A Comprehensive Review on Various Phases of Wastewater Technologies: Trends and Future Perspectives
Abstract
1. Introduction
2. Conventional Wastewater Treatment Plant
3. Preliminary Treatment
4. Primary Treatment
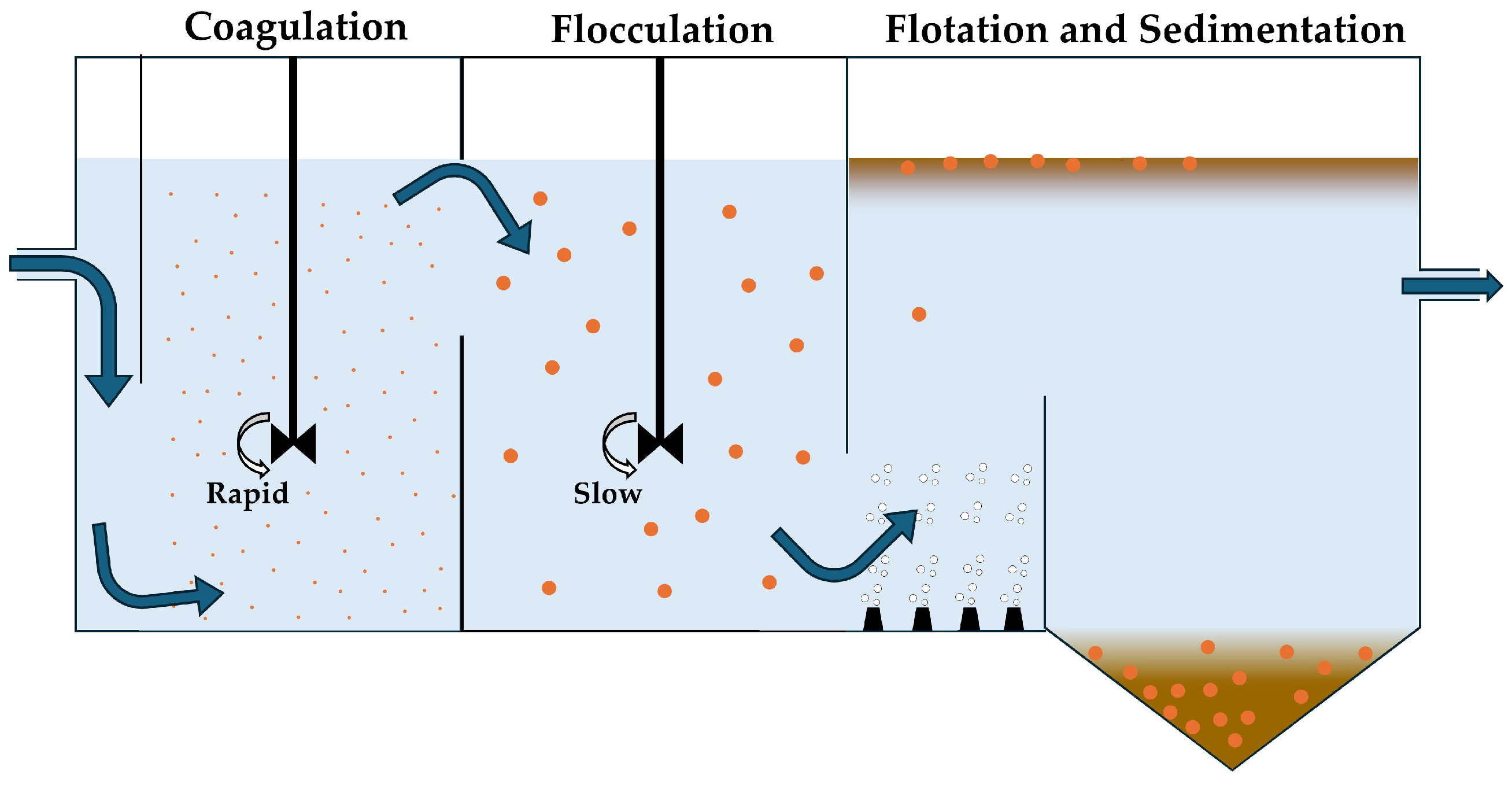
4.1. Coagulation and Flocculation
4.2. Flotation
5. Secondary Treatment
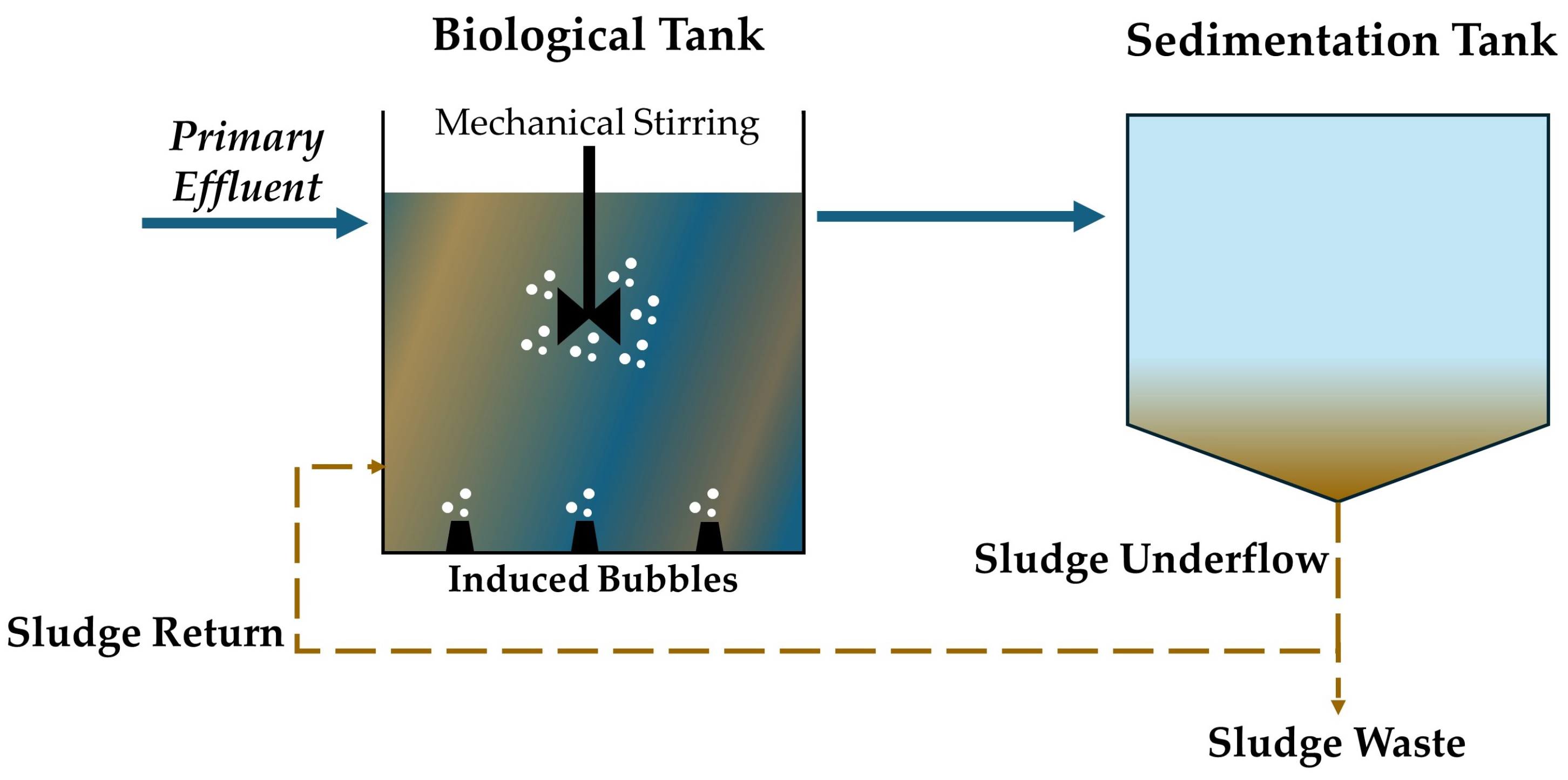
5.1. Activated Sludge
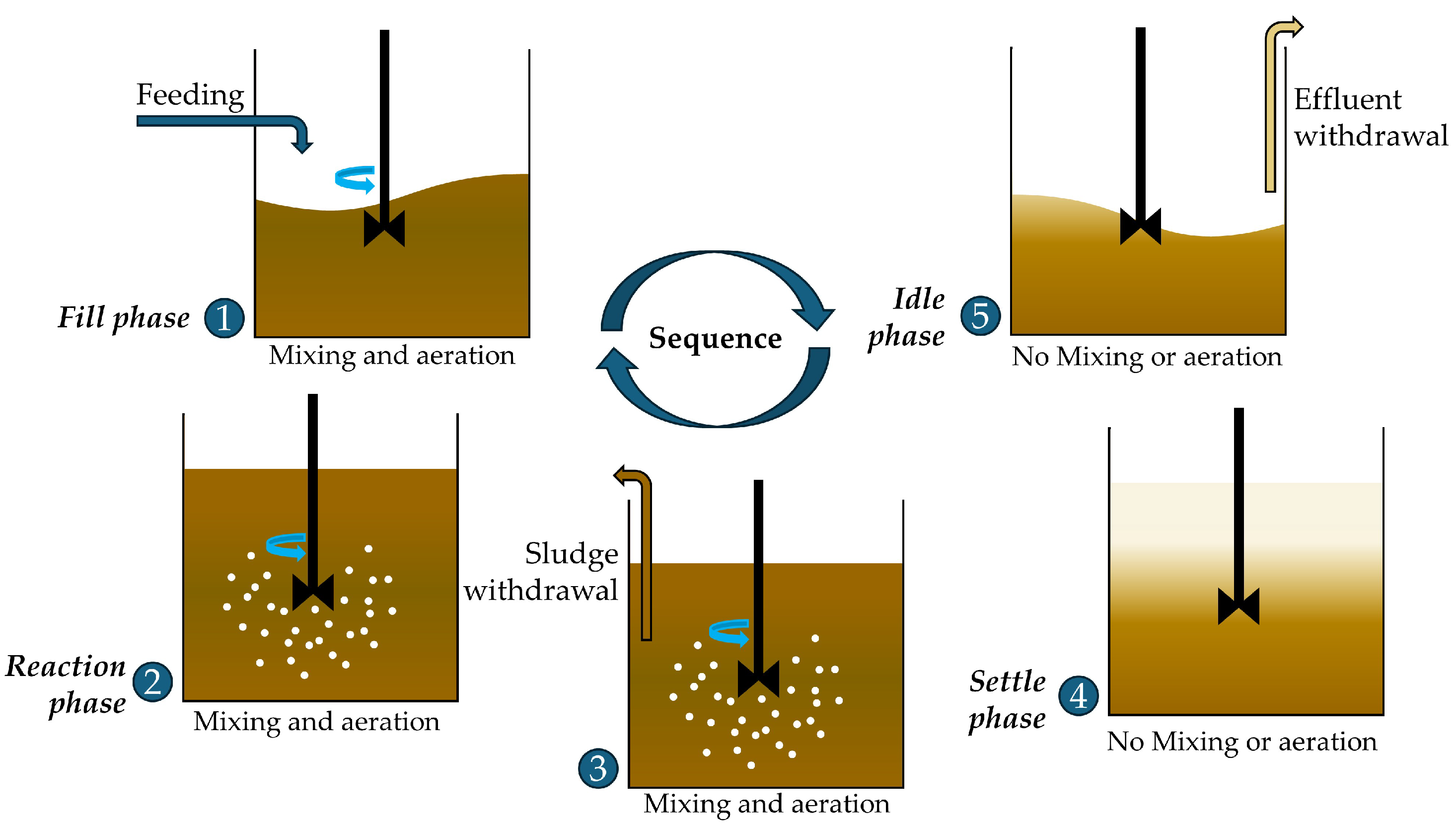
5.2. Sequencing Batch Reactor
5.3. Membrane Bioreactor
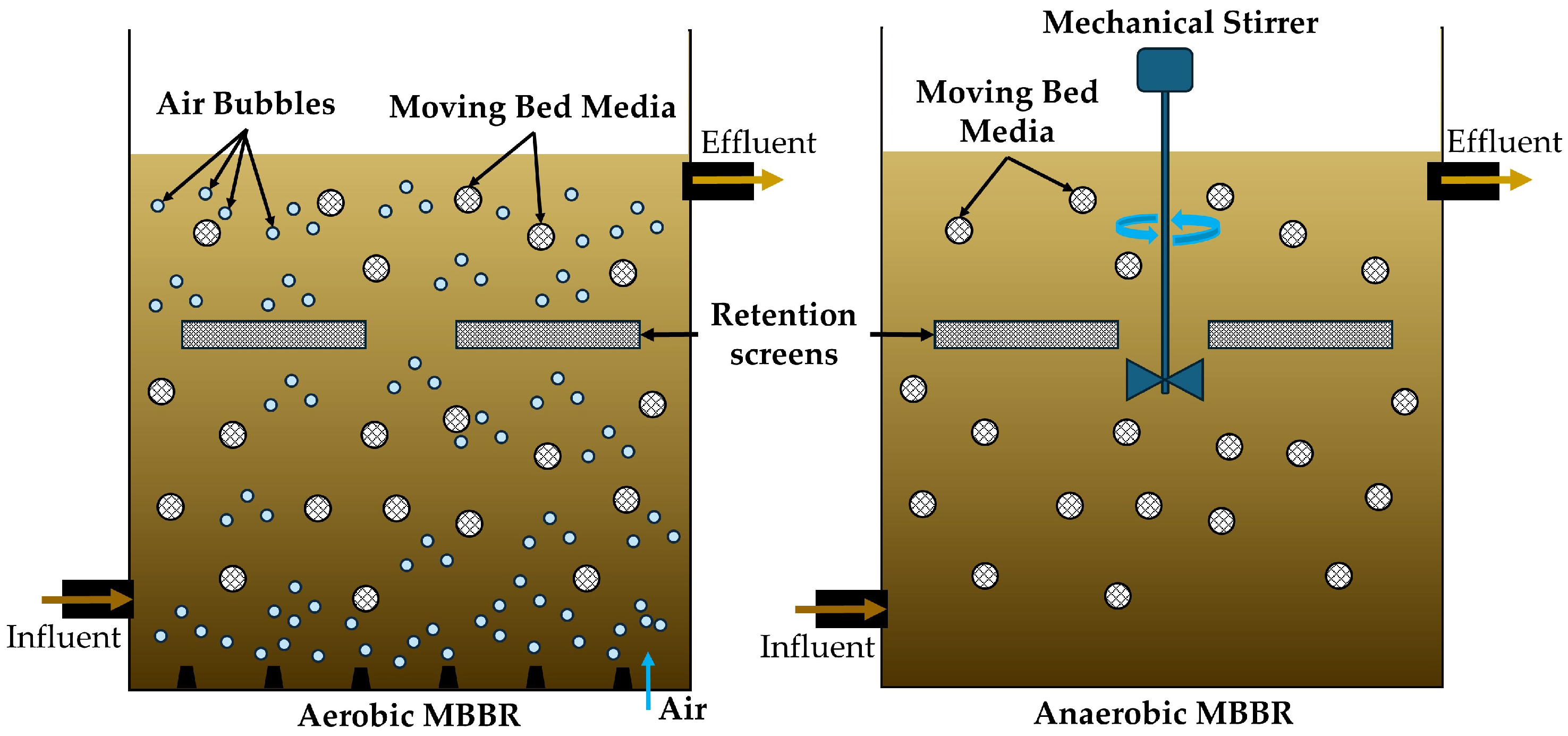
5.4. Biofilm-Based
6. Tertiary Treatment and Advanced Oxidation Processes
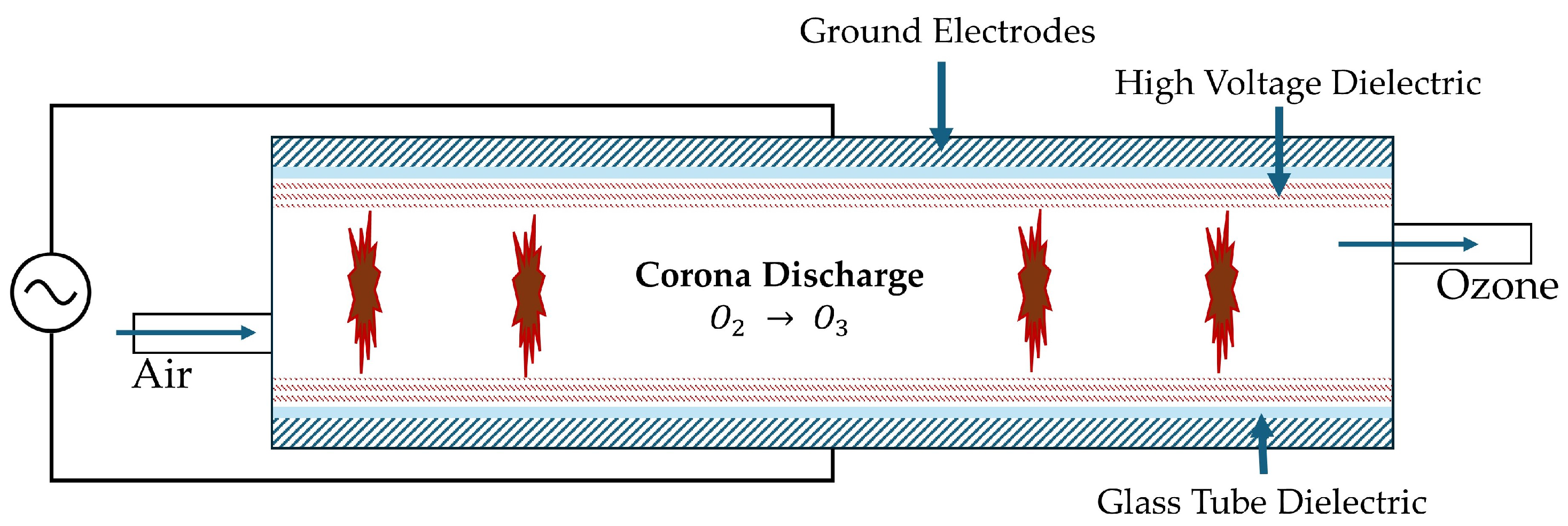
6.1. Chloronation and Ozonation
6.2. UV Disinfection
6.3. Fenton
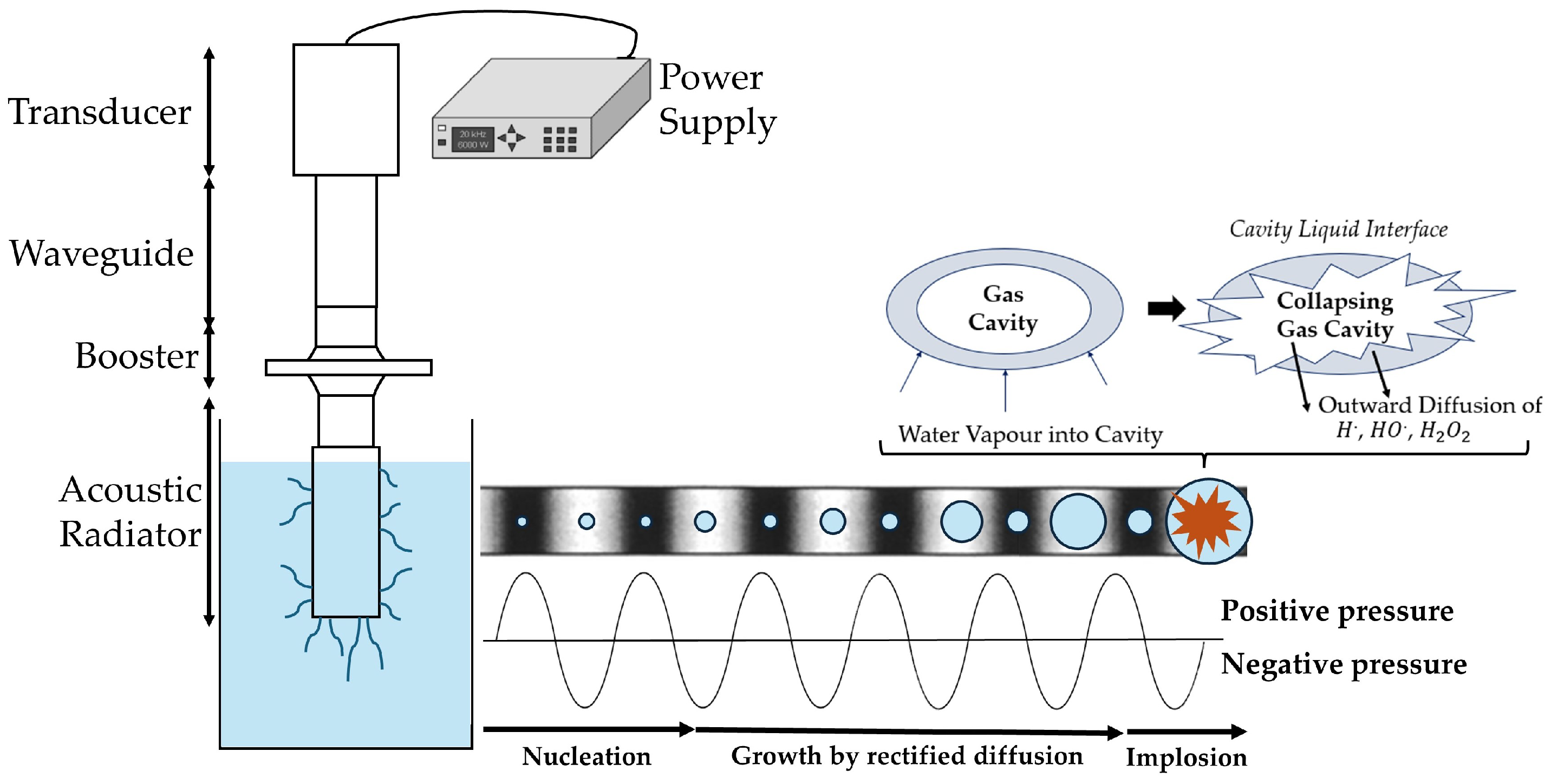
6.4. Ultrasound
6.5. Electrochemical
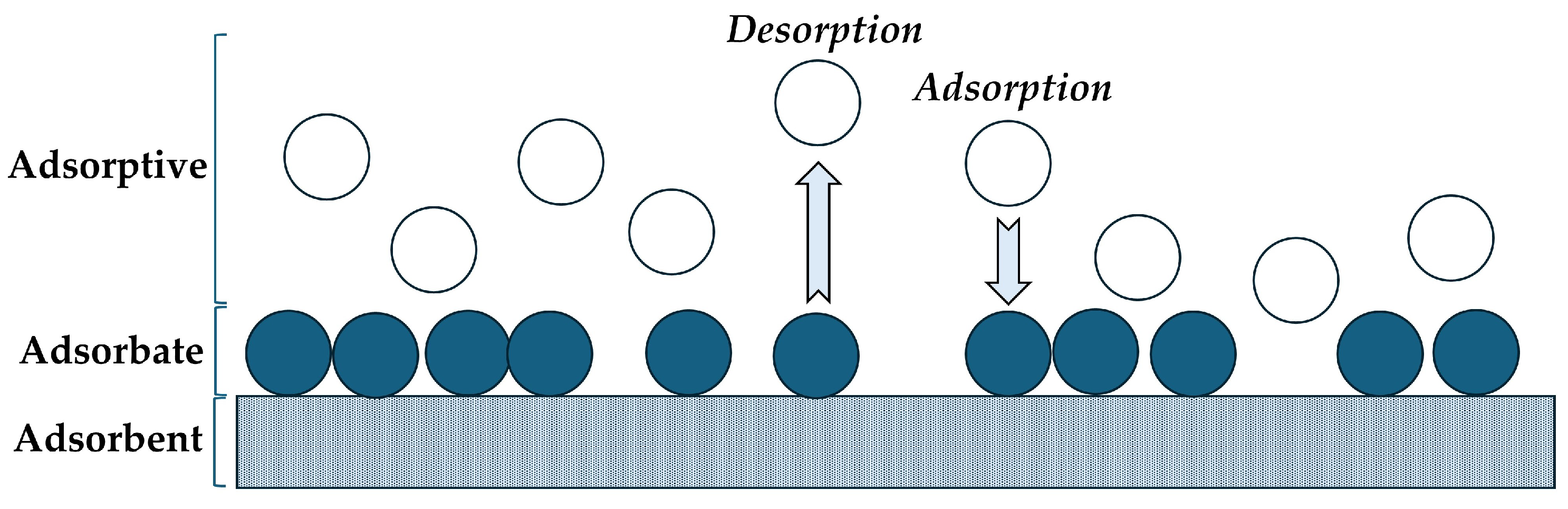
6.6. Adsorption
6.7. Hybrid Processes
| Wastewater | Process | Experimental Conditions | Removal Efficiency |
|---|---|---|---|
| Synthetic | Adsorption/Fenton [166] | 5000 M ; 0.05 g AG; pH 3 | SMT: 78% (60 min) |
| Adsorption/US [167] | 0.5 g/L FeCS; 40 kHz and 300 W US (pre-treatment) | MB: 98% (10 min) | |
| Electro-Fenton/UV [168] | mg/L ; 15.19 mg/L O; pH 3; 0.6 L/min air flow; Felt graphite anode and cathode; 3 mA/ current density | MB: 99% (20 min); TC: 62% (20 min); MG and 90% (14 min) | |
| US/Fe(0)/S(IV) [169] | 0.05 mmol/L S(IV); 0.05 g/L Fe(0); 40 kHz US | TBP: 89.6% (30 min) | |
| US//MC [161] | 100 W US; pH 9; 8.6 kV Discharge Voltage; 15 mL/min flow rate; 0.8 mm microchannel width | MB: 92.7% (14 min) | |
