Abstract
The obtainment of new materials with distinct properties by mixing two or more polymers is a potential strategy in sustainable packaging research. In the present work, a blend of cellulose acetate (CA) and zein (60:40 wt/wt CA:zein) was manufactured by adding glycerol or tributyrin as plasticizers (30% wt/wt), and garlic essential oil (GEO), complexed (IC) or not with β-cyclodextrin (βCD), to produce active packaging. Blends plasticized with tributyrin exhibited a more homogeneous surface than those containing glycerol, which showed major defects. The blends underperformed compared with the CA films regarding mechanical properties and water vapor permeability. The presence of IC also impaired the films’ performance. However, the blends were more flexible than zein brittle films. The films added with GEO presented in vitro activity against Listeria innocua and Staphylococcus aureus. The IC addition into films, however, did not ensure antibacterial action, albeit that IC, when tested alone, showed activity against both bacteria. These findings suggest that the mixture of CA and plasticizers could increase the range of application of zein as a sustainable packaging component, while essential oils act as a natural bioactive to produce active packaging.
1. Introduction
Plastics revolutionized the food packaging sector since they are available at low cost, flexible, durable, versatile, and have distinct mechanical and barrier properties, suitable for food preservation [1,2]. However, the rampant consumption and improper disposal of plastics has resulted in a worldwide problem, causing damage to the environment and human health. In order to reduce the manufacture and use of materials from nonrenewable sources, various strategies are proposed, such as recycling and the total or partial replacement of conventional plastics with bio-based materials [2,3,4,5,6,7,8,9].
Derived from several renewable sources (i.e., agroindustrial byproducts and feedstock), bio-based materials have become increasingly popular among researchers from the packaging area since they fulfill the requirements for sustainable development [2,10]. In this context, several bio-based polymers extracted from different sources have been investigated as potential compounds for packaging. Cellulose derivatives, such as CA, and plant proteins, such as zein, are examples of hydrophobic polymers of natural origin that are already approved by the FDA to be used as food contact substances (CA) or listed as GRAS (zein) [4,11,12,13].
However, despite the growing trend, studies have revealed that films elaborated with bio-based polymers leave much to be desired when compared with the petroleum-based ones. Regarding food packaging and its role in food preservation, the higher water vapor permeability and lower mechanical properties that bio-based films usually present may impair their application as packaging components [2,10]. Aiming at improving the polymers’ performance as a packaging material, several strategies are considered.
The blend or mixture of two or more polymers for obtaining a new material with distinct characteristics is one of these proposed strategies [14,15]. The incorporation of different additives can also allow the elaboration of films with improved properties and/or functions [4,5,16,17,18]. Plasticizers, such as glycerol, for example, are widely used since they can enhance films’ flexibility. They are usually low-molecular weight compounds with low or no volatility, and able to increase polymer chain mobility [16,17].
Essential oils (EOs) are another example of additives for food packaging due to their bioactive properties and natural appeal. Some EOs show antimicrobial activity, which could grant to the packaging a preservative action, contributing to food safety and shelf-life extension [5,6,19,20]. However, the direct incorporation of EOs into polymer matrices is not recommended due to several issues (EOs are prone to thermal and light degradation, for instance) [21]. Thus, their complexation with cyclodextrins (CDs), followed by their addition into polymeric matrices, is considered an interesting way to protect the EOs’ active compounds from the degradation caused by light, temperature exposure, and oxygen presence, as well as to minimize their sensorial impact [18]. βCD, for example, is a low-cost natural CD recognized as safe and already used in food items and drugs [18,19,20].
Within this context, the present work aimed to obtain, characterize, and compare blends of CA and zein with two different plasticizers, glycerol and tributyrin. Although CA:zein blends have already been studied as nanofibers in the biomedical area, their potential as a food packaging material is not elucidated in the literature [22,23]. In addition, GEO was added into the films, free or as an inclusion complex (IC) with βCD, aiming at elaborating active films with antibacterial action. Two hypotheses were investigated: (1) that there would be an improvement in the properties of the new material compared with those of the individual polymers; and (2) that the incorporation of the EO in the complexed form would ensure a better antibacterial action by the films when compared with the ones with added non-complexed EO.
2. Materials and Methods
2.1. Materials
CA (SD = 2.5, and average molar mass of 2,024,000 g·mol−1) was donated by Rhodia Solvay Group (São Paulo, Brazil). Zein was donated by Flo Chemical Corp (F4400, molecular mass of 15–26 kDa) (Ashburnham, MA, USA). Glycerol (LabSynth, Diadema, Brazil) and tributyrin (98% purity degree, Acros Organics, Mumbai, India) were the plasticizers investigated. Anhydrous ethanol (99.8% purity degree, Neon, Brazil) and acetone (99.5% purity degree, LabSynth, Brazil) were the solvents used for polymer dispersion. GEO suitable for food was purchased from Empório Laszlo (Brazil), and βCD (97% purity degree) was acquired from Sigma-Aldrich (Saint Louis, MO, USA).
2.2. Preparation of Geo:βCD Inclusion Complex
IC was prepared using a kneading method following the mass proportion of 88% of βCD and 12% of GEO [19], which approximately matches the 1:1 molar ratio when considering the GEO compound’s average molar mass. The IC preparation was performed according to the methodology of Marques et al. [20], with modifications. In a porcelain mortar, 880 mg of βCD was added to 880 µL of hydroalcoholic solution (1:3 v/v ethanol:water) and homogenized for 5 min. Subsequently, 120 mg of GEO was added to the obtained paste and the mixture was manually macerated for up to 30 min. Samples were dried in a desiccator containing silica gel for 48 h at 25 °C, ground, and stored. A physical mixture (PM) was prepared for comparison purposes by briefly mixing 880 mg of βCD and 120 mg of GEO in a mortar.
