Abstract
Due to the importance of optimizing the manufacture of ceramic pigments, motivated by the increase in prices of both raw materials and energy, and the need to control manufacturing parameters to obtain optimal conditions for the preparation of ceramic inks, two synthesis routes (traditional route and coprecipitation) and two calcination methods (traditional oven and microwave oven) are proposed to obtain the blue ceramic pigment CoAl2O4 with the aim of minimizing the use of mineralizers or flux agents and reducing energy consumption in its manufacturing. The pigments prepared were characterized by thermal analysis and structurally by XRD and SEM, with particle sizes below 300 nm observed. Finally, the colorimetric coordinates of glazed tiles with the pigments obtained were characterized. In all cases, the microwave-assisted synthesis increased the color intensity, considerably decreasing the temperature and calcination time, obtaining a particle size under 300 nm with a very narrow size distribution, and substantially improving the energy cost of its preparation and the color development of the final product. The viability of the combination of synthesis by coprecipitation and microwave calcination as a method of industrial preparation of ceramic pigments has been demonstrated.
1. Introduction
The progress in advancing ceramic pigments has been notably significant in recent years, thanks mainly to the development of digital decoration technology using printing systems. To develop the ceramic inks necessary for use in this digital technology, the need to obtain increasingly smaller particle sizes, less than 1 µm, and the development of higher color intensities has encouraged research into new ways to obtain pigments with these characteristics and with high coloring power and to reduce the high energy costs derived from current grinding processes using attrition mills for long periods of time [1].
The correct development of the properties of the pigments used in this decoration technology is closely linked to their preparation and synthesis conditions. That is the reason why the correct control and optimization of these stages can reduce the manufacturing cost without a loss of color properties, or even with their improvement [2]. Several alternative preparation methodologies have been proposed for the synthesis routes of ceramic pigments [3,4,5,6,7,8], but one of the most useful and easy to move to industrial processes, thanks to its simplicity, is coprecipitation [9,10,11].
Ceramic pigments are based on mixed-metal oxides with a crystalline structure composed, at minimum, by a group of two metals combined with oxygen. At least one of these metals must belong to the transition series and must have free electrons to produce d-d or f-f type transitions, which are responsible for the color along with other mechanisms such as band transfer. We can find several crystalline structures in these mixed-metal oxides. Some examples that can be found are zircon, pyrochlore, garnet, rutile and spinel. These ceramic pigments are formed after mixing, homogenizing and firing the stoichiometric ratio-dosed raw materials. Temperatures needed are very high (ranging from 1000 °C to 1350 °C) and must be accompanied by long reaction periods to promote ion interfusion phenomena and increase the reactivity of the different raw materials used, since they are in a solid state.
Depending on the type of crystalline structure formed, they can be classified into four types or families: structural (chromophore is integrated into the crystalline structure as its main component), solid solution (chromophore ion is hosted into the network, replacing some of the cations that form the crystalline network), encapsulated or occlusion pigments (crystals of the pigment are encapsulated within the host network) and mordant pigments (chromophores are incorporated superficially on a basis crystalline structure) [12].
Spinel-type pigments (Figure 1) are the most used in the ceramic industry. Many of the transition metals used for ceramic colors can be found in its structure, because it can contain divalent and trivalent cations in its structure, providing a wide variety of compositions with which the color can be adapted to production needs [13].
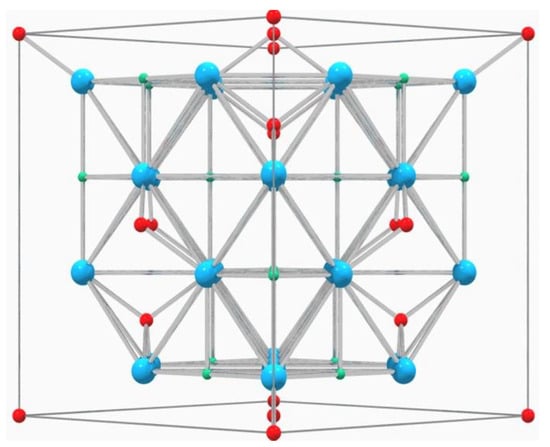
Figure 1.
Structure of spinel (from 011 plane) where blue atoms correspond to oxygen, red atoms to divalent cations, occupying tetrahedral positions, and green atoms to trivalent cations, occupying octahedral positions [13].
