Pomace-Cassava as Antioxidant Bio-Based Coating Polymers for Cheeses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Jabuticaba and Red Cabbage Extract
2.2. Starch Coating Forming Solution
2.3. Minas Frescal Cheese Processing and Coating
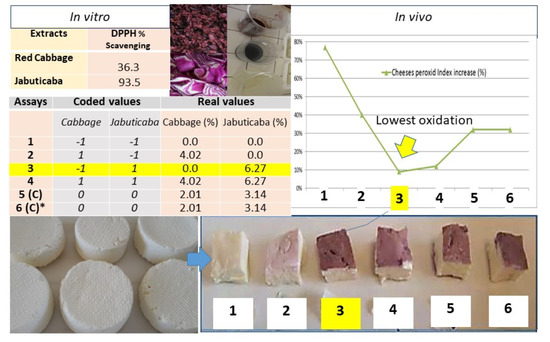
2.4. Experimental Design
2.5. Physico-Chemical Analysis
2.6. In Vitro Antioxidant Capacity
2.7. In Vivo Antioxidant Capacity
3. Results and Discussion
3.1. Cheese Coating
3.2. In Vitro Antioxidant Evaluation
3.3. In Vivo Antioxidant Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2021, 125, 107419. [Google Scholar] [CrossRef]
- De Almeida, P.L.; de Lima, S.N.; Costa, L.L.; de Oliveira, C.C.; Damasceno, K.A.; dos Santos, B.A.; Campagnol, P.C.B. Effect of jabuticaba peel extract on lipid oxidation, microbial stability and sensory properties of Bologna-type sausages during refrigerated storage. Meat Sci. 2015, 110, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Li, Z.; Soladoye, O.P.; Van-Hecke, T. Health Risks of Food Oxidation. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 2; Volume 82, pp. 45–81. [Google Scholar] [CrossRef]
- Ksouda, G.; Sellimi, S.; Merlier, F.; Falcimaigne-Cordin, A.; Thomasset, B.; Nasri, M.; Hajji, M. Composition, antibacterial and antioxidant activities of Pimpinella saxifraga essential oil and application to cheese preservation as coating additive. Food Chem. 2019, 288, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yu, H.; Tian, B.; Jiang, B.; Xu, J.; Li, D.; Feng, Z.; Liu, C. Novel Edible Coating with Antioxidant and Antimicrobial Activities Based on Whey Protein Isolate Nanofibrils and Carvacrol and Its Application on Fresh-Cut Cheese. Coatings 2019, 9, 583. [Google Scholar] [CrossRef] [Green Version]
- Leandro, G.R.; de Souza, O.F.; de Medeiros, T.K.F.; de Oliveira, J.P.F.; de Medeiros, R.S.; de Albuquerque, P.B.S.; de Souza, M.P. Quality and safety of the Coalho cheese using a new edible coating based on the Ziziphus joazeiro fruit pulp. Future Foods 2021, 4, 100089. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Wang, W.; Zhou, Q.; Shi, G.; Wei, F.; Jiang, G. Developmental toxicity of synthetic phenolic antiox-idants to the early life stage of zebrafish. Sci. Total Environ. 2018, 643, 559–568. Available online: https://www.sciencedirect.com/science/article/pii/S0048969718322897 (accessed on 1 March 2022). [CrossRef]
- Masek, A.; Latos, M.; Piotrowska, M.; Zaborski, M. The potential of quercetin as an effective natural antioxidant and indicator for packaging materials. Food Packag. Shelf Life 2018, 16, 51–58. [Google Scholar] [CrossRef]
- Simbine, E.O.; Macuamule, C.M.; Moiane, B.; Filho, W.G.V.; Veiga-Santos, P. Jabuticaba (Myrciaria jaboticaba Vell. Berg) syrup as colouring and pH indicating agent for an intelligent sheep milk yogurt. Braz. J. Food Res. 2018, 9, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Gil, K.A.; Jerković, I.; Marijanović, Z.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G. Evaluation of an innovative sheep cheese with antioxidant activity enriched with different thyme essential oil lecithin liposomes. LWT 2021, 154, 112808. [Google Scholar] [CrossRef]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between Total Antioxidant Capacity, Polyphenol and Fatty Acid Content of Native Grape Seed and Pomace of Four Different Grape Varieties in Hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Barros, L.; Dias, M.I.; Santos-Buelga, C.; Ferreira, I.C.; Asquieri, E.R.; Berrios, J.D.J. Non-fermented and fermented jabuticaba (Myrciaria cauliflora Mart.) pomaces as valuable sources of functional ingredients. Food Chem. 2016, 208, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Aloo, S.