| Municipal | Adsorption/US [170] | 1 mg Cu(BDC)@Wool; 0.5 mL/min flow rate; 7.5 mm bed height; pH 2 | RIF: 98.6% (120 min) |
| //UV [171] | 465 mJ/ UVC; 3 mg/L ; 3 mg/L | FLU: 80%; GMF: 90%; PRM: 50%; CBZ, TMP, SMZ: >99% (continuous) | |
| MBR/ [156] | 5 g/ inlet; NF-90 polyamide membrane | CBZ and SMZ: 100% (15–20 min); TB: 100% (>30 min), APAP, TET: 100% (5 min) | |
| MBR/UV/PS [157] | PVDF flat UF membranes; 254 nm UV; 0.06 mmol/L PS | OMP: 100% (150 min) | |
| US/UV/ [162] | 100 W and 40 kHz US; UVC; 0.5 mM ; 7.8 mM | E. aerogenes: 98.6%; E. coli: 99.1%; Other coliforms: 96.2%; Total coliforms: 98.1%; COD: 91.1% (10 min) | |
| Industrial | (A/O)MBR/Fenton [172] | Alumina microporous membrane; 30 mmol/L ; 6 mmol/L ·O | COD: 90%, AOX: 79%, : 88% (continuous) |
| Adsorption/Fenton [173] | Wood biochar adsorbent; 7 cm bed depth; 15 Ml/min flowrate; 10 mM ; 15 mM ; pH 3 | COD: 94.5%; Sulphide: 97.4%; N: 96.2%, : 83.1%; : 79.3%; Cr(VI): 96.9% (120 min) | |
| Coagulation-AC/UV/Fenton [165] | UVC; 160 ppm/L alum coagulant; 1:100 AC; 1:300 ·7O: | COD: 87.49%; BOD: 87.02%; TSS: 72.45%; Zn: <99%; Cu: 64%; Pb: 96%; Fe: 35% (60 min) | |
| Electroadsorption [174] | 50 mM electrolyte; 0.2 mM Fe (II); pH 3; coconut shell cathode; Iridium and Ruthenium coating anode | TOC: 87% (120 min) | |
| Electro-Fenton [175] | pH 5.95; 1.5 mL ; 1.8 /; Al anode; Iron cathode; 2 A and 24 V current density | COD: 95.8% (60 min) | |
| EC-UV-Fenton [176] | Al anode; Stainless Steel cathode; 120 A/ current density; pH 6.87; UVC 32 W; 0.4 /HP | COD: 75.1%; Color: 93.3%; TSS: 82.0%; Aromatic compounds: 89.8% (30 min) | |