2.3. Characterization of Inclusion Complex
2.3.1. Entrapment Efficiency (EE%)
Determination of GEO amounts in the IC was performed spectrophotometrically according to the method of Hill, Gomes, and Taylor [24] with modifications. Initially, a calibration curve was obtained from GEO solutions ranging from 0.0010 µL·mL−1 to 0.0088 µL·mL−1 in isopropanol:acetonitrile (3:2 v/v) at 209 nm of UV absorbance (UV1800, Shimadzu, Japan). 20 mg of ICs samples was dispersed in 20 mL of isopropanol:acetonitrile and mixed for 48 h. After this, samples were centrifuged at 3200× g (model 4K-15, Sigma, Postfch, Germany), for 15 min. The supernatant was measured at 209 nm of UV absorbance. The percentage of GEO entrapped in ICs was calculated using Equation (1):
in which GEOR and GEOT represent the real and the theoretical amounts of GEO entrapped in the IC, respectively.
2.3.2. Thermal Stability
Thermogravimetric analysis (TGA) was performed on a thermogravimetric analyzer, (Model DTG-60H, Shimadzu, Japan), under a nitrogen atmosphere (50 mL·min−1). Approximately 3 mg of each sample was weighed and heated from 25 °C to 360 °C at a rate of 10 °C·min−1. TG curves were obtained for βCD, IC, PM, and GEO.
The thermal stability of the elaborated films and their components was verified as well. The parameters were the same, with the exception of the final temperature, which was 550 °C.
2.3.3. X-ray Diffraction (XRD)
X-ray diffractograms for βCD, IC, and PM were obtained through a BRUKER X-ray diffractometer (model D8 Discover) equipped with an X-ray tube (Cu-Kα radiation, λ = 0.1514 nm), in a 2θ range from 5° to 70° with a scanning rate of 0.05° s−1.
2.4. Elaboration of Polymer Blends
Aiming to obtain a CA:zein blend, each polymer was, at first, dispersed separately. CA was dispersed in acetone (1:10 wt/v) and allowed to rest for 24 h. Zein was dispersed in ethanol 80% (v/v), at a ratio of 1:10 (wt/v), stirring at 500 rpm at 65 °C for 10 min. After they had cooled back to room temperature (approximately 25 °C), the dispersions were mixed together following the proportion of 60:40 CA:zein (wt/wt), since previous tests conducted in the laboratory revealed that this ratio allowed the obtainment of a more homogeneous film when compared with the others (Figure S1 of Supplementary Material). The plasticizer (glycerol or tributyrin) was also added to the mixed dispersion in a proportion of 30% (wt/wt) based on the polymer mass, as well as the bioactive agents: GEO (10% wt/wt) or IC (10% wt/wt), also based on the polymer mass. The dispersion was homogenized at 5000 rpm for 2 min (model T25, Ultra Turrax, IKA), allowed to rest for 30 min, poured onto a glass plate with machine casting (K-Paint applicator, Litlington, Royston, Hertfordshire, UK), and allowed to dry at room temperature. A blend without a bioactive agent and a blend containing 10% βCD were also investigated as control samples. Therefore, four different blends and four CA-films for each plasticizer were obtained, resulting in 16 assays, as described in Table 1. For comparison purposes, films with only CA and only zein were elaborated as well (Figure S2 of Supplementary Material).

Table 1.
Elaborated films with different plasticizers and additives: cellulose acetate and zein blends (BL) (ratio 60:40 CA:zein wt/wt) and their respective cellulose-acetate-based controls (CA).
2.5. Characterization of Polymer Blends
2.5.1. Scanning Electron Microscopy
Micrographs of films’ surface and films’ cross-sectional area, after liquid-nitrogen brittle fracture, were obtained with a scanning electron Tabletop Microscope (model TM3000, Hitachi High-Technologies, Tokyo, Japan) with a secondary electron detector and operating under low vacuum. Uncoated samples were attached to stubs’ surface with a double-sided carbon tape aid, and the accelerating voltage of 15 KV was used.
2.5.2. Thickness and Mechanical Properties
Film thickness was measured, in µm, with a digital micrometer (model 547–401, Mitutoyo, Japan). Ten specimens of each treatment were analyzed at ten random points per sample [5]. The films’ mechanical properties were also evaluated; factors analyzed were tensile strength (TS, in MPa), elongation at break (E, in %), and modulus of elasticity (Young’s modulus, YM, in MPa) using a Universal Testing Machine (model 3367, Instron Corporation, USA) equipped with a 1 kN load cell. Five rectangular specimens of each treatment (175 mm × 25 mm) were tested. The initial distance of grids’ separation was 125 mm, and the rate of separation was 50 mm·min−1 [25].
2.5.3. Water Vapor Permeability (WVP)
WVP was investigated by the gravimetric method according to ASTM E96/E96M (2010) [26] (with a few modifications), which allowed the calculation of the water vapor transmission rate (WVTR) as a function of weight gain. The films were cut into circles (Ø = 83 mm) and sealed with parafilm in poly(methyl methacrylate) cups containing a saturated solution of lithium chloride (12 ± 5% RH at 25 ± 2 °C). After this, the cups were placed in desiccators containing a saturated solution of sodium chloride (75 ± 5% RH at 25 ± 2 °C). The cups were periodically weighted to provide at least ten data points. Graphs expressing the gain of mass over time allowed the determination of the WVTR according to Equation (2):
in which m/t is the slope of gain of mass (g) over time (h), and A is the permeation area (m²). After this, the WVP was obtained according to Equation (3):
in which XT is the film thickness, PS is the saline water saturation pressure, RH1 is the relative humidity in the desiccator containing NaCl, and RH2 is the relative humidity on the desiccator containing LiCl. WVP was expressed as g·Pa−1·s−1·m−1.
