy (EDS)
2.3.3. Thermogravimetry (TG)
2.3.4. Contact Angle
2.3.5. Light Barrier Property and Moisture Content of Films
2.3.6. Density, Thickness, and Mechanical Performance
2.3.7. Water Vapor Permeability (WVP)
2.3.8. Antibacterial Activity
2.4. Statistical Analysis
3. Results
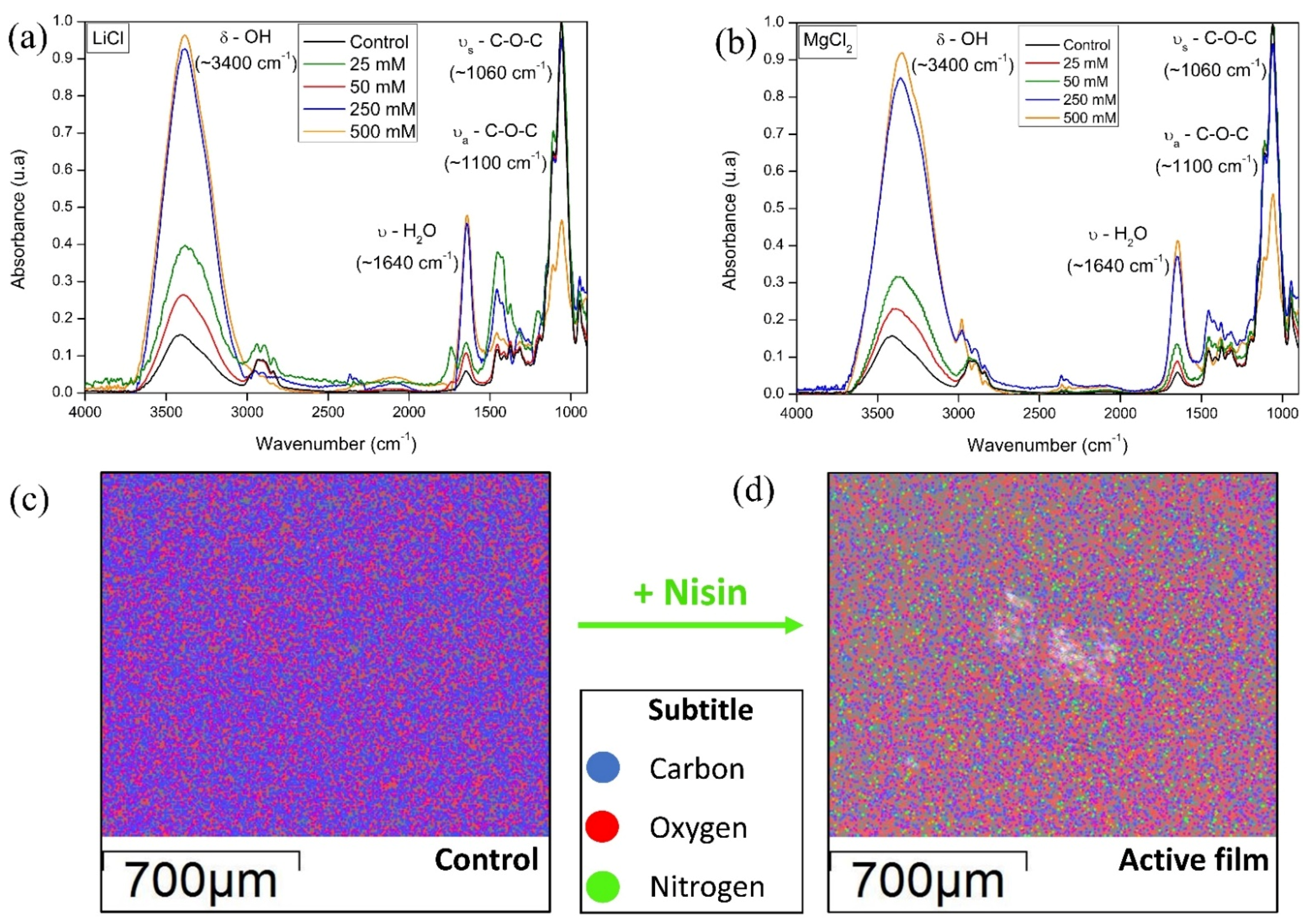
3.1. FTIR, EDS, and Contact Angle
3.2. WVP and Light Barrier Property
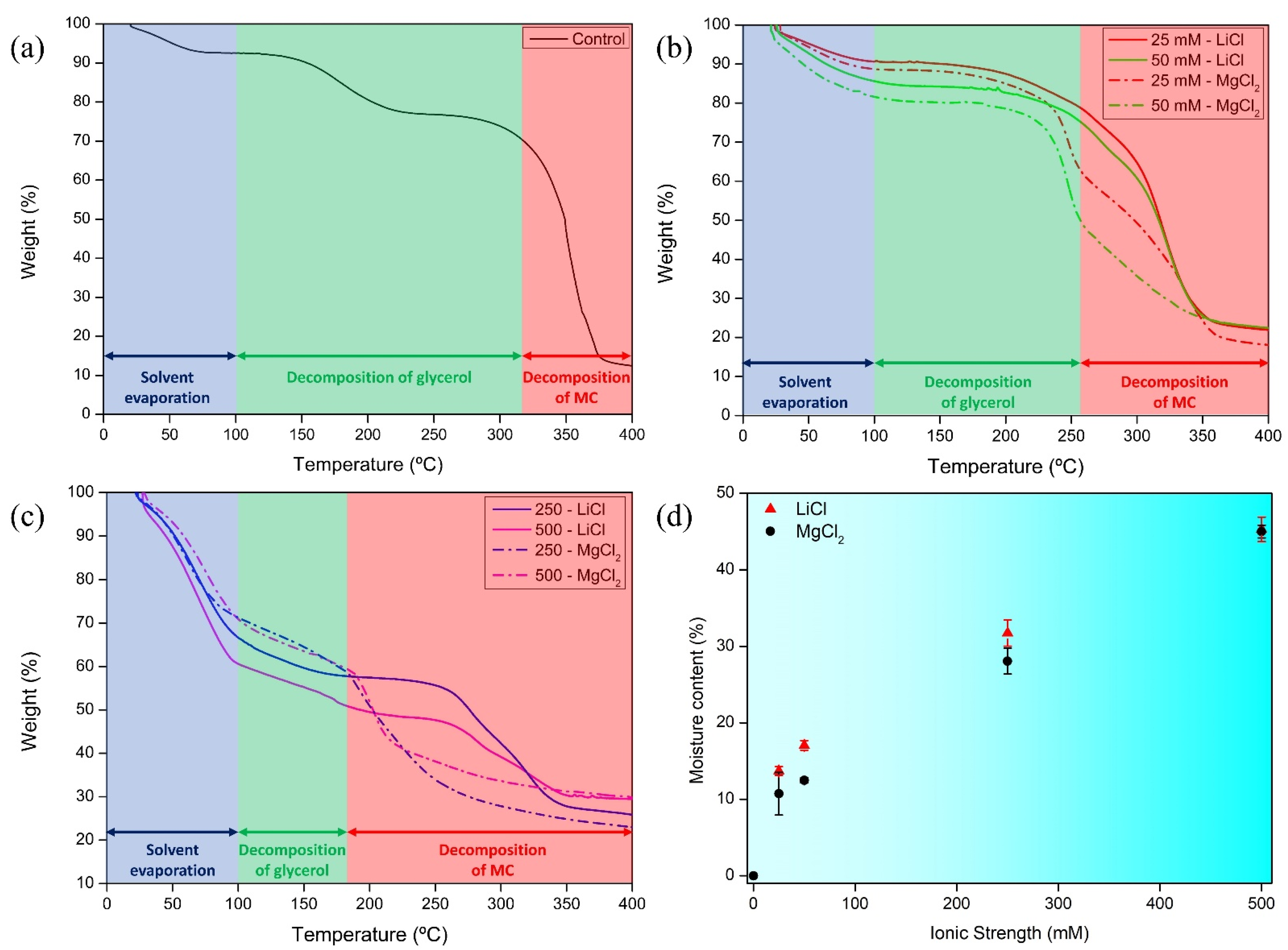
3.3. Thermogravimetry and Moisture Content of Films
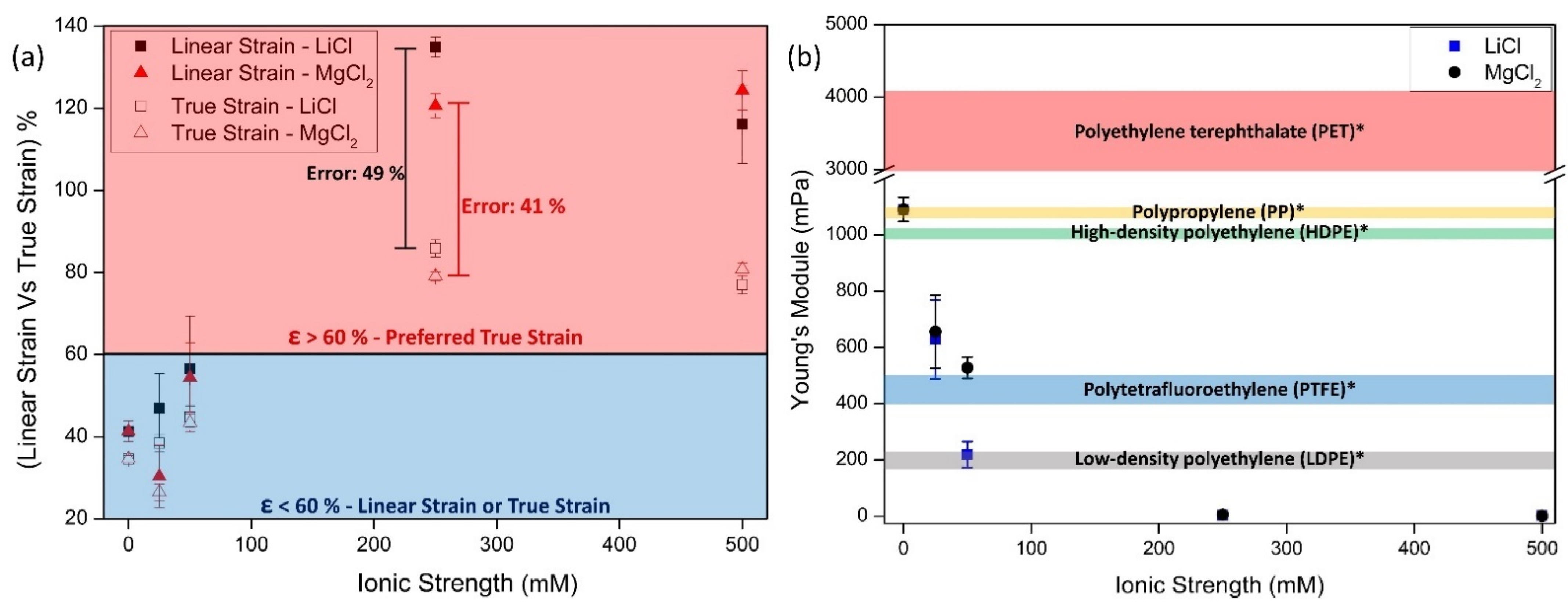
3.4. Density, Thickness, and Mechanical Perfomance
3.5. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; Soares, N.d.F.F. Glycerol and Triethyl Citrate Plasticizer Effects on Molecular, Thermal, Mechanical, and Barrier Properties of Cellulose Acetate Films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Aytac, Z.; Huang, R.; Vaze, N.; Xu, T.; Eitzer, B.D.; Krol, W.; MacQueen, L.A.; Chang, H.; Bousfield, D.W.; Chan-Park, M.B.; et al. Development of Biodegradable and Antimicrobial Electrospun Zein Fibers for Food Packaging. ACS Sustain. Chem. Eng. 2020, 8, 15354–15365. [Google Scholar] [CrossRef]