-O.; Ofosu, F.-K.; Daliri, E.-B.-M.; Oh, D.-H. UHPLC-ESI-QTOF-MS/MS Metabolite Profiling of the Antioxidant and Antidiabetic Activities of Red Cabbage and Broccoli Seeds and Sprouts. Antioxidants 2021, 10, 852. [Google Scholar] [CrossRef]
- Santos, C.T.; Veiga-Santos, P.; Sestari, P.; Sorrini, N.C.; Roça, R.D.O. Protein time–temperature sensor for intelligent starch polymers. J. Food Process. Preserv. 2020, 44, 14428. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Veiga-Santos, P.; Silva, L.T.; de Souza, C.O.; da Silva, J.R.; Albuquerque, E.C.; Druzian, J.I. Coffee-cocoa additives for bio-based antioxidant packaging. Food Packag. Shelf Life 2018, 18, 37–41. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Veiga-Santos, P.; Oliveira, L.; Cereda, M.; Scamparini, A. Sucrose and inverted sugar as plasticizer. Effect on cassava starch–gelatin film mechanical properties, hydrophilicity and water activity. Food Chem. 2007, 103, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Verruck, S.; Prudêncio, E.S.; Müller, C.M.O.; Fritzen-Freire, C.B.; Amboni, R.D.D.M.C. Influence of Bifidobacterium Bb-12 on the physicochemical and rheological properties of buffalo Minas Frescal cheese during cold storage. J. Food Eng. 2015, 151, 34–42. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official methods of analysis. In Method 950.41B, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybidic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. Available online: https://www.ajevonline.org/content/16/3/144 (accessed on 1 March 2022).
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1–F2. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Picque, D.; Leclercq-Perlat, M.-N.; Guillemin, H.; Cattenoz, T.; Corrieu, G.; Montel, M.-C. Impact of packaging on the quality of Saint-Nectaire cheese. Int. Dairy J. 2011, 21, 987–993. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.I.; Rackerby, B.; Goddik, L.; Frojen, R.; Ha, S.-D.; Kim, J.H.; Park, S.H. Microbial communities of a variety of cheeses and comparison between core and rind region of cheeses. J. Dairy Sci. 2020, 103, 4026–4042. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.; Torres, A.G.; Perrone, D.; et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xiao, Y.; Lagnika, C.; Li, D.; Liu, C.; Jiang, N.; Song, J.; Zhang, M. A comparative evaluation of nutritional properties, antioxidant capacity and physical characteristics of cabbage (Brassica oleracea var. Capitate var L.) subjected to different drying methods. Food Chem. 2020, 309, 124935. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Tayebi-Moghaddam, S.; Khatibi, R.; Taklavi, S.; Hosseini-Isfahani, M.; Rezaeinia, H. Sus-tained-release modeling of clove essential oil in brine to improve the shelf life of Iranian white cheese by bioactive electrospun zein. Int. J. Food Microbiol. 2021, 355, 109337. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Salehabadi, A.; Nafchi, A.M.; Oladzadabbasabadi, N.; Jafari, S.M. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends Food Sci. Technol. 2021, 116, 218–231. [Google Scholar] [CrossRef]