2.6. Investigation of Antibacterial Properties
The antibacterial activity of GEO was initially investigated against Gram-positive bacteria Staphylococcus aureus ATCC 6538 and Listeria innocua ATCC 33090, and Gram-negative bacteria Escherichia coli ATCC 11229, Pseudomonas fluorescens 0A7, and Salmonella Choleraesuis ATCC 10708 Since the EO only showed activity against the Gram-positive bacteria, L. innocua and S. aureus were used for investigation of minimal inhibitory concentrations of GEO and IC, as well as for evaluation of the films. P. fluorescens was selected as negative control.
2.6.1. Inocula Preparation
Selected colonies of the bacteria were taken from a non-selective medium (plate count agar, PCA, Oxoid, Basingstoke, Hampshire, England) and were suspended in 0.85% (m/v) saline solution until they reached a visual suspension density similar to 0.5 McFarland turbidity standard (around 1–2 × 107 CFU·mL−1), then diluted at 1:10 [22].
2.6.2. Minimal Inhibitory Concentration (MIC)
The agar dilution method, with a few modifications, was used to investigate the MIC of GEO and IC [27]. Samples of both bioactive agents were dispersed in 10 mL of liquefied Brain Heart Infusion (BHI, Himedia, India) agar (~45 °C), resulting in the final concentrations of 2650 µg·mL−1, 1272 µg·mL−1, 636 µg·mL−1, 318 µg·mL−1, 159 µg·mL−1, 79.5 µg·mL−1, and 39.75 µg·mL−1 for GEO, and 9600 µg·mL−1, 4800 µg·mL−1, 2400 µg·mL−1, 1200 µg·mL−1, 600 µg·mL−1, and 300 µg·mL−1 for IC. Negative controls were BHI agar and BHI agar with 9600 µg·mL−1 of βCD. After allowing the agar to set and solidify, aliquots of 20 µL of the inoculum (Section 2.6.1) were dispensed on the dried agar surface, resulting in a final bacterial concentration of approximately 104 CFU·mg−1 per spot. The plates were incubated at 37 ± 1 °C for 24 h. MIC was determined based on the lowest concentration capable of growth inhibition.
2.6.3. Film Antibacterial Activity by Indirect Contact
The elaborated films were cut into (2 × 4) cm² shapes and fixed onto the lids of Petri dishes. The plates were previously prepared with BHI agar and inoculated with S. aureus or L. innocua (Section 2.6.1) with a swab aid [6]. The incubation conditions were 24 h at 37 °C ± 1 °C (S. aureus and L. innocua) and 10 days at 7 °C ± 1 °C (L. innocua). The presence or absence of microbial growth was evaluated after the incubation period.
2.7. Statistical Analysis
Analysis of variance (ANOVA) was carried out and followed by Tukey’s test at 5% probability, if suitable. The statistical software R was used [28].
3. Results and Discussion
3.1. Inclusion Complex Characterization
The IC preparation of GEO with βCD was achieved by the kneading method, which was chosen due to the practicality and quality of the technique [20,29]. To calculate the GEO entrapped in ICs, the calibration curve was obtained (Equation (4)) with an adjusted R² of 0.9942.
in which Y209 is the measured absorbance at 209 nm and XGEO is the EO concentration. The entrapment efficiency calculated was 87.5%.
The thermogravimetric curves, as well as their derivatives, plus the X-ray diffractograms of IC and pristine materials are displayed in the Supplementary Material, Figures S3 and S4, respectively. Regarding the thermal analyses, the βCD mass losses, exhibited in the TG curve, occurred in two steps: at 25–120 °C, losing 12.6%, due to water loss, and above 309 °C, corresponding to 76.7% of material decomposition, corroborating with Giordano et al. [30]. The EO mass loss displayed in the GEO TG curve started at room temperature (~25 °C) and achieved around 95% of loss until 200 °C. A similar behavior was observed by Piletti et al. [31] when studying the thermal protection of complexed GEO.
Three stages were observed for the TG curve of PM: at 25–120 °C, losing 14.5%, at 120–250 °C (4.0%), and above 300 °C, with 66.2% of material degradation. In the first and second stages, PM exhibited an intermediary behavior between βCD and GEO mass loss under temperature increase. The initial mass loss of the PM system was higher than that of the βCD system, which may have occurred due to water molecule loss besides non-complexed EO volatilization.
The IC TG curve, in turn, presented an initial mass loss of 7%, a lower value than that verified for βCD and PM. This occurrence is discussed in the literature as an indication that the complexation was successfully achieved [31,32]. It can also be observed that ~5% IC mass loss occurred at 100–250 °C, which can be attributed to the degradation of non-complexed EO [20,31]. The material degradation occurred near 300 °C, with 62% of mass loss. A small peak, slightly before material degradation, can be observed in the IC DTG curve, emphasized by a red arrow in Figure S3. This can be attributed to complexed GEO volatilization, which occurred at a higher temperature than for non-complexed GEO, indicating greater thermal stability of the IC compound [31].
Regarding the XRD analyses (Figure S4), the βCD diffractogram exhibited the characteristic peaks for βCD at 2θ = 9.0°, 12.4°, 16.9°, 22.6°, 27.1°, 31.9°, 34.6°, and 35.5° [33]. The PM diffractogram presented peaks similar to those of βCD, albeit at a lesser intensity. On the other hand, in the IC diffractogram, it is possible to observe greater changes in the XDR profile, such as broadening, appearance, and disappearance of some peaks [20,32]. The decrease in the degree of crystallinity, as can be observed when comparing the IC diffractogram with βCD and PM diffractograms, with the disappearance of some well-defined and narrow peaks (pointed out with black arrows in Figure S4), can be a result of changes that occurred in the βCD’s molecular organization, an indicative that the complexation with GEO was achieved [32,34].
