Biofunctionalized Nanomaterials: Alternative for Encapsulation Process Enhancement
Abstract
:1. Introduction
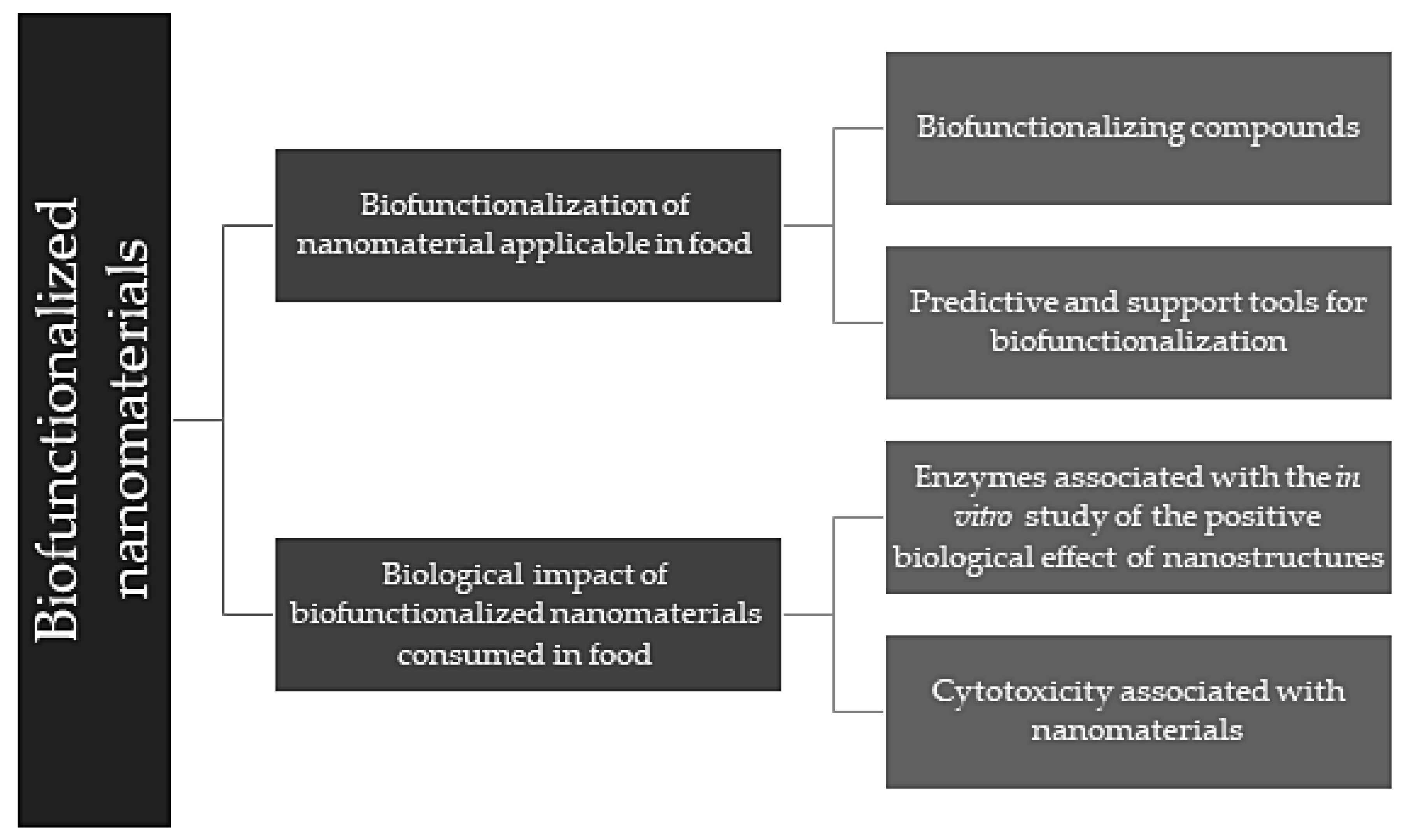
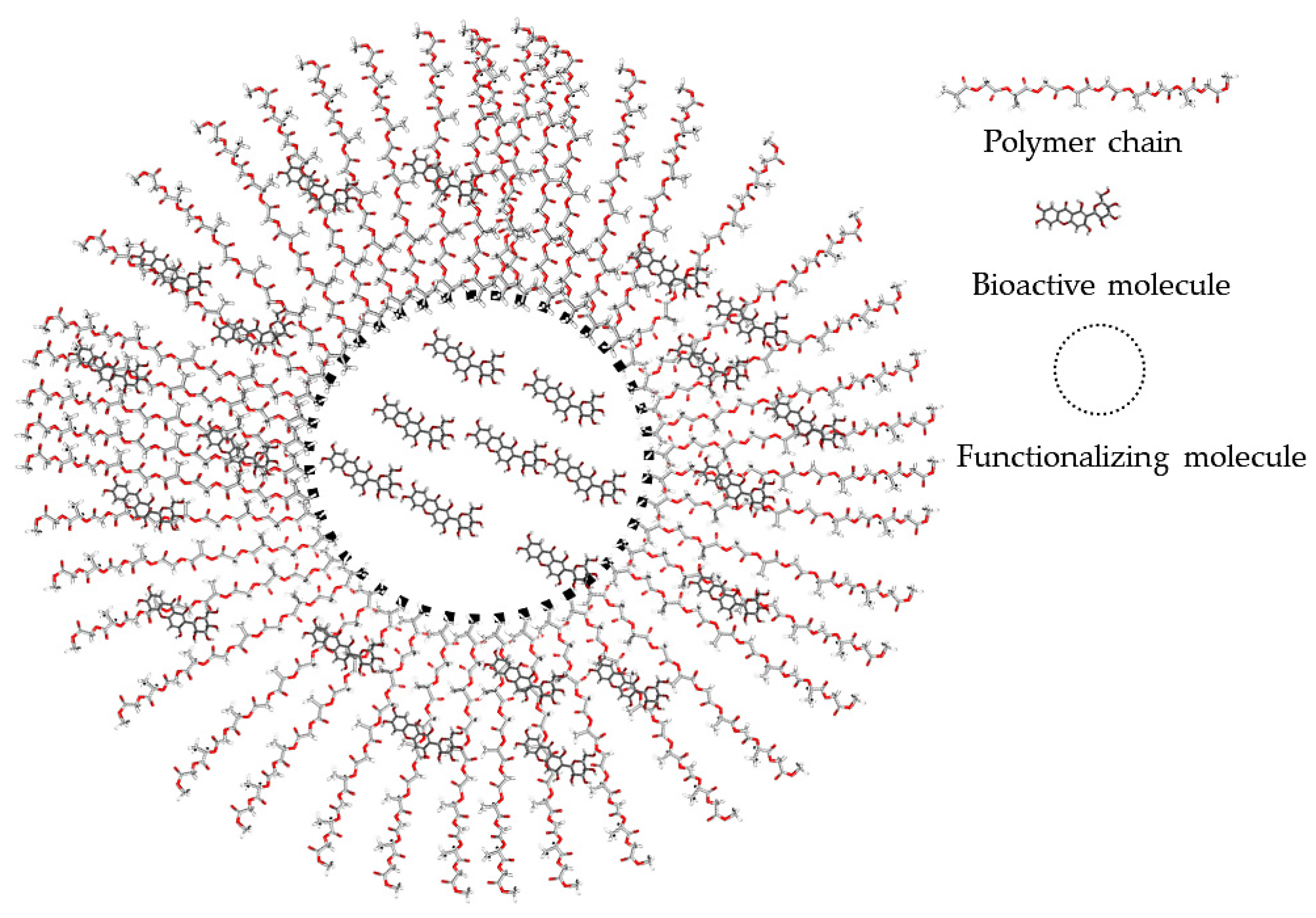
2. Biofunctionalization of Nanomaterials Applicable in Food
2.1. Bio-Functionalizing Compounds
| Material | Load | Generalities | Characterization | Results | Ref |
|---|---|---|---|---|---|
| PLGA | Donepezil | 89.67 ± 6.43 nm Nanoparticles | SEM | Spherical nanoparticles | [35] |
| FTIR | At 3008 cm−1 the characteristic peak of aromatic CH group stretch appeared and at 2924 cm−1 the characteristic peak for aliphatic CH2 stretch appeared. Peaks appeared at 1690 cm−1 and 1589 cm−1 corresponding to the C = O carbonyl stretching and aromatic C = C stretches, respectively | ||||
| XRD | PLGA: 20–20° characteristic signal Load: signals were shown at 5°, 15° y 20° | ||||
| Chitosan | Kaempferol | 137.51–272.91 nm PdI = 0.25 Nanoparticles | ZP | Positive zeta-potential values from + 18.5 to + 38.1 mV. | [36] |
| SEM | Nanoparticles are spherical in shape, and uniform formation. | ||||
| FTIR | Characteristic absorption bands: 3324 cm−1 (O─H stretch), 1661 cm−1 (C = O), 1604 cm−1 (C = C), 1378 cm−1 (C─OH), 1257 cm−1(C─O─C) of chitosan and kaempferol shifted to the 1653 cm−1 and the OH stretch of kaempferol completely disappeared at 3363 cm−1. | ||||
| XRD | Crystalline structure at a diffraction angle of 2°θ 10.80°, 12.50°, 15.90°, 23.95°, 24.36°, 27.46°. | ||||
| PLA | Curcumin | 516 and 601 nm Fibers | FTIR | Characteristic bands corresponding to carbonyl stretching (1750, 1760 cm−1) in PLA and bands corresponding to phenolic, (-OH), C = O and C = C at 1367, 958, 1613, 1505 cm−1 | [37] |
| XRD | PLA: broad peak at 2θ = 21.84°; load: peaks at 2θ = 11.90°, 14.31°, 17.12°, 17.87°,21.03°, 23.14°, 24.34°, 25.35°, 27.15°and 28.76°. | ||||