The traditional route is the most used for the synthesis of ceramic pigments. It consists of the mixture of solid precursors and subsequent firing at high temperatures, but it has the drawback of the high temperatures and long firing times required, due to the slow diffusion processes of atoms in the crystalline network that occur in these solid-state reactions [12]. A traditional way to reduce this great energy consumption is the use of flux agents, also named mineralizers. These types of compounds reduce the energy required to start the solid-state diffusion reaction thanks to the formation of a melted phase at lower temperatures than are otherwise required to start that reaction [14,15].
On the other hand, synthesis by coprecipitation consists of the joint precipitation of the cations corresponding to the crystalline structure to be prepared [9]. Most of the cations of transition metals, alkaline earth metals and the rest of those used in the preparation of ceramic pigments, when found in aqueous solution, can form the corresponding hydroxides with the addition of a basifying agent. Thus, by adding the stoichiometric mixed-metals solution to a basic solution at the appropriate pH, a mixed hydroxide is formed that contains all the metals in the stoichiometric proportions in which they have been mixed. The particle size of the precipitated material is very small, and the homogeneity of all the components is very high, so the energy conditions for the thermal treatment decrease substantially compared to those required in the traditional synthesis method without this compositional homogeneity.
One of the most important ceramic pigments in the industry is cobalt blue, with spinel structure CoAl2O4, due to its color and thermal stability when it is used as a coloring agent in glaze and bulk tile compositions [12]. Due to its importance, several synthesis methods have been studied to obtain it to improve some of the final characteristics, such as hydrothermal [16,17], sol-gel [18,19,20], low-temperature combustion [21,22] and spray pyrolysis [23].
Recent studies in material preparation have used microwave technology in order to reduce temperature synthesis requirements and improve environmental friendliness due to shorter time reactions and the energy consumption saved. Several authors have reported ceramic pigment preparation research using this microwave firing technology [24,25] with very good results, reducing the energy in the synthesis process. The use of microwave technology in the production of ceramic pigments is drawing interest because of its precise, rapid and effective volume heating, offering advantages over traditional firing methods. Some of these advantages include accelerated synthesis rates, uniform heating and a lower temperature needed for the synthesis process [26].
For the present study, we focus on the synthesis of structural pigments of cobalt spinel (CoAl2O4) both by the traditional method and by coprecipitation [27], as well as on different technologies for the firing of said pigments (traditional and microwave kilns) [28]. The main reason for choosing these techniques is because they are susceptible to being applied in industrial production. Other synthesis methods described before are very expensive and difficult to scale in industrial processes, whereas coprecipitation is very easy to carry out. On the other hand, the microwave firing process is used in industrial processes without any problem, and it is an energy-saving solution.
The main objective that we propose in this work is the comparison of the results of the analysis of the pigments obtained by both synthesis routes, and the viability of using microwave firing to obtain these pigments will be verified. Also, we want to demonstrate that the results obtained could be applied in the industrial preparation of ceramic pigments, being much more sustainable and economical than the traditional methods currently used, reaching reductions in energy consumption of close to 95%. This would allow for a process with a lower carbon footprint and would be an environmentally friendly process.
2. Materials and Methods
CoAl2O4 pigments featuring a spinel structure were synthesized with the aim of checking the synthesis parameters of both conventional and coprecipitation methods; also, the results obtained by traditional and microwave firing were compared.
2.1. Synthesis by Traditional Route
The reagents used in the traditional route were cobalt oxide (Co3O4, 99% purity, PANREAC, Darmstadt, Germany) and aluminum hydroxide (Al(OH)3, 99% purity, MERCK, Darmstadt, Germany). The pigments were prepared through solid-solid reactions employing the conventional approach. The raw ingredients were milled into a stoichiometric combination, and this reagent mixture underwent homogenization within a planetary mill using acetone dispersing medium, followed by drying of the resultant material.
2.2. Synthesis by Coprecipitation Route
The reagents used in coprecipitation synthesis were cobalt chloride (CoCl3·6H2O, 99.5% purity, SIGMA-ALDRICH, Darmstadt, Germany), and aluminum chloride (AlCl3·6H2O, 99.5% purity, SIGMA-ALDRICH, Darmstadt, Germany), used to prepare a solution of 0.5 M of each one. These solutions were mixed in a stoichiometric ratio to obtain the final product.