- Silva, R.R.A.; de Freitas, P.A.V.; Teixeira, S.C.; de Oliveira, T.V.; Marques, C.S.; Stringheta, P.C.; dos Santos Pires, A.C.; Ferreira, S.O.; Soares, N.D.F.F. Plasticizer Effect and Ionic Cross-Linking: The Impact of Incorporating Divalent Salts in Methylcellulose Films for Colorimetric Detection of Volatile Ammonia. Food Biophys. 2021, 17, 59–74. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.J.B.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.D.; Freire, C.S.R. Antioxidant and Antimicrobial Films Based on Brewers Spent Grain Arabinoxylans, Nanocellulose and Feruloylated Compounds for Active Packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Siiva, R.R.A.; de Oiiveira, T.V.; Soares, R.R.A.; Soares, N.F.F. Biodegradable Film Development by Nisin Z Addition into Hydroxypropylmethylcellulose Matrix for Mozzarella Cheese Preservation. Int. J. Food Stud. 2020, 9, 360–372. [Google Scholar] [CrossRef]
- Fialho e Moraes, A.R.; Pola, C.C.; Bilck, A.P.; Yamashita, F.; Tronto, J.; Medeiros, E.A.A.; Soares, N.d.F.F. Starch, Cellulose Acetate and Polyester Biodegradable Sheets: Effect of Composition and Processing Conditions. Mater. Sci. Eng. C 2017, 78, 932–941. [Google Scholar] [CrossRef]
- Yan, N.; Capezzuto, F.; Lavorgna, M.; Buonocore, G.G.; Tescione, F.; Xia, H.; Ambrosio, L. Borate Cross-Linked Graphene Oxide-Chitosan as Robust and High Gas Barrier Films. Nanoscale 2016, 8, 10783–10791. [Google Scholar] [CrossRef] [Green Version]