3.2. Polymer Blends Aspect
The manufactured CA:zein blends and the CA-films incorporated with tributyrin or glycerol as plasticizers and the additives (GEO, IC, or βCD) are displayed in Figure 1. The blends presented a yellowish color and a porous appearance when compared with the transparent CA films, probably due to the zein addition (Figure 1 and Figure S2). The films’ aspect also changed as a function of the type of plasticizer used. Tributyrin incorporation resulted in blends with a more homogeneous surface than blends with added glycerol, which in turn presented serious defects.
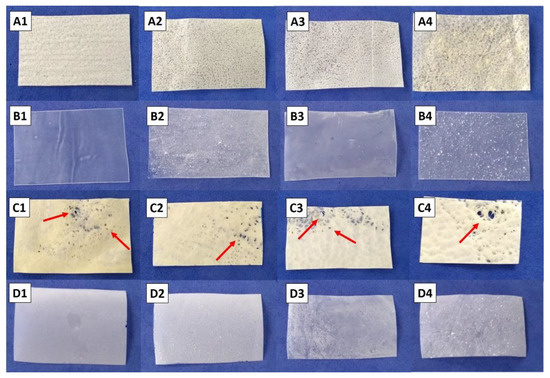
Figure 1.
Images of the obtained blends and cellulose acetate (CA) films. Blends (line A) and their respective CA films (line B) with incorporated tributyrin and additives: control (no additive, A1,B1), βCD (A2,B2), GEO (A3,B3), and inclusion complex (A4,B4). Blends (line C) and their respective CA films (line D) with incorporated glycerol and additives: control (no additive, C1,D1), βCD (C2,D2), GEO (C3,D3), and inclusion complex (C4,D4). Red arrows indicate the presence of macro-holes and tears.
In addition, SEM micrographs revealed the presence of micro-sized globules in the cross-sectional areas of blends with added glycerol, which were not observed in blends with added tributyrin, suggesting a poor compatibility between glycerol and the polymers (Figure 2). Tributyrin is a triglyceride of butyric acid which is more hydrophobic than glycerol, and since both polymers (CA and zein) used have a more hydrophobic nature, the obtained result is coherent [35]. The incorporation of βCD or IC into blends or CA films also resulted in major changes in films’ appearance and thickness, exhibiting more irregular surfaces than the control samples and the ones containing GEO. The presence of lumps and aggregates indicates poor compatibility among these additives and the polymers.
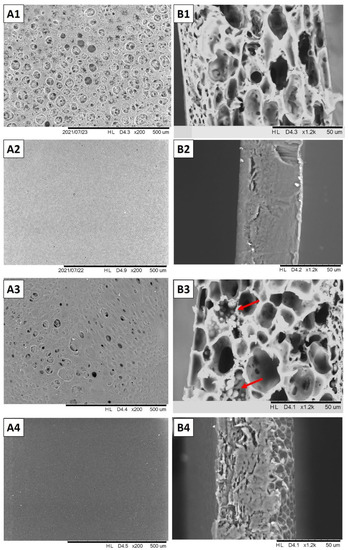
Figure 2.
Scanning Electron micrographs of surface (A) and cross-section (B) of blends and cellulose acetate (CA) films. CA:zein blend with incorporated tributyrin (A1,B1); CA film with tributyrin (A2,B2); CA:zein blend with incorporated glycerol (A3,B3), and CA film with glycerol (A4,B4). Red arrows show glycerol micro-globules.
3.3. Thermogravimetric Analysis
The results obtained from the thermal analysis support the ones observed in the SEM micrographs. TG curves and their respective derivatives of pure materials, CA films, and CA:zein blends are displayed in Figure 3. Moreover, TG curves and their derivatives of all 16 films can be found in the Supplementary Material (Figure S5).
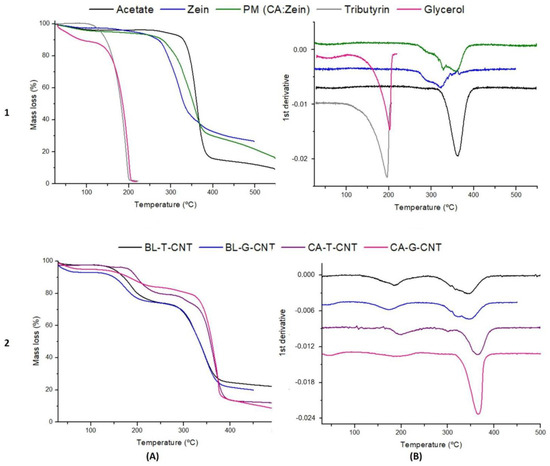
Figure 3.
Thermogravimetric curves (A) and their respective 1st derivative (B) of: (1) pristine polymers (cellulose acetate and zein) and their physical mixture (PM), plus pristine plasticizers (tributyrin and glycerol); (2) control blends with tributyrin (BL-T-CNT) or glycerol (BL-G-CNT) and cellulose acetate films with tributyrin (CA-T-CNT) or glycerol (CA-G-CNT).