| DSC | The glass transition of the polymer with the load is increased | ||||
| PLC | Quercetin | Spheres | ZP | Potential ranged from −120 to 120 mV. | [38] |
| FTIR | Characteristic bans of quercetin as aromatic bending and stretching (1093–1615 cm−1), –OH phenolic bending (1211–1435 cm−1), and –CO stretching (1654 cm−1) and broad phenolic –OH around 3392 cm−1, polymer –CH stretching (2868–2947 cm−1) and –CO stretching (1724 cm−1). | ||||
| XRD | Quercetin: 2θ of 10.80°, 14.16°, 17.23°, 24.33° and 26.97°, but with polymer 21.08°, 23.47° and 26.80°. | ||||
| Gelatin + ZnO | Cefazolin | Diameter 47.5 nm PdI: 0.325 Fibers | ZP | Zeta potential negative −22.67 mV indicated the stable colloidal. | [39] |
| XRD | 2θ values of 32.42°, 34.51°, 36.78°, 48.1° confirmed the crystalline nature. | ||||
| FTIR | Characteristic peaks of cefazolin (1761, 1388, 1285 and 1183 cm−1). | ||||
| PEG | Naproxen | Particles Up to 20 µm | SEM | Exhibited irregularly shaped particles alongside a few spherical microspheres with a smooth surface | [40] |
| FTIR | Absorption peaks of 1724 cm−1 and 1681 cm−1 are related to the carboxylic acid bond and benzene ring and peaks of 1155 cm−1 and 1174 cm−1 are correlated to etheric bond. |
2.2. Predictive and Support Tools for Biofunctionalization
3. Biological Impact of Biofunctionalized Nanomaterials Consumed in Food
3.1. Enzymes Associated with the In Vitro Study of the Positive Biological Effect of Nanostructures
- Cyclooxygenase (COX): COX is related to the inflammatory phenomena, and there are currently immunoassay kits that allow the enzyme inhibition to be quantified from a product associated with inflammation (thromboxanes). Therefore, by reducing the activity of the enzyme, the inflammation can be stopped. There are three types of cyclooxygenase: Cyclooxygenase-1 (COX-1), whose function is to regulate the proliferation of normal or neoplastically