- Caner, C.; Hernandez, R.J.; Harte, B.R. High-Pressure Processing Effects on the Mechanical, Barrier and Mass Transfer Properties of Food Packaging Flexible Structures: A Critical Review. Packag. Technol. Sci. 2004, 17, 23–29. [Google Scholar] [CrossRef]
- Kuwano, K.; Tanaka, N.; Shimizu, T.; Nagatoshi, K.; Nou, S.; Sonomoto, K. Dual Antibacterial Mechanisms of Nisin Z against Gram-Positive and Gram-Negative Bacteria. Int. J. Antimicrob. Agents 2005, 26, 396–402. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.V.; de Freitas, P.A.V.; Pola, C.C.; da Silva, J.O.R.; Diaz, L.D.A.; Ferreira, S.O.; Soares, N.d.F.F. Development and Optimization of Antimicrobial Active Films Produced with a Reinforced and Compatibilized Biodegradable Polymers. Food Packag. Shelf Life 2020, 24, 100459. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Chu, C.; She, Y.; Jiang, S.; Zhai, L.; Jiang, S.; Li, X. Diffusion and Antibacterial Properties of Nisin-Loaded Chitosan/Poly (L-Lactic Acid) Towards Development of Active Food Packaging Film. Food Bioprocess Technol. 2015, 8, 1657–1667. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.d.F.F.; Mattoso, L.H.C. Antimicrobial and Physical-Mechanical Properties of Pectin/Papaya Puree/Cinnamaldehyde Nanoemulsion Edible Composite Films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ahmed, A.A.Q.; Awad, M.F.; Ul-Islam, M.; Subhan, F.; Ullah, M.W.; Yang, G. Development and Characterization of Yeast-Incorporated Antimicrobial Cellulose Biofilms for Edible Food Packaging Application. Polymers 2021, 13, 2310. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; UI-Islam, M.; Ullah, M.W.; Yang, G.A. Biobased Materials for Active Food Packaging: A Review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.A.; El-Haddad, N.; Mahmoud, D.; Miller, M.J.; Karam, L. Invited Review: Advances in Nisin Use for Preservation of Dairy Products. J. Dairy Sci. 2020, 103, 2041–2052. [Google Scholar] [CrossRef]
- Travers, W.; Kelleher, F. Studies of the Highly Potent Lantibiotic Peptide Nisin Z in Aqueous Solutions of Salts and Biological Buffer Components. Biophys. Chem. 2021, 274, 106603. [Google Scholar] [CrossRef]
- ASTM D644-99; Standard Test Method for Moisture Content of Paper and Paperboard by Oven Drying. ASTM, American Society for Testing and Materials, ATSM: West Conshohocken, PA, USA, 1999.