| US/Electro/Fenton [177] | 0.2 mM ; 10 mA/ current density; 100 W US; 4.3 kWh/kg SEC; 0.2 mM | COD: 91.04%; Turbidity: 84.62%; Phenols: 91.67% (56 min) | |
| US/UV/Fenton [160] | 2 g/L FA load; pH 3; 576 kHz US | COD: 40%; Colour: 36.8%; Aromatic Compounds: 50.8% (60 min) |
7. Future Trends
- Coagulants and flocculants pose challenges due to their sourcing and disposal issues. Research should focus on developing eco-friendly biocoagulants and flocculants to address these concerns, prioritizing sustainable raw materials that avoid competition with food supply chains. Reducing extraction complexity and increasing studies on bio-based alternatives are essential. Testing these materials in real-scale projects and improving additive efficiency can help lower energy demands, contributing to more sustainable treatment processes:
- As the demand for removing emerging pollutants increases, there is a shift towards environmentally friendly physical processes that reduce reliance on chemical treatments, enhancing the effectiveness of both and striving for optimal synergy between them. Garrido-Cardenas et al. [178] observed a surge in publications on AOPs since 2015, with future trends focusing on scaling up and implementing these processes in real-world conditions. Current research primarily involves pilot-scale and batch-mode studies, but practical application necessitates a transition to continuous flow evaluations with the integration of fine-tuning operational factors and techno-economic analyses [11,12,101].