- Koop, B.L.; da Silva, M.N.; da Silva, F.D.; Lima, K.T.D.S.; Soares, L.S.; de Andrade, C.J.; Valencia, G.A.; Monteiro, A.R. Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: Sources, classification and enhanced stabilization by encapsulation and adsorption. Food Res. Int. 2022, 153, 110929. [Google Scholar] [CrossRef] [PubMed]
- Sanches, M.A.R.; Camelo-Silva, C.; Tussolini, L.; Tussolini, M.; Zambiazi, R.C.; Pertuzatti, P.B. Development, characterization and optimization of biopolymers films based on starch and flour from jabuticaba (Myrciaria cauliflora) peel. Food Chem. 2021, 343, 128430. [Google Scholar] [CrossRef]
- Rodrigues, M.; Álison, V.; Bertolo, M.R.V.; Marangon, C.A.; Martins, V.D.C.A.; Plepis, A.M.D.G. Chitosan and gelatin materials incorporated with phenolic extracts of grape seed and jabuticaba peel: Rheological, physicochemical, antioxidant, antimicrobial and barrier properties. Int. J. Biol. Macromol. 2020, 160, 769–779. [Google Scholar] [CrossRef]
- Christaki, S.; Moschakis, T.; Kyriakoudi, A.; Biliaderis, C.G.; Mourtzinos, I. Recent advances in plant essential oils and extracts: Delivery systems and potential uses as preservatives and antioxidants in cheese. Trends Food Sci. Technol. 2021, 116, 264–278. [Google Scholar] [CrossRef]


| Assays | Coded Values | Real Values | ||
|---|---|---|---|---|
| Cabbage | Jabuticaba | Cabbage (%) | Jabuticaba (%) | |
| 1 | −1 | −1 | 0.0 | 0.0 |
| 2 | 1 | −1 | 4.02 | 0.0 |
| 3 | −1 | 1 | 0.0 | 6.27 |
| 4 | 1 | 1 | 4.02 | 6.27 |
| 5 (C) | 0 | 0 | 2.01 | 3.14 |
| 6 (C) * | 0 | 0 | 2.01 | 3.14 |
| Assays | TS (%) | Ash (%) | Fat (%) | pH | Acidity (°D) | TP (%) | S (g100 g−1) |
|---|---|---|---|---|---|---|---|
| 1 | 2.47 | 1.58 | 13.66 | 6.34 | 12 | 19.8 | 2.09 |
| 2 | 2.45 | 1.47 | 15.22 | 6.30 | 12 | 20.1 | 2.10 |
| 3 | 2.46 | 1.50 | 14.96 | 6.45 | 13 | 20.0 | 2.09 |
| 4 | 2.51 | 1.48 | 14.01 | 6.42 | 14 | 20.3 | 2.08 |
| 5 (C) | 2.45 | 1.50 | 14.19 | 6.33 | 13 | 20.6 | 2.11 |
| 6 (C) | 2.46 | 1.50 | 14.19 | 6.34 | 13 | 20.5 | 2.10 |
| Extracts | DPPH % | TROLOX (µmols/g) | TEC | TPC (CE mM) | TAC (mg/L) | Total Solids (%) | Ash (%) |
|---|---|---|---|---|---|---|---|
| Red cabbage stirs | 36.3027 | 0.0105 | 0.4345 | 4.4 | 2.1 | 4.0213 | 1.0363 |
| Jabuticaba peels | 93.5079 | 0.0262 | 3.8217 | 12.9 | 8.4 | 6.2717 | 1.4764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veiga-Santos, P.; Antonio, K.d.J.; Santos, C.T.; Arruda, A.A.; Barros, L.B.d.; Gonçalves, L.T. Pomace-Cassava as Antioxidant Bio-Based Coating Polymers for Cheeses. Polysaccharides 2022, 3, 380-387. https://doi.org/10.3390/polysaccharides3020022
Veiga-Santos P, Antonio KdJ, Santos CT, Arruda AA, Barros LBd, Gonçalves LT. Pomace-Cassava as Antioxidant Bio-Based Coating Polymers for Cheeses. Polysaccharides. 2022; 3(2):380-387. https://doi.org/10.3390/polysaccharides3020022
Chicago/Turabian StyleVeiga-Santos, Pricila, Karina de Jesus Antonio, Carolina Toledo Santos, Amanda Alves Arruda, Larissa Bindo de Barros, and Larissa Tulio Gonçalves. 2022. "Pomace-Cassava as Antioxidant Bio-Based Coating Polymers for Cheeses" Polysaccharides 3, no. 2: 380-387. https://doi.org/10.3390/polysaccharides3020022
APA StyleVeiga-Santos, P., Antonio, K. d. J., Santos, C. T., Arruda, A. A., Barros, L. B. d., & Gonçalves, L. T. (2022). Pomace-Cassava as Antioxidant Bio-Based Coating Polymers for Cheeses. Polysaccharides, 3(2), 380-387. https://doi.org/10.3390/polysaccharides3020022