transformed cells and is found in all tissues, especially in the kidney and gastrointestinal tract, and participates in the production of prostaglandins involved in normal physiological processes such as the protection of the gastric epithelium, maintenance of renal flow, and platelet aggregation. Cyclooxygenase-2 (COX-2) modulates inflammation and prostanoids pathways; therefore, the objective of the design of nanomaterials with anti-inflammatory properties seeks the selective inhibition of COX-2, and current existing immunoassays allow for evaluating this type of effect [59,60].
- Topoisomerase (TOP): The exacerbated replication of cells, such as the development of carcinoma, is also associated with enzymatic dysregulation, such as TOP, which is the enzyme responsible for maintaining the tertiary DNA structure throughout the cell cycle, being the one in charge of the winding and unwinding of DNA strands during synthesis, replication, condensation, and recondensation. Three types of topoisomerase DNA have been characterized according to their catalytic properties, energy expenditure, and protein structure: Topoisomerase type I DNA (TOP-I) is involved in the opening of the DNA so that the copy of the material is carried out genetically, and the enzyme known as TOP-Ib or TOP-III isogenic of TOP-I is not found in all eukaryotic cells and is responsible for causing a break in one of the chains and forms a phosphodiester bond between the 5 ‘phosphate and the OH of tyrosine. The type II topoisomerase DNAs (TOP-II) are associated with the closure of the same post-process structure and act together to maintain the appropriate level of supercoiling so that biological processes such as cell development are carried out. It is interesting to inhibit these enzymes in processes associated with cancerous processes due to the overexpression of the TOP [61,62].