- The improvement of MBRs should prioritize enhancing membrane material stability and activity, modifying existing systems, and exploring novel sustainable materials [179,180,181,182]. Coupling MBRs with AOPs or other processes can boost removal efficiency and reduce fouling. Research must shift to real wastewater and pilot-scale testing, focusing on optimizing reactor structures, minimizing energy consumption, and reducing operational costs. Effective membrane cleaning and regeneration are essential for extending lifespan and lowering environmental impacts [102].
- Regarding US technology, sono-chemical oxidation, requires further investigation to understand its potential and integration into WWTPs. Current research focuses on conventional ultrasound devices, but future studies should explore diverse chamber designs and cavitation effects on pollutant removal. Combining ultrasound with other treatments has shown significant potential, and understanding synergies, mechanisms, and optimal configurations will be crucial for improving its application in wastewater treatment.
- Traditional Fenton limitations can be addressed by integrating other AOPs [12,183]. The shift toward heterogeneous Fenton is essential due to the impracticality of the homogeneous process. Future trends focus on using materials like zero-valent iron, iron (hydr)oxides, iron-based metallic glasses, and loaded iron-based materials to improve catalyst efficiency and reduce energy costs [183,184]. Research should optimize catalyst performance, lower operational costs, and explore synergies with other AOPs to enhance treatment efficacy [183].
- Ozone technology has advanced but still faces high energy demands and potential pollutant by-products. Scaling up requires dosage optimization, particularly when combined with Fenton, ultrasound, and UV light. Future research on ozone-based processes should prioritize economic evaluations, cost-effectiveness, reactor designs, and degradation efficiency [163]. Conducting pilot- and field-scale studies is essential to assess feasibility, while a deeper understanding of reaction kinetics and the development of robust models will enhance the optimization of ozonation-based AOPs.
- AC remains a key adsorbent in wastewater treatment, with future trends focusing on optimizing configurations [174,185]. Nanomaterials and bioadsorbents also show promise, though improvements are needed in biosorbent production and scalability for pilot applications in WWTPs. Research should prioritize combining AC with other methods to enhance adsorption capacity, reusability, and removal efficiency. Developing novel adsorbents like carbon nanotubes and metal-organic frameworks, alongside leveraging machine learning and AI for adsorption kinematics study, is crucial for advancing eco-friendly and high-capacity adsorption technologies [114].
- To enhance UV disinfection efficacy and mitigate drawbacks, combining UV with oxidants like hydrogen peroxide, ozone, persulfate, chlorine, and chlorine dioxide is promising. Future research should focus on the mechanisms behind these synergies to improve microorganism removal and reduce by-products [99]. Another key trend is using solar energy as a power source, requiring continued research on optimal conditions, pilot project implementation, and reactor development to maximize solar efficiency.
- Yuan et al. [146] and Li et al. [147] highlight trends in Electrochemical-AOPs (EAOPs) aimed at improving Reactive Oxygen Species efficiency. Key strategies include creating confined micro-environments with metal nano-particles and porous graphite for targeted antibiotic degradation, optimizing cathode properties and addressing low antibiotic concentrations. Future research focuses on developing cost-effective anode materials like carbon and graphite, enhancing toxicity assessment methods, and integrating EAOPs with UV light, ozone, membranes, and biological treatments to boost efficiency.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sangamnere, R.; Misra, T.; Bherwani, H.; Kapley, A.; Kumar, R. A critical review of conventional and emerging wastewater treatment technologies. Sustain. Water Resour. Manag. 2023, 9, 58. [Google Scholar] [CrossRef]
- Vajargah, M.F.; Ramzanipour, M.M.; Mousavi, S.P. An Overview of Municipal Wastewater and Sludge Treatment Process. Ecol. Conserv. Sci. Open Access 2023, 2, 555596. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater Treatment and Reuse: A Review of its Applications and Health Implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- UNESCO World Water Assessment Programme (Ed.) The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; UN: Paris, France, 2023; pp. 1–189. [Google Scholar]
- Sathya, K.; Nagarajan, K.; Malar, G.C.G.; Rajalakshmi, S.; Lakshmi, P.R. A comprehensive review on comparison among effluent treatment methods and modern methods of treatment of industrial wastewater effluent from different sources. Appl. Water Sci. 2022, 12, 70. [Google Scholar] [CrossRef]
- Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 21 June 2024).
- Guillossou, R.; Roux, J.L.; Mailler, R.; Vulliet, E.; Morlay, C.; Nauleau, F.; Gasperi, J.; Rocher, V. Organic micropollutants in a large wastewater treatment plant: What are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 2019, 218, 1050–1060. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of pharmaceuticals from water by adsorption and advanced oxidation processes: State of the art and trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Bracamontes-Ruelas, A.R.; Ordaz-Díaz, L.A.; Bailón-Salas, A.M.; Ríos-Saucedo, J.C.; Reyes-Vidal, Y.; Reynoso-Cuevas, L. Emerging Pollutants in Wastewater, Advanced Oxidation Processes as an Alternative Treatment and Perspectives. Processes 2022, 10, 1041. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Ozaki, N.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Occurrence of per- and polyfluoroalkyl substances in aquatic environments and their removal by advanced oxidation processes. Chemosphere 2023, 330, 138666. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Alvarino, T.; Lema, J.; Omil, F.; Suárez, S. Trends in organic micropollutants removal in secondary treatment of sewage. Rev. Environ. Sci. Bio/Technol. 2018, 17, 447–469. [Google Scholar] [CrossRef]
- Habib, M.L.; Hasan, M.M.; Biswas, S.; Hossain, M.T.; Anwaruzzaman, M.; Kamruzzaman, M. Removal of Organic Micro-Pollutants by Aerobic and Anaerobic Microorganism; Elsevier: Amsterdam, The Netherlands, 2022; pp. 55–78. [Google Scholar] [CrossRef]
- Commission, E. Commission Welcomes Provisional Agreement for More Thorough and More Cost-Effective Urban Wastewater Management. Press Release. 2024. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_24_504 (accessed on 21 June 2024).
- Tušer, I.; Oulehlová, A. Risk assessment and sustainability of wastewater treatment plant operation. Sustainability 2021, 13, 5120. [Google Scholar] [CrossRef]
- Mishra, H.; Gaurav, G.; Khandelwal, C.; Dangayach, G.S.; Rao, P.N. Environmental assessment of an Indian municipal wastewater treatment plant in Rajasthan. Int. J. Sustain. Eng. 2021, 14, 953–962. [Google Scholar] [CrossRef]
- Pandey, B.C.; Gupta, S. Review: Wastewater Treatment in Different Industries. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 563–568. [Google Scholar] [CrossRef]
- Koul, B.; Yadav, D.; Singh, S.; Kumar, M.; Song, M. Insights into the Domestic Wastewater Treatment (DWWT) Regimes: A Review. Water 2022, 14, 3542. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Singh, P.; Carliell-Marquet, C.; Kansal, A. Energy pattern analysis of a wastewater treatment plant. Appl. Water Sci. 2012, 2, 221–226. [Google Scholar] [CrossRef]
- Vieira, Y.; Netto, M.S.; Lima, C.É.; Anastopoulos, I.; Oliveira, M.L.; Dotto, G.L. An overview of geological originated materials as a trend for adsorption in wastewater treatment. Geosci. Front. 2022, 13, 101150. [Google Scholar] [CrossRef]
- Masłoń, A.; Czarnota, J.; Szczyrba, P.; Szaja, A.; Szulżyk-Cieplak, J.; Łagód, G. Assessment of Energy Self-Sufficiency of Wastewater Treatment Plants—A Case Study from Poland. Energies 2024, 17, 1164. [Google Scholar] [CrossRef]
- Riffat, R. Fundamentals of Wastewater Treatment and Engineering; Taylor and Francis Group: London, UK, 2013. [Google Scholar]
- Jasim, N.A. The design for wastewater treatment plant (WWTP) with GPS X modelling. Cogent Eng. 2020, 7, 1723782. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Prabu, S.L.; Kumar, A. Wastewater treatment technologies: A review. Pharma Times 2011, 43, 9–13. [Google Scholar]
- He, L.; Zhang, Y.; Song, D.; Ou, Z.; Xie, Z.; Yang, S.; Guan, W.; Dong, C.; Zhang, Y. Influence of Pretreatment System on Inorganic Suspended Solids for Influent in Wastewater Treatment Plant. J. Environ. Public Health 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Sukmana, H.; Bellahsen, N.; Pantoja, F.; Hodur, C. Adsorption and coagulation in wastewater treatment—Review. Prog. Agric. Eng. Sci. 2021, 17, 49–68. [Google Scholar] [CrossRef]