Both plasticizers lost approximately 100% of their mass close to 200 °C. Regarding the polymers, CA and zein exhibited loss of around 3% of their mass at 100 °C due to water molecule loss [36]. The peak-temperature degradation was 320 °C and 360 °C for zein and CA, respectively. A physical mixture (PM) of zein and CA was also prepared and investigated. In the DTG curve, it is possible to distinguish two peaks in the range of 320–360 °C (Figure 3-1B), indicating that the degradation of each polymer occurred separately from the other. In contrast, when observing the DTG curves of the blends (Figure 3-2B), a single peak around 350 °C is verified for the blend with added tributyrin, while a slight double peak can be seen in the DTG of the blend with added glycerol. This observation is a possible indication that the zein–CA–tributyrin compatibility was higher than the zein–CA–glycerol compatibility. Both CA films and blends containing tributyrin lost mass slower than those plasticized with glycerol, and the mass losses matched around 240 °C. Films plasticized with tributyrin exhibited only two stages of mass loss (plasticizer degradation and polymer degradation), while three stages were observed for films with added glycerol (water loss, plasticizer degradation, and polymer degradation).
3.4. Mechanical and Water Vapor Barrier Properties
The blends’ compatibility can also be evaluated from the quality of the polymer mixing states, and classified as synergism, additivity, or incompatibility [37,38]. Through the mechanical analysis, it was observed that the TS, YM, and E% parameters of the blends were lower than those of CA films, as displayed in Table 2. This behavior is justified by the additivity rule, in which the properties exhibited by the mixed material corresponded with the average properties of isolated components. In this case, the addition of a polymer to another does not entail positive (synergism) or negative (incompatibility) changes to the polymers. It needs to be emphasized that pure zein films, with or without plasticizers, were brittle and difficult to handle, which hindered the obtainment of the specimens required for both the mechanical and permeability assays. The blends with added tributyrin, in turn, were more flexible, allowing the samples to be characterized. Although blends with added glycerol were also more flexible than zein films, the presence of macro-holes and tears on the films’ surface (Figure 1) impaired the obtainment of specimens for the conduction of these assays.

Table 2.
Thickness, mechanical properties (tensile strength, TS, elongation at break, E, and Young’s modulus, YM), and water vapor permeability (WVP) of blends (BL) and control films (CA) plasticized with tributyrin (T) or glycerol (G) and with incorporated garlic essential oil (GEO), β-cyclodextrin (βCD) or inclusion complex (IC).
Besides this, the incorporation of IC or βCD into CA films significantly reduced TS, YM, and E% parameters compared with the control and the film containing GEO. This is justified by the insertion of defects in the matrix and interruption of secondary interactions between polymer chains [39]. However, the same effect was not observed when comparing the mechanical performance among the blends. This suggests that IC and βCD did not impair the structural cohesion between the polymers in the blend.
The elaborated CA:zein blends also underperformed compared with the CA control and GEO films at WVP (Table 2), probably due to their more porous nature when compared with the more solid appearance of the CA films (Figure 2). The presence of IC or βCD also impaired the water vapor barrier property of the films, making them more permeable than the controls. According to Oliveira et al. [3], a film’s thickness heterogeneity may lead to problems with mechanical and barrier properties, which, in fact, was verified herein, since the films presenting the most irregular surfaces performed significantly worse on these assays.
WVP and mechanical properties are important parameters that must be considered when choosing an appropriate packaging for food. WVP, for example, is crucial for food preservation, while TS refers to material endurance and resilience. Although hydrophobic polymers, such as zein and CA, act as a better water vapor barrier than the hydrophilic ones, they are still less effective than the conventional plastics [40,41]. The mechanical properties of the biopolymers evaluated, measured in terms of TS and YM, need to be improved to achieve petrochemical polymer performance. Features such as brittleness, stiffness, and fragility may limit or even prevent their application [16,42]. To overcome these problems, several strategies are proposed, such as the investigation of new polymer nanocomposite-based films [5,14], incorporation of plasticizers [4,17], and mixing two or more polymers aiming for the obtainment of altered materials [15].
In the present work, we verified that the blends resulting from the mixing of zein and CA with tributyrin, despite underperforming compared with the CA control films, showed improvements when compared with the zein films. Incorporation of the nanoparticles βCD and IC impaired the film appearance and performance, while the addition of non-complexed GEO did not affect the films’ properties. Although the direct incorporation of EOs into polymer matrices is not recommended, IC incorporation harmed the films’ performance. Perhaps the choice of a natural βCD may not have been the best due to its insolubility in several organic solvents, such as acetone and ethanol, the solvents used for the filmogenic dispersion preparation [43]. Maybe a derivative CD with a hydrophobic nature or a higher solubility in polar organic solvents would solve the problem [43,44].
We also investigated two kinds of plasticizers: glycerol, widely used to plasticize biopolymer films, and tributyrin, a glycerol ester with three butyric acids of natural occurrence in milkfat [4,17,35,45]. It was observed that the plasticizer choice had an impact on the CA-zein blends. Glycerol has been incorporated into hydrophobic CA-films, as reported by Dias et al. [5], Gonçalves et al. [4] and Teixeira et al. [17]. However, its polar nature may have been the cause of the poor compatibility with the polymeric matrice blends, CA and zein, as verified by SEM micrographs and TG curves. Opposite behavior was observed when tributyrin was added as a plasticizer, resulting in films with a better performance and a more homogeneous appearance.
3.5. Antibacterial Investigation
S. aureus is an important foodborne pathogen, being considered one of the most significant threats to public health [46]. This bacterium is often responsible for foodborne intoxications through the production of heat-stable enterotoxins in various food products [47]. On the other hand, L. innocua has been used by many researchers as a surrogate for Listeria monocytogenes (considered a significant causative agent responsible for severe diseases in both humans and animals) in many food systems [48,49]. Therefore, both microorganisms were chosen to evaluate IC and GEO antibacterial activity. IC and GEO exhibited inhibitory activity against both bacteria, although the GEO antibacterial properties stood out (Table 3). However, it is essential to emphasize that the amount of EO present in IC corresponds to around 10% of the total IC weight (approximately 480 µg·mL−1), thus reducing the discrepancy between the MIC values found for them.

Table 3.