- ASTM D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM, American Society for Testing and Materials, ATSM: West Conshohocken, PA, USA, 2012.
- ASTM E96/E96M-10; Standard Test Method for Water Vapor Transmission of Materials. ASTM, American Society for Testing and Materials, ATSM: West Conshohocken, PA, USA, 2010.
- M02-A12; Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Twelfth Edition. Clinical and Laboratory Standards Institute, CLSI: Filadelfia, PA, USA, 2015.
- Tavares, A.G.; Andrade, J.; Silva, R.R.A.; Marques, C.S.; da Silva, J.O.R.; Vanetti, M.C.D.; de Melo, N.R.; Soares, N.D.F.F. Carvacrol-Loaded Liposome Suspension: Optimization, Characterization and Incorporation into Poly(Vinyl Alcohol) Films. Food Funct. 2021, 12, 6549–6557. [Google Scholar] [CrossRef]
- Barbosa, L.C.A. Espectroscopia No Infravermelho Na Caracterização de Compostos Orgânicos, 1st ed.; Editora UFV: Viçosa, Brazil, 2007; Volume 1, ISBN 978-85-7269-280-9. [Google Scholar]
- Freitas, P.A.V.; Silva, R.R.A.; de Oliveira, T.V.; Soares, R.R.A.; Junior, N.S.; Moraes, A.R.F.; Pires, A.C.D.S.; Soares, N.F.F. Development and Characterization of Intelligent Cellulose Acetate-Based Films Using Red Cabbage Extract for Visual Detection of Volatile Bases. LWT 2020, 132, 109780. [Google Scholar] [CrossRef]
- Kalkan, S.; Otağ, M.R.; Engin, M.S. Physicochemical and Bioactive Properties of Edible Methylcellulose Films Containing Rheum Ribes, L. Extract. Food Chem. 2020, 307, 125524. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Taoudiat, A.; Djenane, D.; Ferhat, Z.; Spigno, G. The Effect of Laurus Nobilis, L. Essential Oil and Different Packaging Systems on the Photo-Oxidative Stability of Chemlal Extra-Virgin Olive Oil. J. Food Sci. Technol. 2018, 55, 4212–4222. [Google Scholar] [CrossRef]
- Boselli, E.; Rodriguez-Estrada, M.T.; Ferioli, F.; Caboni, M.F.; Lercker, G. Cholesterol Photosensitised Oxidation of Horse Meat Slices Stored under Different Packaging Films. Meat Sci. 2010, 85, 500–505. [Google Scholar] [CrossRef]
- Canevarolo, S.V.J. Ciência dos Polímeros. Um Texto Básico Para Tecnólogos e Engenheiros; Artliber: São Paulo, Brazil, 2002; p. 280. [Google Scholar]
- Freitas, P.A.V.; de Oliveira, T.V.; Silva, R.R.A.; e Moraes, A.R.F.; Pires, A.C.D.S.; Soares, R.R.A.; Junior, N.S.; Soares, N.F.F. Effect of PH on the Intelligent Film-Forming Solutions Produced with Red Cabbage Extract and Hydroxypropylmethylcellulose. Food Packag. Shelf Life 2020, 26, 100604. [Google Scholar] [CrossRef]
- Kwon, C.W.; Chang, P.S. Influence of Alkyl Chain Length on the Action of Acetylated Monoglycerides as Plasticizers for Poly (Vinyl Chloride) Food Packaging Film. Food Packag. Shelf Life 2021, 27, 100619. [Google Scholar] [CrossRef]