3.2. Cytotoxicity Associated with Nanomaterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A Review on the Green Synthesis of Silver Nanoparticles and Their Morphologies Studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 68274. [Google Scholar] [CrossRef] [Green Version]
- Urrejola, M.C.; Soto, L.V.; Zumarán, C.C.; Peñaloza, J.P.; Álvarez, B.; Fuentevilla, I.; Haidar, Z.S. Sistemas de Nanopartículas Poliméricas II: Estructura, Métodos de Elaboración, Características, Propiedades, Biofuncionalización y Tecnologías de Auto-Ensamblaje Capa por Capa (Layer-by-Layer Self-Assembly) Polymeric Nanoparticle Systems: Structure, Elabo. Int. J. Morphol. 2018, 36, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Casa, D.M.; Carraro, T.C.M.M.; De Camargo, L.E.A.; Dalmolin, L.F.; Khalil, N.M.; Mainardes, R.M. Poly(L-lactide) Nanoparticles Reduce Amphotericin B Cytotoxicity and Maintain Its In Vitro Antifungal Activity. J. Nanosci. Nanotechnol. 2015, 15, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Larios, A.; Lopez, R.; Hernandez-Gordillo, A.; Tzompantzi, F.; Gómez, R.; Torres-Guerra, L.M. Improved hydrogen production from water splitting using TiO 2-ZnO mixed oxides photocatalysts. Fuel 2012, 100, 139–143. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, D.; Pimentel, A.C.; Mateus, T.; Leitão, J.P.; Soares, J.; Falcão, B.P.; Araújo, A.; Vicente, A.; Filonovich, S.A.; Águas, H.; et al. Influence of the layer thickness in plasmonic gold nanoparticles produced by thermal evaporation. Sci. Rep. 2013, 3, 1469. [Google Scholar] [CrossRef]
- Wang, Y.; Kho, K.; Cheow, W.S.; Hadinoto, K. A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid–polymer hybrid nanoparticles. Int. J. Pharm. 2012, 424, 98–106. [Google Scholar] [CrossRef]
- Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Atwan, M.H.; Tessema, M.M. Solvothermal Synthesis of Platinum Alloy Nanoparticles for Oxygen Reduction Electrocatalysis. J. Am. Chem. Soc. 2012, 134, 8535–8542. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Ponnalagi, A.K.; Nagamuthu, S.; Muralidharan, G. Microwave assisted synthesis of Co3O4 nanoparticles for high-performance supercapacitors. Electrochim. Acta 2013, 106, 500–505. [Google Scholar] [CrossRef]
- Bilek, M. Biofunctionalization of surfaces by energetic ion implantation: Review of progress on applications in implantable biomedical devices and antibody microarrays. Appl. Surf. Sci. 2014, 310, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Van Loo, S.; Stoukatch, S.; Axisa, F.; Destiné, J.; Van Overstraeten-Schlogel, N.; Flandre, D.; Lefevre, O.; Mertens, P. Low temperature assembly method of microfluidic bio-molecules detection device. In Proceedings of the 2012 3rd IEEE International Workshop on Low Temperature Bonding for 3D Integration, LTB-3D 2012, Tokyo, Japan, 22–23 May 2012; pp. 181–184. [Google Scholar]
- Williams, E.H.; Davydov, A.V.; Oleshko, V.P.; Lin, N.J.; Steffens, K.L.; Manocchi, A.K.; Krylyuk, S.; Rao, M.V.; Schreifels, J.A. Biofunctionalization of Si nanowires using a solution based technique. Nanoepitaxy Mater. Devices IV 2012, 8467, 846702. [Google Scholar] [CrossRef]
- Jain, D.; Athawale, R.; Bajaj, A.; Shrikhande, S.; Goel, P.N.; Gude, R.P. Studies on stabilization mechanism and stealth effect of poloxamer 188 onto PLGA nanoparticles. Colloids Surfaces B Biointerfaces 2013, 109, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Speed, D.; Westerhoff, P.; Sierra-Alvarez, R.; Draper, R.; Pantano, P.; Aravamudhan, S.; Chen, K.L.; Hristovski, K.; Herckes, P.; Bi, X.; et al. Physical, chemical, and in vitro toxicological characterization of nanoparticles in chemical mechanical planarization suspensions used in the semiconductor industry: Towards environmental health and safety assessments. Environ. Sci. Nano 2015, 2, 227–244. [Google Scholar] [CrossRef]
- Madhavi, V. Synthesis and Spectral Characterization of Iron Based Micro and Nanoparticles. Iran. J. Energy Environ. 2013, 4, 385–390. [Google Scholar] [CrossRef]