Garlic essential oil (GEO) and inclusion complex (IC) minimal inhibitory concentration (MIC) against S. aureus and L. innocua after incubation at 37 °C for 24 h.
MIC values also indicated that S. aureus was more susceptible to the GEO antibacterial effect than L. innocua. One of the main action mechanisms of GEO is altering the permeability of the cell membrane due to the hydrophobic nature of the compounds [50,51] Therefore, the higher GEO effect in S. aureus than L. innocua can be related to the microorganisms’ composition surface. Listeria sp. are Gram-positive bacteria; in spite of this, the microorganism has a peptidoglycan structure similar to Gram-negative bacteria, such as E. coli [52]. Thus, the greater hydrophilicity of the surface probably acted as a barrier to the EOs’ components [52,53].
Regarding the films, their antibacterial activity was evaluated by the indirect contact methodology, also using S. aureus and L. innocua as target microorganisms at their optimal growth temperature (37 °C). Furthermore, L. innocua was tested under refrigeration conditions (7 °C) since this microorganism has the ability to grow under refrigeration. The polymer matrix did not exert significative influence on the antibacterial properties of the films, and a similar pattern was observed when evaluating CA films and the blends. However, the kind of plasticizer used and the complexation or not of EO compounds did influence the outcomes. The results are displayed in Figure 4.
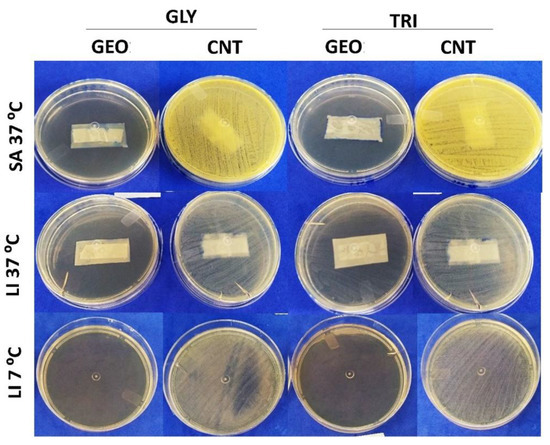
Figure 4.
Antibacterial properties of active cellulose acetate films with 10% garlic essential oil incorporated (GEO) and control films (CNT), produced with glycerol (GLY) and tributyrin (TRI) as plasticizers, tested by indirect contact against S. aureus (SA) and L. innocua (LI) at 37 °C/24 h and 7 °C/10 day.
The plasticized films with incorporated GEO completely inhibited the growth of S. aureus after 24 h at 37 °C. On the other hand, differences in the kind of plasticizer were observed in L. innocua growth, tested under the same conditions. The active films produced with tributyrin (BL-T-GEO and CA-T-GEO) only partially inhibited the target bacteria, while the films with added glycerol completely inhibited L. innocua growth. The plasticized films containing the IC, in turn, did not affect bacteria growth, showing a similar outcome to the control films.
The differences found for plasticizer type can be attributed to the hydrophobic character of tributyrin [35]. This non-polar profile of tributyrin may enhance the entrapment of the GEO into the film matrix and, therefore, reduce the release of antibacterial agents. As a result, the films produced with this plasticizer exhibited lower antibacterial performance during the tests. Corroborating with this hypothesis, the GEO MIC results, previously discussed, indicated higher susceptibility of S. aureus to GEO components, which may explain the complete inhibition achieved with the active film; meanwhile, L. innocua exhibited a certain resistance, being partially inhibited.
Under refrigeration (7 °C/10 day), L. innocua, a psychrotrophic bacteria, did not exhibit visual growth when incubated in the presence of films with 10% GEO incorporated, independent of the plasticizer (Figure 4). In this case, despite the ability to grow under refrigeration temperatures [54], the cold conditions contributed to the GEO antibacterial activity. GEO thermal stability at lower temperatures was not assessed in the present research; however, low temperatures were already associated with stabilizing some GEO compounds [55]. This improved activity minimized the possible entrapment effect of tributyrin, and the films were able to control the bacteria growth during the incubation period. In addition, films with IC incorporated once again underperformed compared with the films with added GEO and only partially inhibited L. innocua growth when under refrigerated conditions (results not shown). These results are in agreement with the MIC values found, since it was verified that a much higher concentration of IC was necessary to inhibit microbial growth, indicating that more IC should be added to the filmogenic dispersion in order to obtain films with a similar active property to the films with added GEO. However, this would hardly be viable.
4. Conclusions
In the present work, CA:zein active blends were successfully manufactured by the incorporation of GEO, as the bioactive agent, and tributyrin, as the plasticizer, into bio-based polymeric matrices. Despite the blends underperforming compared with the CA control films, they showed considerable improvements over zein films, which were brittle and fragile to the extent of impairing their manipulation. This could open a wider range of applications of zein as a food packaging component. Besides this, the results indicated that the films elaborated with non-complexed GEO could be used as a potential ally in food preservation, assisting in maintaining the microbiological quality and safety of food products. Films with IC incorporated, on the other hand, underperformed compared with those with added GEO regarding antibacterial activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polysaccharides3010016/s1, Figure S1. Cellulose acetate and zein blends prepared with different polymer ratios (wt/wt). Figure S2. Cellulose acetate and zein as pellets and films. Figure S3. Thermogravimetric (TG) curves and their derivative (DTG) of βCD, inclusion complex (IC), garlic essential oil (GEO), and physical mixture (PM). * The red arrow indicates GEO loss in the inclusion complex. Figure S4. X-ray diffractograms of βCD, inclusion complex (IC), and physical mixture (PM). * Black arrows indicate the characteristic peaks for βCD. Figure S5. Thermogravimetric (TG) curves and their derivative (DTG) of cellulose acetate (CA) films and CA:zein blends incorporated with tributyrin (T) or glycerol (G) as plasticizers, and garlic essential oil (GEO), β-cyclodextrin (βCD), or inclusion complex (IC).