- Ullah, A.; Hussain, S.; Wasim, A.; Jahanzaib, M. Development of a decision support system for the selection of wastewater treatment technologies. Sci. Total Environ. 2020, 731, 139158. [Google Scholar] [CrossRef]
- Ho, Y.C.; Chua, S.C.; Chong, F.K. Coagulation-Flocculation Technology in Water and Wastewater Treatment. In Handbook of Research on Resource Management for Pollution and Waste Treatment; IGI Global: Hershey, PA, USA, 2020; pp. 432–457. [Google Scholar] [CrossRef]
- Agunbiade, M.O.; Pohl, C.H.; Ashafa, A.O. A review of the application of biofloccualnts in wastewater treatment. Pol. J. Environ. Stud. 2016, 25, 1381–1389. [Google Scholar] [CrossRef]
- Abujazar, M.S.S.; Karaağaç, S.U.; Amr, S.S.A.; Alazaiza, M.Y.; Bashir, M.J. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Rachid, E.B.; Abderrahim, S.; Hafid, A.; Souad, R. Water treatment: Aluminum sulfate, polymer, and activated carbon between efficacy and overdosing. Case of Oum Er-Rbia River, Morocco. Desalin. Water Treat. 2024, 317, 100273. [Google Scholar] [CrossRef]
- Hafidi, E.M.E.; Mortadi, A.; Chahid, E.G.; Laasri, S. Optimization of Domestic Wastewater Treatment Using Ferric Chloride Coagulant: Physicochemical Analysis and Impedance Spectroscopy Studies. Water Air Soil Pollut. 2024, 235, 68. [Google Scholar] [CrossRef]
- Ilias, M.K.M.; Hossain, M.S.; Ngteni, R.; Al-Gheethi, A.; Ahmad, H.; Omar, F.M.; Naushad, M.; Pandey, S. Environmental Remediation Potential of Ferrous Sulfate Waste as an Eco-Friendly Coagulant for the Removal of NH3-N and COD from the Rubber Processing Effluent. Int. J. Environ. Res. Public Health 2021, 18, 12427. [Google Scholar] [CrossRef]
- He, W.; Luo, J.; Wu, Y.; Luo, T.; Tang, C. Ballasted flocculation pretreatment for mitigating ultrafiltration membrane fouling: Role of differently charged polyacrylamides at typical coagulant dosages. Sep. Purif. Technol. 2024, 328, 125029. [Google Scholar] [CrossRef]
- Du, P.; Li, X.; Yang, Y.; Fan, X.; Fang, X.; Zhou, Z. Enhanced coagulation by two-stage alum addition: The role of solution pH, floc breakage and assistant of non-ionic polyacrylamide. Environ. Technol. 2021, 42, 4456–4465. [Google Scholar] [CrossRef]
- Maddela, N.R.; Cruzatty, L.C.G.; Chakraborty, S. (Eds.) Advances in the Domain of Environmental Biotechnology; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Rathilal, S.; Tetteh, E.K. Coagulation treatment of wastewater: Kinetics and natural coagulant evaluation. Molecules 2021, 26, 698. [Google Scholar] [CrossRef]
- Kurniawan, S.; Abdullah, S.; Imron, M.; Said, N.; Ismail, N.; Hasan, H.; Othman, A.; Purwanti, I. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Fourmentin, M.; Zemmouri, H.; do Carmo Nascimento, I.O.; Queiroz, L.M.; Tadza, M.Y.M.; Picos-Corrales, L.A.; Pei, H.; Wilson, L.D.; et al. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019, 17, 1603–1621. [Google Scholar] [CrossRef]
- Lee, C.S.; Chong, M.F.; Robinson, J.; Binner, E. A Review on Development and Application of Plant-Based Bioflocculants and Grafted Bioflocculants. Ind. Eng. Chem. Res. 2014, 53, 18357–18369. [Google Scholar] [CrossRef]
- Das, N.; Ojha, N.; Mandal, S.K. Wastewater treatment using plant-derived bioflocculants: Green chemistry approach for safe environment. Water Sci. Technol. 2021, 83, 1797–1812. [Google Scholar] [CrossRef]
- Pasciucco, F.; Pasciucco, E.; Castagnoli, A.; Iannelli, R.; Pecorini, I. Comparing the effects of Al-based coagulants in waste activated sludge anaerobic digestion: Methane yield, kinetics and sludge implications. Heliyon 2024, 10, e29282. [Google Scholar] [CrossRef]
- Lee, E.; Min, K.J.; Choi, H.; Park, K.Y. Impact of dewatering inorganic coagulants on anaerobic digestion treating food waste leachate. Bioresour. Technol. 2024, 393, 130136. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Liu, X.; Xu, Q.; Chen, Y.; Yang, Q.; Li, H.; Ni, B. Effect of poly aluminum chloride on dark fermentative hydrogen accumulation from waste activated sludge. Water Res. 2019, 153, 217–228. [Google Scholar] [CrossRef]
- Cainglet, A.; Kujala, K.; Liimatainen, M.; Prokkola, H.; Piippo, S.; Postila, H.; Ronkanen, A.K.; Heiderscheidt, E. The influence of coagulant type on the biological treatment of sewage sludge. Sci. Total Environ. 2023, 869, 161706. [Google Scholar] [CrossRef]
- Muñoz-Alegría, J.A.; Muñoz-España, E.; Flórez-Marulanda, J.F. Dissolved Air Flotation: A Review from the Perspective of System Parameters and Uses in Wastewater Treatment. TecnoLógicas 2021, 24, e2111. [Google Scholar] [CrossRef]
- Shen, W.; Mukherjee, D.; Koirala, N.; Hu, G.; Lee, K.; Zhao, M.; Li, J. Microbubble and nanobubble-based gas flotation for oily wastewater treatment: A review. Environ. Rev. 2022, 30, 359–379. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.; Ibraheem, I. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Abyar, H.; Nowrouzi, M. Trickling filter systems for sustainable water supply: An evaluation of eco-environmental burdens and greenhouse gas emissions. Environ. Res. 2023, 237, 117011. [Google Scholar] [CrossRef]
- Katare, A.K.; Tabassum, A.; Sharma, A.K.; Sharma, S. Treatment of pharmaceutical wastewater through activated sludge process—a critical review. Environ. Monit. Assess. 2023, 195, 1466. [Google Scholar] [CrossRef]
- Dutta, A.; Sarkar, S. Sequencing Batch Reactor for Wastewater Treatment: Recent Advances. Curr. Pollut. Rep. 2015, 1, 177–190. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, A.; Saidulu, D.; Gupta, A.K. Advancements of sequencing batch reactor for industrial wastewater treatment: Major focus on modifications, critical operational parameters, and future perspectives. J. Environ. Manag. 2022, 317, 115305. [Google Scholar] [CrossRef]
- Rasheed, A.A.; Ciroma, S. A Review on Sequencing Batch Reactors: Process Design, Operation and Modelling. Niger. Res. J. Eng. Environ. Sci. 2020, 5, 223–238. [Google Scholar]
- Asante-Sackey, D.; Rathilal, S.; Tetteh, E.K.; Armah, E.K. Membrane Bioreactors for Produced Water Treatment: A Mini-Review. Membranes 2022, 12, 275. [Google Scholar] [CrossRef]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Rotating biological contactors: A review on main factors affecting performance. Rev. Environ. Sci. Biotechnol. 2008, 7, 155–172. [Google Scholar] [CrossRef]
- Waqas, S.; Harun, N.Y.; Sambudi, N.S.; Bilad, M.R.; Abioye, K.J.; Ali, A.; Abdulrahman, A. A Review of Rotating Biological Contactors for Wastewater Treatment. Water 2023, 15, 1913. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Klaysom, C.; Jaafar, J.; Khan, A.L. An integrated rotating biological contactor and membrane separation process for domestic wastewater treatment. Alex. Eng. J. 2020, 59, 4257–4265. [Google Scholar] [CrossRef]
- Mpongwana, N.; Rathilal, S. Exploiting Biofilm Characteristics to Enhance Biological Nutrient Removal in Wastewater Treatment Plants. Appl. Sci. 2022, 12, 7561. [Google Scholar] [CrossRef]
- Khoja, I.; Ladhari, T.; Sakly, A.; M’sahli, F. Parameter Identification of an Activated Sludge Wastewater Treatment Process Based on Particle Swarm Optimization Method. Math. Probl. Eng. 2018, 2018, 7823930. [Google Scholar] [CrossRef]
- Smol, M. Circular Economy in Wastewater Treatment Plant—Water, Energy and Raw Materials Recovery. Energies 2023, 16, 3911. [Google Scholar] [CrossRef]
- Castagnoli, A.; Falcioni, S.; Touloupakis, E.; Pasciucco, F.; Pasciucco, E.; Michelotti, A.; Iannelli, R.; Pecorini, I. Influence of Aeration Rate on Uncoupled Fed Mixed Microbial Cultures for Polyhydroxybutyrate Production. Sustainability 2024, 16, 2961. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef]
- Kacanski, M.; Pucher, L.; Peral, C.; Dietrich, T.; Neureiter, M. Cell Retention as a Viable Strategy for PHA Production from Diluted VFAs with Bacillus megaterium. Bioengineering 2022, 9, 122. [Google Scholar] [CrossRef]
- Lorini, L.; Munarin, G.; Salvatori, G.; Alfano, S.; Pavan, P.; Majone, M.; Valentino, F. Sewage sludge as carbon source for polyhydroxyalkanoates: A holistic approach at pilot scale level. J. Clean. Prod. 2022, 354, 131728. [Google Scholar] [CrossRef]
- Pathak, N.; Tran, V.H.; Merenda, A.; Johir, M.A.J.; Phuntsho, S.; Shon, H. Removal of organic micro-pollutants by conventional membrane bioreactors and high-retention membrane bioreactors. Appl. Sci. 2020, 10, 2969. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, M.; Dong, J.; Sun, C.; Chen, G. Removal of Pharmaceutical and Personal Care Products (PPCPs) from Municipal Waste Water with Integrated Membrane Systems, MBR-RO/NF. Int. J. Environ. Res. Public Health 2018, 15, 269. [Google Scholar] [CrossRef]
- Hoinkis, J.; Deowan, S.A.; Panten, V.; Figoli, A.; Huang, R.R.; Drioli, E. Membrane Bioreactor (MBR) Technology – a Promising Approach for Industrial Water Reuse. Procedia Eng. 2012, 33, 234–241. [Google Scholar] [CrossRef]