- Karagoz, B.; Esser, L.; Duong, H.T.; Basuki, J.S.; Boyer, C.; Davis, T.P. Polymerization-Induced Self-Assembly (PISA)–control over the morphology of nanoparticles for drug delivery applications. Polym. Chem. 2013, 5, 350–355. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Yu, S.-M.; Benseny-Cases, N.; Stürzenbaum, S.; Roig, A.; Laromaine, A. Toxicogenomics of iron oxide nanoparticles in the nematode C. elegans. Nanotoxicology 2017, 11, 647–657. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, Z.; Dogan, A.L.; Ozdemir, O.; Serper, A. Evaluation of the cytotoxicity of different root canal sealers on L929 cell line by MTT assay. Dent. Mater. J. 2012, 31, 1028–1032. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef]
- Wang, A.; Gu, F.; Zhang, L.; Chan, J.; Radovic-Moreno, A.; Shaikh, M.R.; Farokhzad, O.C. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin. Biol. Ther. 2008, 8, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Valero-Valdivieso, M.F.; Ortegón, Y.; Uscategui, Y. Biopolímeros: Avances Y Perspectivas Biopolymers: Progress And Prospects. Dyna 2013, 80, 171–180. [Google Scholar]
- Azmin, S.N.H.M.; Hayat, N.A.B.M.; Nor, M.S.M. Development and characterization of food packaging bioplastic film from cocoa pod husk cellulose incorporated with sugarcane bagasse fibre. J. Bioresour. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brel, V.K.; Artyushin, O.I.; Moiseeva, A.; Sharova, E.V.; Buyanovskaya, A.G.; Nelyubina, Y.V. Functionalization of bioactive substrates with a F5SCH = CH moiety. J. Sulfur Chem. 2019, 41, 29–43. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Guo, S.; Raya, J.; Bianco, A.; Menard-Moyon, C. Strategies for the Controlled Covalent Double Functionalization of Graphene Oxide. Chem. Eur. J. 2020, 26, 6591–6598. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Maria das Graças, B.Z.; Maia, J.G.S.; Fabricius, H.; Marx, F. Chemical Characterization of the Fruit of Annona squamosa L. Occurring in the Amazon. J. Food Compos. Anal. 2001, 14, 227–232. [Google Scholar] [CrossRef]
- Noel, S.; Liberelle, B.; Robitaille, L.; De Crescenzo, G. Quantification of Primary Amine Groups Available for Subsequent Biofunctionalization of Polymer Surfaces. Bioconj. Chem. 2011, 22, 1690–1699. [Google Scholar] [CrossRef] [Green Version]
- Samadarsi, R.; Mishra, D.; Dutta, D. Mangiferin nanoparticles fortified dairy beverage as a low glycemic food product: Its quality attributes and antioxidant properties. Int. J. Food Sci. Technol. 2020, 55, 589–600. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. In vitro bactericidal and drug release properties of vancomycin-amino surface functionalized bioactive glass nanoparticles. Mater. Chem. Phys. 2020, 241, 122423. [Google Scholar] [CrossRef]
- Brahmachari, G.; Choo, C.; Ambure, P.; Roy, K. In vitro evaluation and in silico screening of synthetic acetylcholinesterase inhibitors bearing functionalized piperidine pharmacophores. Bioorgan. Med. Chem. 2015, 23, 4567–4575. [Google Scholar] [CrossRef]
- Vedani, A.; Dobler, M.; Hu, Z.; Smieško, M. OpenVirtualToxLab-A platform for generating and exchanging in silico toxicity data. Toxicol. Lett. 2015, 232, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekha, R.; Mahboob, S.; Ramya, A.K.; Kerthekeyan, S.; Govindarajan, M.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Vaseeharan, B. Synthesis and Bio-physical Characterization of Crustin Capped Zinc Oxide Nanoparticles, and Their Photocatalytic, Antibacterial, Antifungal and Antibiofilm Activity. J. Clust. Sci. 2020, 32, 843–855. [Google Scholar] [CrossRef]
- Avvakumova, S.; Colombo, M.; Tortora, P.; Prosperi, D. Biotechnological approaches toward nanoparticle biofunctionalization. Trends Biotechnol. 2014, 32, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bhavna; Md, S.; Ali, M.; Baboota, S.; Sahni, J.K.; Bhatnagar, A.; Ali, J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2014, 40, 278–287. [Google Scholar]
- Ilk, S.; Sağlam, N.; Özgen, M.; Korkusuz, F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol. 2016, 94, 653–662. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Nandkumar, A.M.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA—Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Kumar, V.D.; Verma, P.R.P.; Singh, S.K. Development and evaluation of biodegradable polymeric nanoparticles for the effective delivery of quercetin using a quality by design approach. LWT Food Sci. Technol. 2015, 61, 330–338. [Google Scholar] [CrossRef]