For the coprecipitation process, the Al/Co solution was introduced dropwise into a basic NaOH solution (99.5% purity, SIGMA-ALDRICH, Darmstadt, Germany) with a pH of 10 to prevent fractional precipitation. This led to the formation of a mixed Co-Al(OH)3 hydroxide precipitate from the precursor compounds present in the solution. To maintain a consistent pH of 10, a saturated NaOH solution was continuously added while stirring until a colloidal gel was formed. The gel was then filtered, washed until all chloride residues were completely removed, and subsequently dried. The dried material was further refined using a planetary ball mill with acetone as the dispersing medium in preparation for subsequent heat treatment.
2.3. Traditional Heat Treatment
The powders produced through both synthesis methods were carefully measured and placed into mullite crucibles. They were then subjected to firing cycles in a Nanetti Mod. FCN furnace, with temperatures ranging from 1000 °C to 1200 °C, using a heating rate of 8 °C per minute and a 20 min dwelling period at the maximum temperature followed by slow cooling for 18 h. Following the firing process, the resulting material was further processed and purified in an agate mortar to break up possible agglomerates formed.
2.4. Microwave-Assisted Heat Treatment
Microwave-assisted thermal treatment was conducted employing a household microwave oven rated at 800 W to heat an Al2O3 container internally coated with SiC (refer to Figure 2) [10]. Various samples were positioned within this container, and microwave radiation at 800 W was employed for durations of 20 and 40 min, resulting in peak temperatures of 900 °C and 970 °C, respectively. Temperature measurements were determined using FERRO’s process temperature control rings (PTCR) of the ETH type, designed for a temperature range of 850 °C to 1100 °C.

Figure 2.
Al2O3 container for the microwave kiln coated with SiC.
2.5. Characterization of Samples
To assess the color development of all prepared pigments, a 2%-by-weight inclusion of these pigments was incorporated into an alkaline transparent industrial glaze (provided by ESMALDUR, Sant Joan de Moró, Spain, Ref. CP-900). This glaze mixture was then applied onto a single-fired ceramic test piece, featuring a glaze thickness of 0.6 mm. Subsequently, the glazed tiles underwent an industrial firing cycle, reaching 1100 °C with a 5 min hold at the maximum temperature and a total firing time of 50 min (from cold to cold).
The chromatic characteristics of the pigments generated in this investigation and the glazed surfaces were determined using an ultraviolet-visible spectrophotometer that adheres to the CIE L*a*b* color system (KONICA MINOLTA (Tokyo, Japan), CM-3600A, utilizing SpectraMagicNX software, d65 illumination, and a 2° observer angle).
The crystalline phases present in the specimens were identified via X-ray diffraction (BRUKER AXS (Billerica, MA, USA), EndeavorD4) with a scanning range of 10–75° 2θ and an acquisition time of 2 s per 0.05° 2θ using a Cu anode emitting in Kα radiation with a wavelength of 1.54056 Å.
Thermal analysis was carried out using TG-DTA (Thermogravimetric Analysis–Differential Thermal Analysis) equipment (BÄHR STA503) featuring a Pt crucible, and the samples were heated at a rate of 5 °C per minute in an air atmosphere.
For morphological examination and size distribution analysis, scanning electron microscopy (SEM) was employed (JEOL7001) with an FED warm cathode, operating at 15 kV, and the samples were coated with a thin layer of carbon prior to imaging.
2.6. Sample References
Table 1 shows the different sample references used in the present work, relating the synthesis and firing method with the temperature reached during each firing method.

Table 1.
Samples references.
3. Results
3.1. Differential Thermal Analysis and Thermogravimetric Sample Characterization
Thermal analysis shows different reactions through the firing process. Figure 3 shows DTA (red line) and TG (black line) analysis of the sample prepared by the traditional route and Figure 4 shows the one corresponding to the sample prepared by coprecipitation.
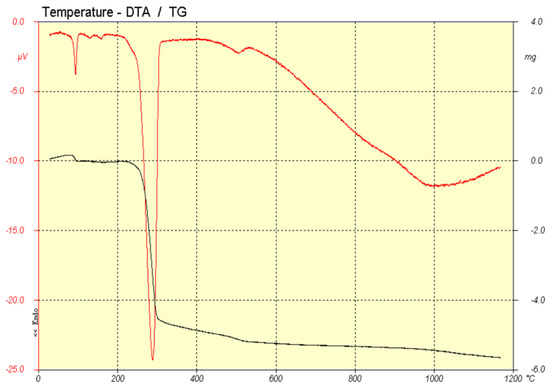
Figure 3.
DTA (red) and TG (black) analysis of pigment prepared by traditional method.