- Iliopoulou, A.; Arvaniti, O.S.; Deligiannis, M.; Gatidou, G.; Vyrides, I.; Fountoulakis, M.S.; Stasinakis, A.S. Combined use of strictly anaerobic MBBR and aerobic MBR for municipal wastewater treatment and removal of pharmaceuticals. J. Environ. Manag. 2023, 343, 118211. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, Y.; Mamrol, N.; Ren, L.; Li, X.; Shao, J.; Yang, X.; van der Bruggen, B. Membrane bioreactors for hospital wastewater treatment: Recent advancements in membranes and processes. Front. Chem. Sci. Eng. 2022, 16, 634–660. [Google Scholar] [CrossRef]
- Zhang, M.; Lee, E.; Vonghia, E.; Hong, Y.; Liao, B. Introduction to Aerobic Membrane Bioreactors: Current Status and Recent Developments; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–23. [Google Scholar] [CrossRef]
- Kharraz, J.A.; Khanzada, N.K.; Farid, M.U.; Kim, J.; Jeong, S.; An, A.K. Membrane distillation bioreactor (MDBR) for wastewater treatment, water reuse, and resource recovery: A review. J. Water Process Eng. 2022, 47, 102687. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane bioreactor for wastewater treatment: A review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef]
- Roccaro, P.; Vagliasindi, F.G. Membrane Bioreactors for Wastewater Reclamation: Cost Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 311–322. [Google Scholar] [CrossRef]
- Vinardell, S.; Astals, S.; Peces, M.; Cardete, M.A.; Fernández, I.; Mata-Alvarez, J.; Dosta, J. Advances in anaerobic membrane bioreactor technology for municipal wastewater treatment: A 2020 updated review. Renew. Sustain. Energy Rev. 2020, 130, 109936. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Suleman, H.; Nordin, N.A.H.; Jaafar, J.; Othman, M.H.D.; Elma, M. An energy-efficient membrane rotating biological contactor for wastewater treatment. J. Clean. Prod. 2021, 282, 124544. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Hossain, M.I.; Cheng, L. High-strength wastewater treatment using microbial biofilm reactor: A critical review. World J. Microbiol. Biotechnol. 2020, 36, 75. [Google Scholar] [CrossRef]
- Aslam, Z.; Alam, P.; Islam, R.; Khan, A.H.; Samaraweera, H.; Hussain, A.; Zargar, T.I. Recent developments in moving bed biofilm reactor (MBBR) for the treatment of phenolic wastewater—A review. J. Taiwan Inst. Chem. Eng. 2024. [Google Scholar] [CrossRef]
- Maurya, A.; Kumar, R.; Raj, A. Biofilm-based technology for industrial wastewater treatment: Current technology, applications and future perspectives. World J. Microbiol. Biotechnol. 2023, 39, 112. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Kanafina, D.; Malamis, S.; Katsou, E.; Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E. Anaerobic membrane bioreactors for municipal wastewater treatment: A literature review. Membranes 2021, 11, 967. [Google Scholar] [CrossRef]
- Foroughi, M.; Khiadani, M.; Kakhki, S.; Kholghi, V.; Naderi, K.; Yektay, S. Effect of ozonation-based disinfection methods on the removal of antibiotic resistant bacteria and resistance genes (ARB/ARGs) in water and wastewater treatment: A systematic review. Sci. Total Environ. 2022, 811, 151404. [Google Scholar] [CrossRef]
- Cerreta, G.; Roccamante, M.A.; Plaza-Bolaños, P.; Oller, I.; Aguera, A.; Malato, S.; Rizzo, L. Advanced treatment of urban wastewater by UV-C/free chlorine process: Micro-pollutants removal and effect of UV-C radiation on trihalomethanes formation. Water Res. 2020, 169, 115220. [Google Scholar] [CrossRef]
- Mecha, A.C.; Chollom, M.N. Photocatalytic ozonation of wastewater: A review. Environ. Chem. Lett. 2020, 18, 1491–1507. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced Oxidation Processes for Water and Wastewater Viral Disinfection. A Systematic Review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef]
- Milani, S.J.; Bidhendi, G.N. A Review on the Potential of Common Disinfection Processes for the Removal of Virus from Wastewater. Int. J. Environ. Res. 2022, 16, 6. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Chen, S.; Zhou, W.; Chen, J. Problems of conventional disinfection and new sterilization methods for antibiotic resistance control. Chemosphere 2020, 254, 126831. [Google Scholar] [CrossRef]
- Gelete, G.; Gokcekus, H.; Ozsahin, D.U.; Uzun, B.; Gichamo, T. Evaluating disinfection techniques of water treatment. Desalin. Water Treat. 2020, 177, 408–415. [Google Scholar] [CrossRef]
- Amin, M.M.; Hashemi, H.; Boyini, A.M. A review on wastewater disinfection. Int. J. Environ. Health Eng. 2013, 2, 22. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Xiang, J.L.; Wang, J.J.; Du, H.S.; Wang, T.T.; Huo, Z.Y.; Wang, W.L.; Liu, M.; Du, Y. Ultraviolet-based synergistic processes for wastewater disinfection: A review. J. Hazard. Mater. 2023, 453, 131393. [Google Scholar] [CrossRef]
- Umar, M. From Conventional Disinfection to Antibiotic Resistance Control—Status of the Use of Chlorine and UV Irradiation during Wastewater Treatment. Int. J. Environ. Res. Public Health 2022, 19, 1636. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. Critical perspective on advanced treatment processes for water and wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 2020, 10, 4549. [Google Scholar] [CrossRef]
- Rostam, A.B.; Taghizadeh, M. Advanced oxidation processes integrated by membrane reactors and bioreactors for various wastewater treatments: A critical review. J. Environ. Chem. Eng. 2020, 8, 104566. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; Gaayda, J.E.; Akbour, R.A.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process.-Process Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q.; Jin, Z.; Xiao, J.; et al. Recent advances in waste water treatment through transition metal sulfides-based advanced oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef]
- Radwan, E.K.; Ghafar, H.H.A.; Ibrahim, M.B.; Moursy, A.S. Recent trends in treatment technologies of emerging contaminants. Environ. Qual. Manag. 2023, 32, 7–25. [Google Scholar] [CrossRef]
- Bampos, G.; Petala, A.; Frontistis, Z. Recent Trends in Pharmaceuticals Removal from Water Using Electrochemical Oxidation Processes. Environments 2021, 8, 85. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, H.; Wei, K.; Liu, L.; Sun, M.; Zhou, M. Comprehensive analysis of research trends and prospects in electrochemical advanced oxidation processes (EAOPs) for wastewater treatment. Chemosphere 2023, 341, 140083. [Google Scholar] [CrossRef] [PubMed]
- García-Espinoza, J.D.; Robles, I.; Durán-Moreno, A.; Godínez, L.A. Photo-assisted electrochemical advanced oxidation processes for the disinfection of aqueous solutions: A review. Chemosphere 2021, 274, 129957. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Albolafio, S.; Marín, A.; Allende, A.; García, F.; Simón-Andreu, P.J.; Soler, M.A.; Gil, M.I. Strategies for mitigating chlorinated disinfection byproducts in wastewater treatment plants. Chemosphere 2022, 288, 132583. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Freitas, B.; de Souza Leite, L.; Daniel, L.A. Chlorine and peracetic acid in decentralized wastewater treatment: Disinfection, oxidation and odor control. Process Saf. Environ. Prot. 2021, 146, 620–628. [Google Scholar] [CrossRef]
- Xu, P.; Janex, M.L.; Savoye, P.; Cockx, A.; Lazarova, V. Wastewater disinfection by ozone: Main parameters for process design. Water Res. 2002, 36, 1043–1055. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, Z.; Liu, H.; Lu, Y.; Li, K.; Shi, Y.; Mao, Y.; Hu, H.Y. Efficient synergistic disinfection by ozone, ultraviolet irradiation and chlorine in secondary effluents. Sci. Total Environ. 2021, 758, 143641. [Google Scholar] [CrossRef]
- Kong, J.; Lu, Y.; Ren, Y.; Chen, Z.; Chen, M. The virus removal in UV irradiation, ozonation and chlorination. Water Cycle 2021, 2, 23–31. [Google Scholar] [CrossRef]
- Hussain, K.; Khan, N.A.; Vambol, V.; Vambol, S.; Yeremenko, S.; Sydorenko, V. Advancement in Ozone base wastewater treatment technologies: Brief review. Ecol. Quest. 2022, 33, 7–19. [Google Scholar] [CrossRef]
- Veremeychik, A.; Dmukhaylo, E.; Onysko, S.; Sazonov, M.; Khvisevich, V. High-performance electric arc source of ultraviolet radiation to ozone generate. E3S Web Conf. 2020, 212, 01018. [Google Scholar] [CrossRef]
- Meher, P.; Deshmukh, N.; Mashalkar, A.; Kumar, D. Ozone (O3) generation and its applications: A review. AIP Conf. Proc. 2023, 2764, 070011. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; et al. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Qasim, M.; Rafique, M.S.; Naz, R. Water purification by ozone generator employing non-thermal plasma. Mater. Chem. Phys. 2022, 291, 126442. [Google Scholar] [CrossRef]
- Alsheyab, M.A.; Muñoz, A.H. Optimisation of ozone production for water and wastewater treatment. Desalination 2007, 217, 1–7. [Google Scholar] [CrossRef]