Author Contributions
Conceptualization, C.S.M. and N.d.F.F.S.; methodology, C.S.M., R.R.A.S., and A.L.V.F.; validation, C.S.M. and R.R.A.S.; investigation, C.S.M.; resources, N.d.F.F.S.; writing—original draft preparation, C.S.M., R.R.A.S., and T.R.A.; writing—review and editing, C.S.M. and T.V.d.O.; supervision, M.V.D., A.R.F.M., M.C.D.V., and N.d.F.F.S.; project administration, N.d.F.F.S.; funding acquisition, N.d.F.F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (grant number 142564/2018-4). In addition, this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors are grateful to colleagues from the Food Packaging Laboratory of Federal University of Viçosa (UFV), for exchange of experiences and knowledge, and to the Physics Department (UFV) for the use of experimental facilities. They also would like to thank Rhodia Solvay Group, for donating cellulose acetate, and Flo Chemical Corp, for donating zein.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kato, L.S.; Conte-Junior, C.A. Safety of plastic food packaging: The challenges about non-intentionally added-substances (NIAS) discovery, identification and risk assessment. Polymers 2021, 13, 2077. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Oliveira, T.V.; Freitas, P.A.V.; Pola, C.C.; Silva, J.O.R.; Diaz, L.D.A.; Ferreira, S.O.; Soares, N.F.F. Development and optimization of antimicrobial active films produced with a reinforced and compatibilized biodegradable polymers. Food Packag. Shelf. Life 2020, 24, 100459. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Santos, D.C.; Motta, J.F.G.; Santos, R.R.; Chávez, D.W.H.; Melo, N.R. Structure and functional properties of cellulose acetate incorporated with glycerol. Carbohydr. Polym. 2019, 209, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.V.; Sousa, M.M.; Lara, B.R.B.; Azevedo, V.M.; Soares, N.F.F.; Borges, S.V.; Queiroz, F. Thermal and morphological properties and kinetics of diffusion of antimicrobial films on food and a simulant. Food Packag. Shelf. Life 2018, 16, 15–22. [Google Scholar] [CrossRef]
- Pola, C.C.; Medeiros, E.A.A.; Pereira, O.L.; Souza, V.G.L.; Otoni, C.G.; Camilloto, G.P.; Soares, N.F.F. Cellulose acetate active films incorporated with oregano (Origanum vulgare) essential oil and organophilic montmorillonite clay control the growth of phytopathogenic fungi. Food Packag. Shelf. Life 2016, 9, 69–78. [Google Scholar] [CrossRef]
- Malm, M.; Liceaga, A.M.; San Martin-Gonzalez, F.; Jones, O.G.; Garcia-Bravo, J.M.; Kaplan, I. Development of Chitosan Filmsfrom Edible Crickets and Their Performance as a Bio-Based Food Packaging Material. Polysaccharides 2021, 2, 744–758. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Development and characterization of plant oil-incorporated carboxymethyl cellulose/bacterial cellulose/glycerol-based antimicrobial edible films for food packaging applications. Adv. Compos. Hybrid. Mater. 2022, 1–17. [Google Scholar] [CrossRef]
- Yuvarai, D.; Iyyappan, J.; Gnanasekaran, R.; Ishwarya, G.; Harshini, R.P.; Dhithya, V.; Chandran, M.; Kanishka, V.; Gomathi, K. Advances in bio food packaging—An overview. Heliyon 2021, 7, e07998. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Huang, R.; Vaze, N.; Xu, T.; Eitzer, B.D.; Krol, W.; MacQueen, K.A.; Chang, H.; Bousfield, D.W.; Chan-Park, M.B.; et al. Development of Biodegradable and Antimicrobial Electrospun Zein Fibers for Food Packaging. ACS Sustain. Chem. Eng. 2020, 8, 15354–15365. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=FCN&id=2043&sort=FCN_No&order=DESC&startrow=1&type=basic&search=cellulose%20acetate (accessed on 20 January 2022).
- US Food and Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1984 (accessed on 20 January 2022).
- Lara, B.R.B.; Andrade, P.S.; Guimarães Junior, M.; Dias, M.V.; Alcântara, L.A.P. Novel Whey Protein Isolate/Polyvinyl Biocomposite for Packaging: Improvement of Mechanical and Water Barrier Properties by Incorporation of Nano-silica. J. Polym. Environ. 2021, 29, 2397–2408. [Google Scholar] [CrossRef]
- Moraes, A.R.F.; Pola, C.C.; Bilck, A.P.; Yamashita, F.; Tronto, J.; Medeiros, E.A.A.; Soares, N.F.F. Starch, cellulose acetate and polyester biodegradable sheets: Effect of composition and processing conditions. Mater. Sci. Eng. C 2017, 78, 932–941. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, S.C.; Silva, R.R.A.; Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.; Soares, N.F.F. Glycerol and triethyl citrate plasticizer effects on molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Arruda, T.R.; Marques, C.S.; Soares, N.F.F. Native Cyclodextrins and Their Derivatives as Potential Additives for Food Packaging: A Review. Polysaccharides 2021, 2, 825–842. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; González-Aguilar, G.A. Optimizing the use of garlic oil as antimicrobial agent on fresh-cut tomato through a controlled release system. J. Food Sci. 2010, 75, M398–M405. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control 2022, 136, 108883. [Google Scholar] [CrossRef]
- Ali, S.; Khatri, Z.; Oh, K.W.; Ki, I.S.; Kim, S.H. Zein/cellulose acetate hybrid nanofibers: Electrospinning and characterization. Macromol. Res. 2014, 22, 971–977. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: As efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery application. LWT-Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- ASTM. ASTM D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2012.