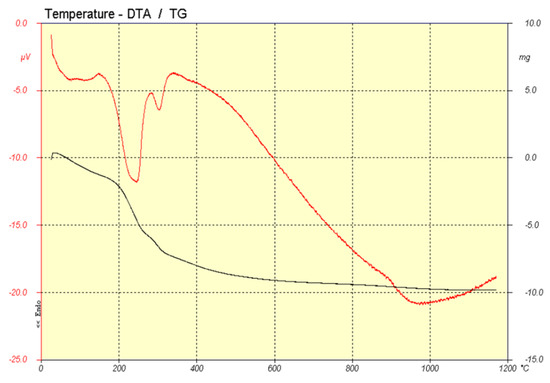
Figure 4.
DTA (red) and TG (black) analysis of pigment prepared by coprecipitation.
In the sample prepared by the traditional method (Figure 3), a small weight loss with an endothermic signal was observed at 100 °C, corresponding to the loss of residual moisture in the sample. And especially at 300 °C, a significant weight loss was observed, accompanied by an endothermic signal, corresponding to the dehydroxylation of Al(OH)3.
In the case of the coprecipitation method, several endothermic signals could be observed between 200 °C and 400 °C that are due to the dehydroxylation of the Co-Al mixed hydroxide formed.
3.2. X-ray Diffraction Analysis of Crystalline Phases
Below are the results regarding the development of the crystalline phases of the different samples prepared, analyzed using X-ray diffraction, in the traditional way or with coprecipitation and using a traditional kiln or microwave.
Figure 5 shows XRD analysis of the sample prepared through the traditional method and with a traditional kiln fired to 1000 °C. It can be observed that the formation of the CoAl2O4 phase was not obtained, but the Co3O4 remains unreacted, while the Al(OH)3 has reacted to Al2O3, but with no formation of the spinel phase.
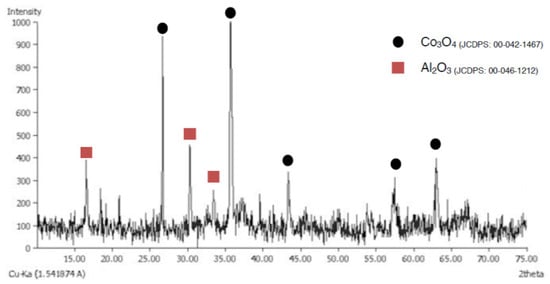
Figure 5.
XRD pattern of sample TK1000.
When the firing temperature was increased to 1200 °C using the traditional method (Figure 6), the formation of spinel Co2AlO4 could be observed, instead of spinel CoAl2O4. This is because there is still an Al2O3 phase present, so, although the spinel formed, it did not do so completely.
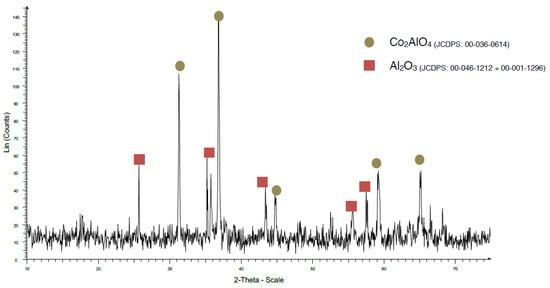
Figure 6.
XRD pattern of sample TK1200.
On the other hand, when the coprecipitation method was used (Figure 7), a CoAl2O4 crystalline phase was obtained even at 1000 °C, albeit with broad and low defined peaks, maybe due to poor crystallization because the dwelling time at the maximum temperature was only 20 min, and it might be necessary to increase it.
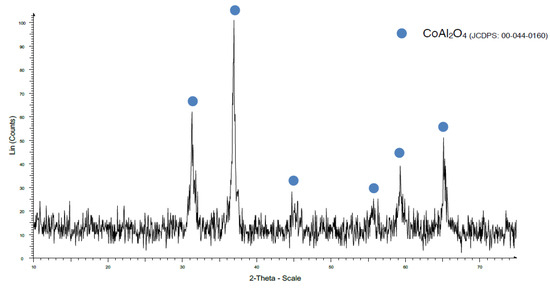
Figure 7.
XRD pattern of sample CK1000.
When the maximum firing temperature was increased to 1200 °C (Figure 8), the coprecipitation method showed a very good CoAl2O4 crystalline development without any secondary phase. This is very important to highlight because the dwelling time was only 20 min, whereas in the industrial process using the traditional method, it is necessary to use flux agents to reduce the firing temperature, and a long time process is required to obtain good crystallization without secondary phases.