- Prombud, T.; Wisassakwichai, C.; Anusurain, E.; Methavithit, W. Ozone Treatment Strategies for Efficient Color Removal in Wastewater. In Proceedings of the 2024 12th International Electrical Engineering Congress (iEECON), Pattaya, Thailand, 6–8 March 2024; IEEE: New York, NY, USA, 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Hafeez, A.; Shezad, N.; Javed, F.; Fazal, T.; ur Rehman, M.S.; Rehman, F. Developing multiplexed plasma micro-reactor for ozone intensification and wastewater treatment. Chem. Eng. Process.-Process Intensif. 2021, 162, 108337. [Google Scholar] [CrossRef]
- González, Y.; Gómez, G.; Moeller-Chávez, G.E.; Vidal, G. UV Disinfection Systems for Wastewater Treatment: Emphasis on Reactivation of Microorganisms. Sustainability 2023, 15, 11262. [Google Scholar] [CrossRef]
- Li, B.; Cheng, X.; Zou, R.; Su, Y.; Zhang, Y. Dynamic coordination of two-phase reactions in heterogeneous Fenton for selective removal of water pollutants. J. Hazard. Mater. 2023, 454, 131554. [Google Scholar] [CrossRef]
- Dat, N.D.; Huynh, Q.S.; Tran, K.A.T.; Nguyen, M.L. Performance of heterogeneous Fenton catalyst from solid wastes for removal of emerging contaminant in water: A potential approach to circular economy. Results Eng. 2023, 18, 101086. [Google Scholar] [CrossRef]
- Yi, C.; Lu, Q.; Wang, Y.; Wang, Y.; Yang, B. Degradation of organic wastewater by hydrodynamic cavitation combined with acoustic cavitation. Ultrason. Sonochemistry 2018, 43, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ameta, S.; Ameta, R. Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Elsevier Science: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hassani, A.; Malhotra, M.; Karim, A.V.; Krishnan, S.; Nidheesh, P.V. Recent progress on ultrasound-assisted electrochemical processes: A review on mechanism, reactor strategies, and applications for wastewater treatment. Environ. Res. 2022, 205, 112463. [Google Scholar] [CrossRef] [PubMed]
- Mason, T. Advances in Sonochemistry; Elsevier Science: Amsterdam, The Netherlands, 1999; Volume 5. [Google Scholar]
- Ameta, S.; Ameta, R.; Ameta, G. Sonochemistry: An Emerging Green Technology; Apple Academic Press: Oakville, ON, Canada, 2018. [Google Scholar]
- Nie, E.; Yang, M.; Wang, D.; Yang, X.; Luo, X.; Zheng, Z. Degradation of diclofenac by ultrasonic irradiation: Kinetic studies and degradation pathways. Chemosphere 2014, 113, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Camargo-Perea, A.L.; Rubio-Clemente, A.; Peñuela, G.A. Use of Ultrasound as an Advanced Oxidation Process for the Degradation of Emerging Pollutants in Water. Water 2020, 12, 1068. [Google Scholar] [CrossRef]
- Mason, T.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications; Woodhead: Philadelphia, PA, USA, 2002. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Martínez-Pachón, D.; Moncayo-Lasso, A.; Ibáñez, M.; Hernández, F.; Torres-Palma, R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Recent Developments in Sonochemical Synthesis of Nanoporous Materials. Molecules 2023, 28, 2639. [Google Scholar] [CrossRef]
- Peshkovsky, A.; Peshkovsky, S. Acoustic Cavitation Theory and Equipment Design Principles for Industrial Applications of High-intensity Ultrasound; Physics Research and Technology; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Matei, N.; Scarpete, D. The Use of Ultrasound in the Treatment Process of Wastewater: A Review. Ann. “Dunarea Jos” Univ. Galati Fascicle IX Metall. Mater. Sci. 2015, 38, 45–50. [Google Scholar]
- Sikalidis, C. Advances in Ceramics—Electric and Magnetic Ceramics, Bioceramics, Ceramics and Environment; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Vijaya, M. Piezoelectric Materials and Devices: Applications in Engineering and Medical Sciences; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Fernandes, J.; Ramísio, P.J.; Puga, H. Unveiling Acoustic Cavitation Characterization in Opaque Chambers through a Low-Cost Piezoelectric Sensor Approach. Electronics 2024, 13, 1581. [Google Scholar] [CrossRef]
- Yuan, Q.; Qu, S.; Li, R.; Huo, Z.Y.; Gao, Y.; Luo, Y. Degradation of antibiotics by electrochemical advanced oxidation processes (EAOPs): Performance, mechanisms, and perspectives. Sci. Total Environ. 2023, 856, 159092. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Xiong, Z.; Yao, G.; Lai, B. The electrochemical advanced oxidation processes coupling of oxidants for organic pollutants degradation: A mini-review. Chin. Chem. Lett. 2019, 30, 2139–2146. [Google Scholar] [CrossRef]
- Gisi, S.D.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.; Al-Balakocy, N.; Ola, S.A.E.; Bilyakova, M. Development of Pilot Scale System for Production of Nylon-6 Fibers Grafted with Polydimethylaminoethylmathacrylate (PDMAEMA) for the Application as Ion Exchange. Egypt. J. Chem. 2019, 63, 27–36. [Google Scholar] [CrossRef]
- Ramísio, P.; Vieira, J. Heavy metal removal efficiency in a kaolinite–sand media filtration pilot-scale installation. In Highway and Urban Environment. Alliance For Global Sustainability Bookseries; Springer: Dordrecht, The Netherland, 2007; pp. 319–329. [Google Scholar]
- Hamad, H.; AbdElhafez, S.; Elsenety, M.; Sorour, M.K.; Amin, N.; Abdelwahab, O.; El-Ashtoukhy, E.S. Fabrication and characterization of functionalized lignin-based adsorbent prepared from black liquor in the paper industry for superior removal of toxic dye. Fuel 2022, 323, 124288. [Google Scholar] [CrossRef]
- Ali, R.; Elsagan, Z.; AbdElhafez, S. Lignin from Agro-Industrial Waste to an Efficient Magnetic Adsorbent for Hazardous Crystal Violet Removal. Molecules 2022, 27, 1831. [Google Scholar] [CrossRef] [PubMed]
- Elhafez, S.A.; Taha, N.; El-Maghraby, A. Adsorption studies of cationic dye on raw and modified sugarcane bagasse from aqueous solutions: Kinetic and Isotherm aspects. Egypt. J. Chem. 2021, 64, 1593–1600. [Google Scholar] [CrossRef]
- Corpus, R.M.B.; Bayani, M.S.; Aguilar, J.L.; Aguilar, J.C.B.; Aguilar, H.B. Emerging pollutants in waste water: Challenges and advancements in treatment technology. IOP Conf. Ser. Earth Environ. Sci. 2024, 1372, 012037. [Google Scholar] [CrossRef]
- Yacouba, Z.A.; Mendret, J.; Lesage, G.; Zaviska, F.; Brosillon, S. Removal of organic micropollutants from domestic wastewater: The effect of ozone-based advanced oxidation process on nanofiltration. J. Water Process Eng. 2021, 39, 101869. [Google Scholar] [CrossRef]
- Li, M.; Wen, Q.; Chen, Z.; Tang, Y.; Yang, B. Comparison of ozonation and UV based oxidation as pre-treatment process for ultrafiltration in wastewater reuse: Simultaneous water risks reduction and membrane fouling mitigation. Chemosphere 2020, 244, 125449. [Google Scholar] [CrossRef]
- Wang, A.; Xu, H.; Chen, C.; Chen, L.; Lin, T.; Ma, J.; Ding, M. Critical review on advances and perspectives of ultrasound assisted membrane technologies for water purification. Chem. Eng. J. 2024, 482, 148873. [Google Scholar] [CrossRef]
- Bognár, S.; Jovanović, D.; Despotović, V.; Finčur, N.; Putnik, P.; Šojić Merkulov, D. Advancing Wastewater Treatment: A Comparative Study of Photocatalysis, Sonophotolysis, and Sonophotocatalysis for Organics Removal. Processes 2024, 12, 1256. [Google Scholar] [CrossRef]
- Poblete, R.; Cortes, E.; Pérez, N.; Rodríguez, C.; Luna-Galiano, Y. Treatment of landfill leachate by combined use of ultrasound and photocatalytic process using fly ash as catalyst. J. Environ. Manag. 2024, 349, 119552. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, J.; Liu, X. Study on the ozonation degradation of methylene blue enhanced by microchannel and ultrasound. Water Sci. Technol. 2023, 87, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Gole, V.L.; Sharma, J.; Yadav, R.K. Treatment and Disinfection of Municipal Wastewater Using Synergy of Ultrasound, LEDs-UVC, and Oxidants. Ind. Eng. Chem. Res. 2024, 63, 11090–11098. [Google Scholar] [CrossRef]
- Liu, Z.; Demeestere, K.; Hulle, S.V. Comparison and performance assessment of ozone-based AOPs in view of trace organic contaminants abatement in water and wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105599. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Karimian, M.; Chen, Z.; Ni, B.J. Combination of coagulation and adsorption technologies for advanced wastewater treatment for potable water reuse: By ANN, NSGA-II, and RSM. J. Environ. Manag. 2024, 349, 119429. [Google Scholar] [CrossRef]
- Agustina, T.E.; Arita, S.; Melwita, E.; Bahrin, D.; Marcelia, R.; Irdiansyah, H.; Ramadhini, T.K. Laboratory Wastewater Treatment by Using Combination Methods of AOPs and Chemical-Physical Pretreatments. Ecol. Eng. Environ. Technol. 2024, 25, 216–226. [Google Scholar] [CrossRef]
- Serna-Carrizales, J.C.; Zárate Guzmán, A.I.; Forgionny, A.; Acelas, N.; Pérez, S.; Muñoz-Saldaña, J.; Ocampo-Perez, R. Production of activated carbon from agave residues and its synergistic application in a hybrid adsorption-AOPs system for effective removal of sulfamethazine from aqueous solutions. Environ. Res. 2024, 250, 118559. [Google Scholar] [CrossRef]
- Chakinala, N.; Gogate, P.R. Ultrasound assisted removal of methylene blue using functionalized mesoporous biochar composites. Chem. Eng. Process.-Process Intensif. 2024, 196, 109684. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, W.; Zhang, J.; Liang, J.; Xie, L.; Guo, J.; Zhong, J.; Li, Z.; Yang, K.; Zhang, C.; et al. The performance and applicability study of a photovoltaic-driven electro-Fenton wastewater treatment system. Energy Convers. Manag. 2024, 314, 118617. [Google Scholar] [CrossRef]