- ASTM. ASTM E96/E96M-10; Standard Test Method for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2010.
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 20 December 2021).
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT-Food Sci. Technol. 2015, 60, 583–592. [Google Scholar] [CrossRef]
- Giordano, F.; Novak, C.; Moyano, J.R. Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta 2001, 380, 123–151. [Google Scholar] [CrossRef]
- Piletti, R.; Zanetti, M.; Jung, G.; Melo, J.M.M.; Dalcanton, F.; Soares, C.; Riella, H.G.; Fiori, M.A. Microencapsulation of garlic oil by β-cyclodextrin as thermal protection method for antibacterial action. Mater. Sci. Eng. C 2019, 94, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Boy, R.; Gupta, B.S.; Tonelli, A.E. Analytical techniques for characterizing cyclodextrins and their inclusion complexes with large and small molecular weight guest molecules. Polym. Test. 2017, 62, 402–439. [Google Scholar] [CrossRef]
- Takahashi, A.I.; Veiga, F.J.B.; Ferraz, H.G. A literature review of cyclodextrin inclusion complexes characterization—Part II: X-ray diffraction, infrared spectroscopy and nuclear magnetic resonance. Int. J. Pharm. Sci. Rev. Res. 2012, 1, 8–15. [Google Scholar]
- Iglesias Montes, M.L.; D’amico, D.A.; Manfredi, L.B.; Cyras, V.P. Effect of Natural Glyceryl Tributyrate as Plasticizer and Compatibilizer on the Performance of Bio-Based Polylactic Acid/Poly(3-Hydroxybutyrate) Blends. J. Polym. Environ. 2019, 27, 1429–1438. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Silva, R.R.A.; Oliveira, T.V.; Soares, R.R.A.; Junior, N.S.; Moraes, A.R.F.; Pires, A.C.S.; Soares, N.F.F. Development and characterization of intelligent cellulose acetate-based films using red cabbage extract for visual detection of volatile bases. LWT-Food Sci. Technol. 2020, 132, 109780. [Google Scholar] [CrossRef]
- Meister, J. Polymer Modification: Principles, Techniques and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; 914p. [Google Scholar]
- Shanks, R.A. Concepts and classification of compatibilization processes. In Compatibilization of Polymer Blends, 1st ed.; Ajitha, A.R., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–56. [Google Scholar]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; John Willey & Sons: Hoboken, NJ, USA, 2010; 992p. [Google Scholar]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Togashi, Y.; Hara, M. Water vapor permeability of polypropylene. Fusion Sci. Technol. 2011, 60, 1471–1474. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Abdul Wahab, N.I. Corn Starch (Zea mays) Biopolymer Plastic Reaction in Combination with Sorbitol and Glycerol. Polymers 2021, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.C.; Martins, T.E.A.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef] [Green Version]
- Przybyla, M.A.; Yilmaz, G.; Becer, R. Natural cyclodextrins and their derivatives for polymer synthesis. Polym. Chem. 2020, 11, 7582–7602. [Google Scholar] [CrossRef]
- Vazquez, R.; Nogueira, R.; Orfão, M.; Mata, J.L.; Saramago, B. Stability of triglyceride liquid films on hydrophilic and hydrophobic glasses. J. Colloid Interface Sci. 2006, 299, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Rubab, M.; Shahbaz, H.M.; Olaimat, A.N.; Oh, D.H. Biosensors for Rapid and Sensitive Detection of Staphylococcus aureus in Food. Biosens. Bioelectron. 2018, 105, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and Its Food Poisoning Toxins: Characterization and Outbreak Investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamloo, E.; Hosseini, H.; Moghadam, A.Z.; Larsen, H.M.; Haslberger, A.; Alebouyeh, M. Importance of Listeria Monocytogenes in Food Safety: A Review of Its Prevalence, Detection, and Antibiotic Resistance. Iran. J. Vet. Res. 2019, 20, 241–254. [Google Scholar] [PubMed]
- Friedly, E.C.; Crandall, P.G.; Ricke, S.; O’Bryan, C.A.; Martin, E.M.; Boyd, L.M. Identification of Listeria Innocua Surrogates for Listeria Monocytogenes in Hamburger Patties. J. Food Sci. 2008, 73, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Münchberg, U.; Anwar, A.; Mecklenburg, S.; Jacob, C. Polysulfides as Biologically Active Ingredients of Garlic. Org. Biomol. Chem. 2007, 5, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Arbach, M.; Santana, T.M.; Moxham, H.; Tinson, R.; Anwar, A.; Groom, M.; Hamilton, C.J. Antimicrobial Garlic-Derived Diallyl Polysulfanes: Interactions with Biological Thiols in Bacillus Subtilis. Biochim. Biophys. Acta—Gen. Subj. 2019, 1863, 1050–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bierne, H.; Cossart, P. Listeria Monocytogenes Surface Proteins: From Genome Predictions to Function. Microbiol. Mol. Biol. Rev. 2007, 71, 377–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedler, F. Biochemistry of the Cell Surface of Listeria Strains: A Locating General View. Infection 1988, 16, S92–S97. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Shen, X.; Su, Y.; Xue, Y.; Gao, H.; Mendoza, M.; Green, T.; Hanrahan, I.; Zhu, M.-J. Effects of 1-Methylcyclopropene and Gaseous Ozone on Listeria Innocua Survival and Fruit Quality of Granny Smith Apples during Long-Term Commercial Cold Storage. Food Microbiol. 2022, 102, 103922. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of Allicin Determined by Chemical and Biological Assays. Biosci. Biotechnol. Biochem. 2008, 72, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