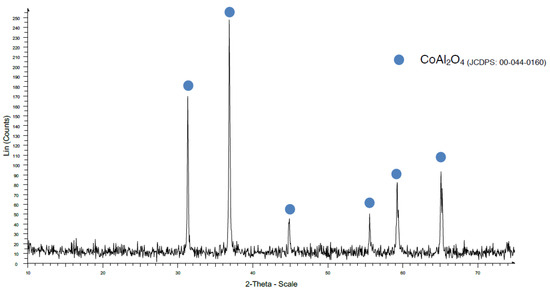
Figure 8.
XRD pattern of sample CK1200.
Microwave treatment substantially improved crystallization in all cases; lower temperatures than those used in traditional firing were used. At 900 °C, by using both traditional and coprecipitation methods, CoAl2O4 was obtained, although with traditional synthesis, Co3O4 and Al2O3 were still present (Figure 9).
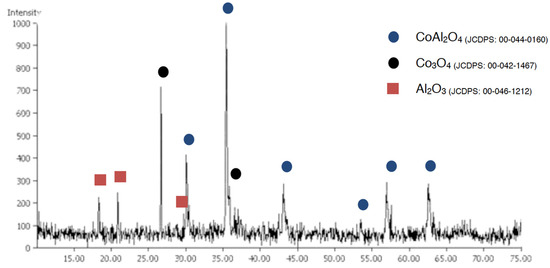
Figure 9.
XRD pattern of sample TMW900.
When a temperature of 970 °C was used, some Al2O3 was detected with the traditional method (Figure 10), but when the coprecipitation method was used, the CoAl2O4 crystallization was very good (Figure 11).
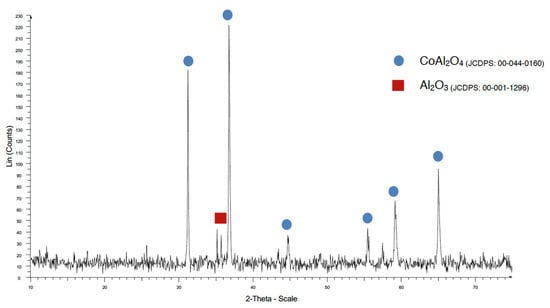
Figure 10.
XRD pattern of sample TMW970.
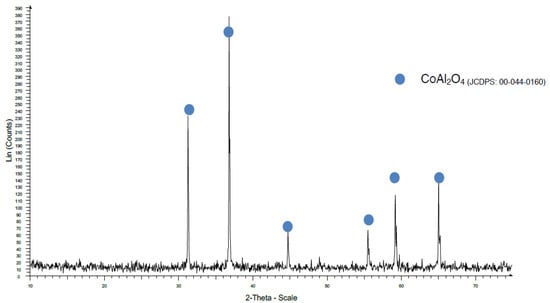
Figure 11.
XRD pattern of sample CMW970.
3.3. Scanning Electron Microscopy Characterization of Microstructure
As seen in the previous paragraph, the best results were obtained at the maximum firing temperature with both firing methods, so, at this point, we limit our discussion to these synthesis conditions.
Figure 12 shows SEM images of samples produced by traditional firing, (a) prepared by the traditional method, and (b) by coprecipitation. In both cases, an irregular size distribution was obtained, but in the coprecipitation case, it can be observed that the crystal size is smaller than that produced in the traditional way.
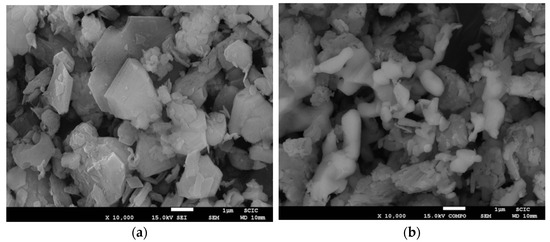
Figure 12.
SEM micrographies (magnification ×10,000) of samples (a) TK1200 and (b) CK1200.
Figure 13 shows particle aggregates with a nominal size under 500 nm obtained by the coprecipitation method using traditional firing to 1200 °C (sample CK1200).

Figure 13.
SEM micrography (magnification ×20,000) of sample CK1200.
When microwave heating was used, the final firing temperature was 970 °C. The microstructure of the sample obtained by the traditional method is very similar to the one obtained by traditional firing (Figure 14a). But in the coprecipitation method, a significant change can be observed in the microstructure (Figure 14b) because there are no large crystalline growths.