- De Oliveira, T.V.; de Freitas, P.A.V.; Pola, C.C.; Terra, L.R.; da Silva, J.O.R.; Badaró, A.T.; Junior, N.S.; de Oliveira, M.M.; Silva, R.R.A.; Soares, N.d.F.F. The Influence of Intermolecular Interactions between Maleic Anhydride, Cellulose Nanocrystal, and Nisin-Z on the Structural, Thermal, and Antimicrobial Properties of Starch-PVA Plasticized Matrix. Polysaccharides 2021, 2, 40. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Xiao, L.; Lin, D.; Yang, Y.; Wang, H.; Yang, Y.; Wu, D.; Chen, H.; Zhang, Q.; et al. Physical Properties and Structural Characterization of Starch/Polyvinyl Alcohol/Graphene Oxide Composite Films. Int. J. Biol. Macromol. 2019, 123, 569–575. [Google Scholar] [CrossRef]
- Bartolomei, S.S.; Santana, J.G.; Valenzuela Díaz, F.R.; Kavaklı, P.A.; Guven, O.; Moura, E.A.B. Investigation of the Effect of Titanium Dioxide and Clay Grafted with Glycidyl Methacrylate by Gamma Radiation on the Properties of EVA Flexible Films. Radiat. Phys. Chem. 2020, 169, 107973. [Google Scholar] [CrossRef]
- Prateepchanachai, S.; Thakhiew, W.; Devahastin, S.; Soponronnarit, S. Mechanical Properties Improvement of Chitosan Films via the Use of Plasticizer, Charge Modifying Agent and Film Solution Homogenization. Carbohydr. Polym. 2017, 174, 253–261. [Google Scholar] [CrossRef]
- Lim, H.; Hoag, S.W. Plasticizer Effects on Physical-Mechanical Properties of Solvent Cast Soluplus® Films. AAPS PharmSciTech 2013, 14, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Jiang, B.; Chen, J.; Jin, Z. Blend-Modification of Soy Protein/Lauric Acid Edible Films Using Polysaccharides. Food Chem. 2014, 151, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science & Engineering—An Introduction, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; p. 721. [Google Scholar]
- Irkin, R.; Esmer, O.K. Novel Food Packaging Systems with Natural Antimicrobial Agents. J. Food Sci. Technol. 2015, 52, 6095–6111. [Google Scholar] [CrossRef]
- Remedio, L.N.; Silva dos Santos, J.W.; Vieira Maciel, V.B.; Yoshida, C.M.P.; de Carvalho, R.A. Characterization of Active Chitosan Films as a Vehicle of Potassium Sorbate or Nisin Antimicrobial Agents. Food Hydrocoll. 2019, 87, 830–838. [Google Scholar] [CrossRef]
- Helander, I.M.; Mattila-Sandholm, T. Permeability Barrier of the Gram-Negative Bacterial Outer Membrane with Special Reference to Nisin. Int. J. Food Microbiol. 2000, 60, 153–161. [Google Scholar] [CrossRef]
- Sperber, W.H. Influence of Water Activity on Foodborne Bacteria-A Review. J. Food. Prot. 1983, 46, 142–150. [Google Scholar] [CrossRef]
- Zimet, P.; Mombrú, Á.W.; Mombrú, D.; Castro, A.; Villanueva, J.P.; Pardo, H.; Rufo, C. Physico-Chemical and Antilisterial Properties of Nisin-Incorporated Chitosan/Carboxymethyl Chitosan Films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef]