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mater. Sci. Eng. C 2016, 58, 242–253. [Google Scholar] [CrossRef]
- Adibkia, K.; Barzegar-Jalali, M.; Maheri-Esfanjani, H.; Ghanbarzadeh, S.; Shokri, J.; Sabzevari, A.; Javadzadeh, Y. Physicochemical characterization of naproxen solid dispersions prepared via spray drying technology. Powder Technol. 2013, 246, 448–455. [Google Scholar] [CrossRef]
- Vedani, A.; Dobler, M.; Smieško, M. VirtualToxLab-A platform for estimating the toxic potential of drugs, chemicals and natural products. Toxicol. Appl. Pharmacol. 2012, 261, 142–153. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin Loaded-PLGA Nanoparticles Conjugated with Tet-1 Peptide for Potential Use in Alzheimer’s Disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamdari, R.F.; Hajimirsadeghi, S.S.; Kohsari, I. Synthesis of silver chromate nanoparticles: Parameter optimization using Taguchi design. Inorg. Mater. 2010, 46, 60–64. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Electrospinning of Cyclodextrin Functional Nanofibers for Drug Delivery Applications. Pharmaceutics 2018, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, J.; Sundar, R.; John, A.; Abraham, A. Phytochemical Incorporated Drug Delivery Scaffolds for Tissue Regeneration. Regen. Eng. Transl. Med. 2018, 4, 167–176. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- Heng, B.C.; Zhao, X.; Xiong, S.; Ng, K.W.; Boey, F.Y.-C.; Loo, J.S.-C. Toxicity of zinc oxide (ZnO) nanoparticles on human bronchial epithelial cells (BEAS-2B) is accentuated by oxidative stress. Food Chem. Toxicol. 2010, 48, 1762–1766. [Google Scholar] [CrossRef]
- Di Pasqua, A.J.; Sharma, K.K.; Shi, Y.-L.; Toms, B.B.; Ouellette, W.; Dabrowiak, J.C.; Asefa, T. Cytotoxicity of mesoporous silica nanomaterials. J. Inorg. Biochem. 2008, 102, 1416–1423. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Qiao, Z.-A.; Dai, S. Recent advances in carbon nanospheres: Synthetic routes and applications. Chem. Commun. 2015, 51, 9246–9256. [Google Scholar] [CrossRef]
- Sanna, V.; Lubinu, G.; Madau, P.; Pala, N.; Nurra, S.; Mariani, A.; Sechi, M. Polymeric Nanoparticles Encapsulating White Tea Extract for Nutraceutical Application. J. Agric. Food Chem. 2015, 63, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Nasirpour, A.; Fathi, M. Application of Cellulosic Nanofibers in Food Science Using Electrospinning and Its Potential Risk. Compr. Rev. Food Sci. Food Saf. 2015, 14, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, W.; Li, D.; Liu, T.; Zhang, Y.S.; Ding, J.; Chen, X. Locally Deployable Nanofiber Patch for Sequential Drug Delivery in Treatment of Primary and Advanced Orthotopic Hepatomas. ACS Nano 2018, 12, 6685–6699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Zhang, D.; Zhang, X.; Ma, Y.; Zhou, Y.; Qi, L. Biotemplated synthesis of cold nanoparticle-bacteria cellulose nanofiber nanocomposites and their application in biosensing. Adv. Funct. Mater. 2010, 20, 1152–1160. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [Green Version]
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Durán, G.; Paula, A.J.; Durán, N. Advances in Dental Materials through Nanotechnology: Facts, Perspectives and Toxicological Aspects. Trends Biotechnol. 2015, 33, 621–636. [Google Scholar] [CrossRef]
- Créminon, C.; Habib, A.; Maclouf, J.; Pradelles, P.; Grassi, J.; Frobert, Y. Differential measurement of constitutive (COX-1) and inducible (COX-2) cyclooxygenase expression in human umbilical vein endothelial cells using specific immunometric enzyme immunoassays. Biochim. Et Biophys. Acta Lipids Lipid Metab. 1995, 1254, 341–348. [Google Scholar] [CrossRef]
- Moro, M.G.; Oliveira, M.D.D.S.; De Oliveira, L.R.; Teixeira, S.A.; Muscará, M.N.; Spolidorio, L.C.; Holzhausen, M. Effects of Selective Versus Non-Selective COX-2 Inhibition on Experimental Periodontitis. Braz. Dent. J. 2019, 30, 133–138. [Google Scholar] [CrossRef]
- Sheng, C.; Miao, Z.; Zhang, W. Topoisomerase I Inhibitors Derived from Natural Products: Structure–Activity Relationships and Antitumor Potency. Stud. Nat. Prod. Chem. 2016, 47, 1–28. [Google Scholar] [CrossRef]