- Feng, J.; Luo, Y.; Chen, Y.; Liu, Z.; Shi, J.; Shao, Q.; Xie, P.; Ma, J. Combination with ultrasound and Fe0 in sulfite activation to degrade Tribromophenol: Synergistic performance and mechanism. Chem. Eng. J. 2024, 494, 152974. [Google Scholar] [CrossRef]
- Barzegarzadeh, M.; Amini-Fazl, M.S.; Sohrabi, N. Efficient Ultrasound-Assisted Rifampicin Removal Using Cu(BDC)@Wool Biocomposite in Batch Adsorption Column and Fixed Bed. J. Inorg. Organomet. Polym. Mater. 2024, 34, 207–220. [Google Scholar] [CrossRef]
- Sgroi, M.; Anumol, T.; Vagliasindi, F.G.; Snyder, S.A.; Roccaro, P. Comparison of the new Cl2/O3/UV process with different ozone- and UV-based AOPs for wastewater treatment at pilot scale: Removal of pharmaceuticals and changes in fluorescing organic matter. Sci. Total Environ. 2021, 765, 142720. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Huang, J.; Zhang, L.; Zhang, T.; Yu, X.; Liu, W.; Huang, C. Treatment of printing and dyeing wastewater using Fenton combined with ceramic microfiltration membrane bioreactor. Biochem. Eng. J. 2024, 201, 109143. [Google Scholar] [CrossRef]
- Singh, K.; Prasad, B.; Kumar, A.; Kumari, M.; Dubey, D.; Sillanpää, M.; Prasad, K.S. Hyphenated Fenton-column packed nMnO-modified wood biochar for tannery effluent treatment: Adsorption mechanism and reusability study. Environ. Res. 2024, 252, 118786. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Dong, Y.; Jiang, H.; Loh, W.H.; Imbrogno, J.; Swenson, T.M.; Garcia-Rodriguez, O.; Lefebvre, O. A new approach of simultaneous adsorption and regeneration of activated carbon to address the bottlenecks of pharmaceutical wastewater treatment. Water Res. 2024, 252, 121180. [Google Scholar] [CrossRef]
- Mohmmad, A.; Mosavian, M.T.H.; Khodaparast, M.H.H. Electro-Fenton technology for dairy wastewater treatment. Int. J. Environ. Sci. Technol. 2024, 21, 35–42. [Google Scholar] [CrossRef]
- Kösem, E.; Nur Ersöz, Ö.; Yılmaz, R.N.; Babaoğlu, D.; Yazici Guvenc, S.; Can-Güven, E.; Varank, G. Sequential electrocoagulation and Fenton/Photo-Fenton processes for the treatment of membrane bioreactor effluent of landfill leachate. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 689, 133740. [Google Scholar] [CrossRef]
- Ghjeer, A.Y.; Abbar, A.H. A comparative study of four technologies (Fenton, Sono-Fenton (SF), Electro-Fenton (EF), and Sono-Electro-Fenton (SEF)) for hospital wastewater treatment. Case Stud. Chem. Environ. Eng. 2023, 8, 100519. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and advanced membrane technology for wastewater treatment: A review. J. Basic Microbiol. 2022, 62, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Gorjizadeh, S.; Rahbari-Sisakht, M.; Emadzadeh, D. Surface modification of membrane bioreactor by hybrid halloysite nanotubes for industrial wastewater treatment containing heavy metals. Iran. Polym. J. 2024, 33, 1001–1016. [Google Scholar] [CrossRef]
- Gul, B.Y.; Teber, O.O.; Tuncay, G.; Pekgenc, E.; Arabi, N.; Hemmati-Eslamlu, P.; Habibi-Yangjeh, A.; Vatanpour, V.; Koyuncu, I. Modification of PAN electrospun nanofiber membranes with g-C3N4 nanotubes/carbon dots to enhance MBR performance. Chemosphere 2024, 349, 140866. [Google Scholar] [CrossRef]
- Lee, B.; Kim, C. Innovative membrane technology for water treatment solutions: Current status and future prospects of carbon nanotube membranes. Environ. Eng. Res. 2024, 29, 240104. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Bie, Y. Study on Adsorption Properties of Activated Carbon for Advanced Treatment of Chemical Wastewater. Highlights Sci. Eng. Technol. 2024, 83, 627–632. [Google Scholar] [CrossRef]

| Process | Advantages | Disadvantages |
|---|---|---|
| Coagulation/Flocculation [22,23,32,33,34] | Process simplicity | Requires non-reusable materials |
| Integrated physicochemical process | Physicochemical monitoring required | |
| Wide range of chemicals available | Increased sludge volume generation | |
| Inexpensive and easily accessible materials | Low removal of arsenic, dissolved impurities, ions, and pathogens | |
| Good sludge settling | Further processing is required | |
| Flotation [23,35] | Integrated physicochemical process | High initial capital costs |
| Non-ionic or ionic collectors | High energy, maintenance, and operation costs | |
| Efficient removal of small and low-density particles | pH dependent | |
| Low retention time | Cannot remove colloidal or dissolved solids and nutrients |
| Process | Advantages | Disadvantages |
|---|---|---|
| Activated Sludge [22,58] | Lower installation cost | Long hydraulic retention time |
| High removal of organics and pathogens | Higher operation cost | |
| Low area needed | Large amount of sludge | |
| Applicable to large- and small-scale WWTPs | Shock loads impact stability | |
| Sequencing Batch Reactor [35,59,60,61] | Can be fully automated | Sludge bulking issues |
| Short aeration time | Time required for sludge settling | |
| Low area requirements and manpower | High CAPEX and OPEX | |
| Low energy consumption | ||
| Membrane Bioreactor [22,35,58,62,63,64] | Lower footprint | High operational cost |
| Effective for pathogen, solids, and biological waste | Membrane fouling | |
| Higher efficiency than AS | Short operational life of the membrane | |
| No chemical usage | Requires skilled manpower | |
| Direct recycling of effluent | High energy consumption | |
| Biofilm-Based [1,65,66,67,68] | High treatment efficiency | Low adaptability under varying conditions |
| No sludge recirculation | Low efficiency when clogged or fouling occurs | |
| Low energy consumption | Regular maintenance required | |
| Cost-effective | Limited full-scale implementation | |
| Effective for high-strength wastewater under extreme conditions |
| Process | Advantages | Disadvantages |
|---|---|---|
| Ozonation [58,94,95,96,97] | High efficiency for a variety of pollutants | Low effectiveness for heavy metals |
| No sludge production | Highly toxic gas | |
| Possible to combine with various catalysts | Generation of toxic by-products | |
| Strong oxidation ability | High capital and operating costs | |
| Contribution of oxygen to water after disinfection | Short half-life | |
| UV [22,58,94,95,96,98,99,100] | No formation of disinfection by-products | Efficiency dependent on suspended particles |
| Short retention time | Non-effective for antibiotic-resistant bacteria | |
| Effective on a wide range of resilient viruses | Cannot remove soluble impurities | |
| Economical | Photoreactivation post-UV exposure | |
| Produces hydroxyl radicals | ||
| No chemical usage and compact | ||
| Ultrasound [34,101,102,103] | Compact | Requires high energy |
| Environmentally friendly | Need for supplemental oxidants | |
| Produces hydroxyl radicals | Not commercially applicable yet | |
| No chemical usage | ||
| Fenton [94,102,104,105,106,107] | Not expensive | pH dependent |
| Efficient for organic pollutants | High amount of Fenton agents required | |
| Total mineralization | Difficulties in transporting chemicals like | |
| Simple implementation | High amounts of iron sludge | |
| Environmentally friendly | ||
| Shortest reaction time among AOPs | ||
| Electro-Chemical [108,109,110,111,112,113] | Degrades a wide range of contaminants | Initial cost of electrode materials |
| Simple setup and operation procedures | Formation of secondary pollutants | |
| Easily combined with other AOPs | Expensive and inefficient electrodes | |
| Production of toxic intermediate products | ||
| Adsorbents [108,109,110,111,112,113,114] | Cost-effectiveness | Adsorption capacity tends to decrease |
| Versatility | Non-specific binding | |
| Environmental friendliness | Difficult to recover and reuse some adsorbents | |
| High efficiency in removing heavy metals | Use, disposal, and management of adsorbents | |
| Sustainability and long-term cost-effectiveness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, J.; Ramísio, P.J.; Puga, H. A Comprehensive Review on Various Phases of Wastewater Technologies: Trends and Future Perspectives. Eng 2024, 5, 2633-2661. https://doi.org/10.3390/eng5040138
Fernandes J, Ramísio PJ, Puga H. A Comprehensive Review on Various Phases of Wastewater Technologies: Trends and Future Perspectives. Eng. 2024; 5(4):2633-2661. https://doi.org/10.3390/eng5040138
Chicago/Turabian StyleFernandes, José, Paulo J. Ramísio, and Hélder Puga. 2024. "A Comprehensive Review on Various Phases of Wastewater Technologies: Trends and Future Perspectives" Eng 5, no. 4: 2633-2661. https://doi.org/10.3390/eng5040138
APA StyleFernandes, J., Ramísio, P. J., & Puga, H. (2024). A Comprehensive Review on Various Phases of Wastewater Technologies: Trends and Future Perspectives. Eng, 5(4), 2633-2661. https://doi.org/10.3390/eng5040138