- Panina, I.; Krylov, N.; Nolde, D.; Efremov, R.; Chugunov, A. Environmental and Dynamic Effects Explain How Nisin Captures Membrane-Bound Lipid II. Sci. Rep. 2020, 10, 8821. [Google Scholar] [CrossRef]
- Hsu, S.T.D.; Breukink, E.; Tischenko, E.; Lutters, M.A.G.; de Kruijff, B.; Kaptein, R.; Bonvin, A.M.J.J.; van Nuland, N.A.J. The Nisin-Lipid II Complex Reveals a Pyrophosphate Cage That Provides a Blueprint for Novel Antibiotics. Nat. Struct. Mol. Biol. 2004, 11, 963–967. [Google Scholar] [CrossRef] [Green Version]
- Collins, K.D.; Brauman, J.I. Sticky Ions in Biological Systems (Ion Hydration/Potassium Ion/Ammonium Ion/Protein Structure/Hofmeister Series). Proc. Natl. Aca. Sci. USA 1995, 92, 5553–5557. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.S.; Dinadayalane, T.C.; Leszczynski, J.; Sastry, G.N. Comprehensive Study on the Solvation of Mono- and Divalent Metal Cations: Li+, Na+, K+, Be2+, Mg2+ and Ca2+. J. Phys. Chem. A 2008, 112, 12944–12953. [Google Scholar] [CrossRef] [PubMed]
- Clifton, L.A.; Skoda, M.W.A.; le Brun, A.P.; Ciesielski, F.; Kuzmenko, I.; Holt, S.A.; Lakey, J.H. Effect of Divalent Cation Removal on the Structure of Gram-Negative Bacterial Outer Membrane Models. Langmuir 2015, 31, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Schneck, E.; Schubert, T.; Konovalov, O.V.; Quinn, B.E.; Gutsmann, T.; Brandenburg, K.; Oliveira, R.G.; Pink, D.A.; Tanaka, M. Quantitative Determination of Ion Distributions in Bacterial Lipopolysaccharide Membranes by Grazing-Incidence X-Ray Fluorescence. Proc. Natl. Acad. Sci. USA 2010, 107, 9147–9151. [Google Scholar] [CrossRef] [Green Version]
- Demishtein, K.; Reifen, R.; Shemesh, M. Antimicrobial Properties of Magnesium Open Opportunities to Develop Healthier Food. Nutrients 2019, 11, 2363. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Sun, X.; Yang, H. The Role of Antibacterial Metallic Elements in Simultaneously Improving the Corrosion Resistance and Antibacterial Activity of Magnesium Alloys. Mater. Des. 2021, 198, 109350. [Google Scholar] [CrossRef]
- Wolf, F.I.; Torsello, A.; Fasanella, S.; Cittadini, A. Cell Physiology of Magnesium. Mol. Asp. Med. 2003, 24, 11–26. [Google Scholar] [CrossRef]


| Treatment | Tck (mm) | TS (mPa) | YM (mPa) | εl (%) | D (g·mL−1) |
|---|---|---|---|---|---|
| Control | 73.1 (11) c | 46.7 (7.3) a | 1090.9 (42) a | 41.3 (2.5) bc | 1.14 (0.1) e |
| 25 mM-LiCl | 81.1 (6.5) c | 25.0 (2.9) bc | 629.3 (140) b | 46.9 (8.4) bc | 1.39 (0.1) d |
| 50 mM-LiCl | 97.7 (12.1) c | 20.4 (4.7) c | 219.5 (46) c | 56.6 (13) b | 1.40 (0.1) d |
| 250 mM-LiCl | 156.2 (7.4) b | 7.0 (0.4) d | 4.1 (0.6) d | 134.9 (2.4) a | 2.40 (0.2) b |