- Hevener, K.; Verstak, T.A.; Lutat, K.E.; Riggsbee, D.L.; Mooney, J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B 2018, 8, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles: Promises for diagnosis and treatment of cancer. Int. J. Mol. Epidemiol. Genet. 2011, 2, 367–390. [Google Scholar]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Decome, L.; Rose, J.; Orsiere, T.; De Meo, M.; Briois, V.; Chaneac, C.; Olivi, L.; Berge-Lefranc, J.-L.; Botta, A.; et al. In Vitro Interactions between DMSA-Coated Maghemite Nanoparticles and Human Fibroblasts: A Physicochemical and Cyto-Genotoxical Study. Environ. Sci. Technol. 2006, 40, 4367–4373. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-cabello, J. Liver and brain imaging through dimercaptosuccinic acid-coated iron oxide nanoparticles R esearch A rticle. Future Med. 2010, 5, 397–408. [Google Scholar]
- Luengo, Y.; Nardecchia, S.; Morales, M.P.; Serrano, M.C. Different cell responses induced by exposure to maghemite nanoparticles. Nanoscale 2013, 5, 11428–11437. [Google Scholar] [CrossRef] [Green Version]
- Geppert, M.; Hohnholt, M.C.; Thiel, K.; Nürnberger, S.; Grunwald, I.; Rezwan, K.; Dringen, R. Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Nanotechnology 2011, 22, 145101. [Google Scholar] [CrossRef]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and Cancer: Mechanisms of Action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Körstgens, V.; Yang, D.; Hohn, N.; Roth, S.V.; Müller-Buschbaum, P. Morphology control of low temperature fabricated ZnO nanostructures for transparent active layers in all solid-state dye-sensitized solar cells. J. Mater. Chem. A 2018, 6, 4405–4415. [Google Scholar] [CrossRef]
- Fu, Y.-S.; Du, X.-W.; Kulinich, S.A.; Qiu, J.-S.; Qin, W.-J.; Li, R.; Sun, A.J.; Liu, J. Stable Aqueous Dispersion of ZnO Quantum Dots with Strong Blue Emission via Simple Solution Route. J. Am. Chem. Soc. 2007, 129, 16029–16033. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K.A. The Evolution of the Protein Corona around Nanoparticles: A Test Study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Ali, L.M.A.; Cáceres-Vélez, P.R.; Cornudella, R.; Gutiérrez, M.; Moreno, J.A.; Piñol, R.; Palacio, F.; Fascineli, M.L.; de Azevedo, R.B.; et al. Hematotoxicity of magnetite nanoparticles coated with polyethylene glycol: In vitro and in vivo studies. Toxicol. Res. 2015, 4, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Ghaffar, F.R.A.; Hassouna, I.A.; Ibrahim, H.M.; Elelaimy, I.A.; Abd, H.M. The protective effect of hesperidin or garlic oil against the hemotoxicity of diazinon in male albino rats The protective effect of hesperidin or garlic oil against the hemotoxicity of diazinon in male albino rats. J. Biosci. Appl. Res. 2017, 3, 23–36. [Google Scholar]
- Liu, T.; Bai, R.; Zhou, H.; Wang, R.; Liu, J.; Zhao, Y.; Chen, C. The effect of size and surface ligands of iron oxide nanoparticles on blood compatibility. RSC Adv. 2020, 10, 7559–7569. [Google Scholar] [CrossRef]
- Sasidharan, A.; Chandran, P.; Monteiro-Riviere, N.A. Biocorona Bound Gold Nanoparticles Augment Their Hematocompatibility Irrespective of Size or Surface Charge. ACS Biomater. Sci. Eng. 2016, 2, 1608–1618. [Google Scholar] [CrossRef]
- Dahdouh, F.; Bendjeffal, H.; Nouacer, Z.; Moumene, W.; Zeminour, M.E.-H.; Naous, M.; Djebar, H. Selenium Nanoparticles Attenuate Gentamycin-Induced Nephrotoxicity and Hematotoxicity in Female Swiss Albino Mice. BioNanoScience 2019, 9, 356–364. [Google Scholar] [CrossRef]
- Khan, M.S.; Qureshi, N.A.; Jabeen, F.; Shakeel, M.; Asghar, M.S. Assessment of Waterborne Amine-Coated Silver Nanoparticle (Ag-NP)-Induced Toxicity in Labeo rohita by Histological and Hematological Profiles. Biol. Trace Elem. Res. 2017, 182, 130–139. [Google Scholar] [CrossRef]
- Asghar, M.S.; Qureshi, N.A.; Jabeen, F.; Khan, M.S.; Shakeel, M.; Chaudhry, A.S. Ameliorative Effects of Selenium in ZnO NP-Induced Oxidative Stress and Hematological Alterations in Catla catla. Biol. Trace Elem. Res. 2018, 186, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Mazumdar, H.; Haloi, N.A. study on biosynthesis of iron nanoparticles by Pleurotus sp. Veg. Crops Res. Bull. 2013, 8, 5–19. [Google Scholar]