| 500 mM-LiCl | 251.5 (2.4) a | 1.6 (0.3) d | 1.6 (0.2) d | 116.1 (9.5) a | 4.25 (0.2) a |
| 25 mM-MgCl2 | 81.3 (1.5) c | 31.6 (4.0) bc | 703.2 (189) b | 38.3 (14.2) c | 1.35 (0.1) d |
| 50 mM-MgCl2 | 94.2 (8.2) c | 38.8 (6.5) b | 618.2 (79) b | 46.0 (4.2) bc | 1.40 (0.2) d |
| 250 mM-MgCl2 | 164.6 (9.1) b | 12.1 (0.9) d | 13.6 (1.8) d | 110.5 (6.7) a | 1.75 (0.1) c |
| 500 mM-MgCl2 | 249.3 (2.7) a | 5.2 (0.3) d | 3.7 (0.9) d | 119.5 (11) a | 2.17 (0.2) b |
| Inhibition Zone (mm) | |||
|---|---|---|---|
| Treatment | S. aureus (mm) | L. plantarum (mm) | E. coli (mm) |
| Control | 21.7 (0.6) ns | 15.7 (1.2) bcd | NA |
| 25 mM-LiCl | 20.6 (0.5) ns | 14.2 (1.6) cd | NA |
| 50 mM-LiCl | 21.2 (0.3) ns | 15.5 (0.8) cd | NA |
| 250 mM-LiCl | 20.1 (0.6) ns | 16.3 (1.2) abc | NA |
| 500 mM-LiCl | 20.0 (0.4) ns | 17.8 (1.3) ab | 11.4 (0.5) |
| 25 mM-MgCl2 | 21.1 (1.4) ns | 14.5 (1.0) cd | NA |
| 50 mM-MgCl2 | 21.7 (1.6) ns | 13.8 (0.7) d | NA |
| 250 mM-MgCl2 | 20.2 (0.3) ns | 14.3 (1.9) cd | NA |
| 500 mM-MgCl2 | 20.1 (0.6) ns | 18.5 (0.6) a | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V.; Stringheta, P.C.; dos Santos Pires, A.C.; de Fátima Ferreira Soares, N. Ionic Strength of Methylcellulose-Based Films: An Alternative for Modulating Mechanical Performance and Hydrophobicity for Potential Food Packaging Application. Polysaccharides 2022, 3, 426-440. https://doi.org/10.3390/polysaccharides3020026
Silva RRA, Marques CS, Arruda TR, Teixeira SC, de Oliveira TV, Stringheta PC, dos Santos Pires AC, de Fátima Ferreira Soares N. Ionic Strength of Methylcellulose-Based Films: An Alternative for Modulating Mechanical Performance and Hydrophobicity for Potential Food Packaging Application. Polysaccharides. 2022; 3(2):426-440. https://doi.org/10.3390/polysaccharides3020026
Chicago/Turabian StyleSilva, Rafael Resende Assis, Clara Suprani Marques, Tarsila Rodrigues Arruda, Samiris Cocco Teixeira, Taíla Veloso de Oliveira, Paulo Cesar Stringheta, Ana Clarissa dos Santos Pires, and Nilda de Fátima Ferreira Soares. 2022. "Ionic Strength of Methylcellulose-Based Films: An Alternative for Modulating Mechanical Performance and Hydrophobicity for Potential Food Packaging Application" Polysaccharides 3, no. 2: 426-440. https://doi.org/10.3390/polysaccharides3020026
APA StyleSilva, R. R. A., Marques, C. S., Arruda, T. R., Teixeira, S. C., de Oliveira, T. V., Stringheta, P. C., dos Santos Pires, A. C., & de Fátima Ferreira Soares, N. (2022). Ionic Strength of Methylcellulose-Based Films: An Alternative for Modulating Mechanical Performance and Hydrophobicity for Potential Food Packaging Application. Polysaccharides, 3(2), 426-440. https://doi.org/10.3390/polysaccharides3020026