- Yahya, R.A.M.; Azab, A.E.; Shkal, K.E.M.; Jm, J. Hepatotoxicity Induced By Copper Oxide and Zinc Oxide Nanoparticles and Their Mixtures in Male Albino Rats Hepatotoxicity Induced By Copper Oxide and Zinc Oxide Nanoparticles and Their Mixtures in Male Albino Rats. J. Biotechnol. 2019, 3, 1–17. [Google Scholar]
| Biofunctionalization Nanomaterials | Synthesis Type | Size (nm) | Results | Ref |
|---|---|---|---|---|
| PLA + quercetin | Solvent evaporation method | 130 | Enhancing solubility and stability (ZP 15 mV), spheres by SEM. | [43] |
| PLGA + curcumin | 150–200 | Enhancing solubility and increase of antioxidant activity (ABTS). | [44] | |
| PLGA + cucurbitacin | 399 ± 66 | Promote the encapsulation efficiency until 45% | [45] | |
| Cyclodextrin + voriconazole | Electrospinning | ˂800 | drug delivery is a dynamic and complex process and can be adapted to meet the needs of the targeted application | [46] |
| Polycarpone + plants phytochemicals | ˂700 | Non-toxic formulation of phytochemicals for tissue regeneration and repair. | [47] | |
| Lecitin + | 354 ± 12 | Improvement of drug loading in the lipid nanoparticles with lecitin. | [48] |
| Shape | Synthesis | Generalities | Application | Ref |
|---|---|---|---|---|
| Nanocapsules/nanospheres | emulsion and solvent evaporation | Size: 200–300 nm. | Cutaneous | [51] |
| Hydrothermal carbonization (HTC) | Size: 20–50 nm. monodispersed and mesopores | Electrocatalytic | [52] | |
| Nanoprecipitation method | Size: 380.80 ± 37.97 PdI: 0.15 ± 0.06 | Nutraceutical | [53] | |
| Nanofibers | Electrospinning | Diameter: 400–600 nm. | Active and intelligent food packaging products | [54] |
| Electrospinning | Diameter: 139.0 ± 25.6 nm. | Nutraceutical patch | [55] | |
| Coprecipitation | Diameter 100–200 nm. | Immobilize enzymes, bioelectroanalysis, and bioelectrocatalysis | [56] |
| Cell Line | Size | Essays | Results | Ref |
|---|---|---|---|---|
| Fibroblasts humans Normal | 450 ± 20 | WST | Low effect toxic. | [65] |
| Macrophages murine RAW264.7 | 80 ± 18 nm | MTT | No significant alterations. | [66] |
| HeLa | 30 to 70 nm | MTT | No significant alterations after 24 h. | [67] |
| Fibroblasts L929 | 49 nm | MTT | No toxic seven days after. | [68] |
| Crops primary of Astrocytes | 60 nm | Action enzymatic of LDH | No significant alterations. | [69] |
| Cells oligodendroglia’s OLN-93 | 60 nm | Action enzymatic of LDH | No significant alterations. | [70] |
| Stem cells derivate of adipocyte | 20 nm | Kit-8 for counting mobil | No significant alterations. | [71] |
| Material | Size | Effect | Cite |
|---|---|---|---|
| Gold | 40 nm | Promotes erythrocyte scavenging. | [78] |
| Selenium | 30 to 100 nm | Hematological alterations in female mice. | [79] |
| Selenium and zinc oxide | 36–40 nm | Ameliorative effect on hematology and antioxidant systems. | [80] |
| Silver | 17–22 nm | Alterations in the production or synthesis of hematological contents. | [81] |
| Zinc oxide | 30–50 nm | Induced changes in hematological parameters. | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razura-Carmona, F.F.; Perez-Larios, A.; Sáyago-Ayerdi, S.G.; Herrera-Martínez, M.; Sánchez-Burgos, J.A. Biofunctionalized Nanomaterials: Alternative for Encapsulation Process Enhancement. Polysaccharides 2022, 3, 411-425. https://doi.org/10.3390/polysaccharides3020025
Razura-Carmona FF, Perez-Larios A, Sáyago-Ayerdi SG, Herrera-Martínez M, Sánchez-Burgos JA. Biofunctionalized Nanomaterials: Alternative for Encapsulation Process Enhancement. Polysaccharides. 2022; 3(2):411-425. https://doi.org/10.3390/polysaccharides3020025
Chicago/Turabian StyleRazura-Carmona, Francisco Fabián, Alejandro Perez-Larios, Sonia Guadalupe Sáyago-Ayerdi, Mayra Herrera-Martínez, and Jorge Alberto Sánchez-Burgos. 2022. "Biofunctionalized Nanomaterials: Alternative for Encapsulation Process Enhancement" Polysaccharides 3, no. 2: 411-425. https://doi.org/10.3390/polysaccharides3020025
APA StyleRazura-Carmona, F. F., Perez-Larios, A., Sáyago-Ayerdi, S. G., Herrera-Martínez, M., & Sánchez-Burgos, J. A. (2022). Biofunctionalized Nanomaterials: Alternative for Encapsulation Process Enhancement. Polysaccharides, 3(2), 411-425. https://doi.org/10.3390/polysaccharides3020025