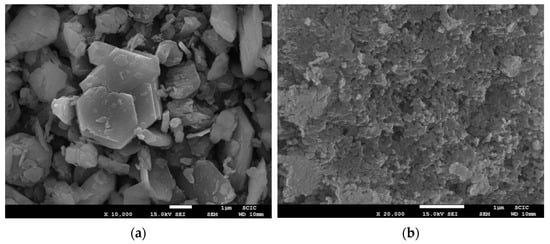
Figure 14.
SEM micrographies of samples (a) TMW970 with magnification ×10,000 and (b) CMW970 with magnification ×20,000.
The high-speed firing and low dwelling times in the microwave process and the homogeneity of the temperatures reached inside the crucibles prevented crystalline growth, and in all cases, a distribution of nanometric-sized particles close to 100 nm was obtained (Figure 15). Although the material is agglomerated, there was no sintering between the particles.

Figure 15.
SEM micrography (magnification ×50,000) of sample CMW970.
3.4. Color Characterization
As in the previous section on microstructural characterization, the only pigments that were studied were those obtained at higher temperatures, since they are the ones that show the best crystallizations, and, therefore, the best results in color development.
Figure 16 shows the final aspect of glazed tiles prepared with different pigment samples. The most intense colors were obtained by the coprecipitation method in both traditional and microwave firing. It can also be observed that, regardless of the preparation method, microwave calcination also improves the intensity of the color obtained.
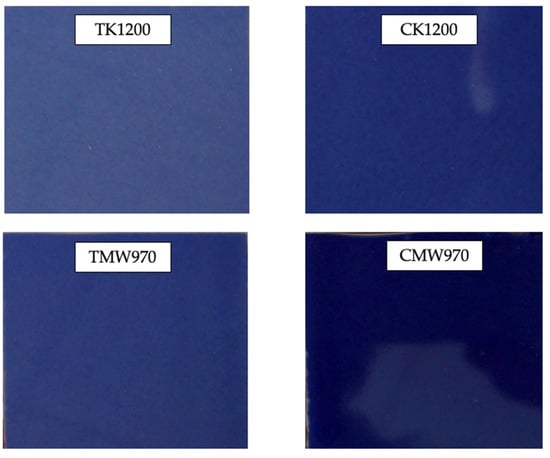
Figure 16.
Glazed tiles prepared with different pigment samples.
In Table 2, color coordinates using the CIE-L*a*b* reference are shown. The darkest blue result is the one corresponding to the sample prepared by coprecipitation and microwave firing, as expected from the previous results.

Table 2.
Color coordinates (CIE-L*a*b*) of glazes prepared with different pigment samples.
It can also be observed that the chromatic coordinates are practically the same for the sample prepared with coprecipitation and fired in a traditional kiln to 1200 °C and for the sample prepared by the traditional route and fired by microwave to 970 °C (CT1200 and TMW970, respectively).
In fact, the chromatic coordinate that indicates the blue hue (−b) is almost identical in these three glazes (CT1200, TMW970 and CMW950), with differences appearing both in the L coordinate (which decreases as the intensity of the glaze increases) and in the chromatic coordinate indicated by red (+a), being greater in those of the coprecipitation route than in those of the traditional route.
These differences in the chromatic coordinates (and therefore in the blue shade) are probably due to the lower percentage of spinel (CoAl2O4) formed at lower temperatures, especially in the traditional method, as observed in the XRD analysis.
4. Discussion
Several blue ceramic pigment compositions were synthesized, from different synthesis routes (traditional and coprecipitation) and different firing techniques (traditional kiln and microwave treatment), as we sought to obtain pigments with the structure of cobalt spinel (CoAl2O4).
Blue pigments were obtained in all cases without the use of mineralizing agents in any of them, although the shade varied quite a bit depending on the amount of spinel formed, so we concluded that in those resulting in lower intensity, mineralizing agents should be used if you do not want to vary the synthesis conditions to obtain pigments with better properties (intensity, morphology, structure, etc.).
Through X-ray diffraction, it can be observed that the pigments prepared by the traditional method develop a CoAl2O4 phase with worse crystallinity than those prepared by coprecipitation, since the raw materials remained unreacted. This is the main reason that explains the need to use mineralizing agents in the current synthesis processes of industrial pigments, which are based on the traditional method [14].
X-ray diffraction results also allow us to conclude that the higher the firing temperature, the greater the amount of spinel formed in the pigments (which translates into a greater color intensity) and that microwave firing improves the development of the crystalline phases with respect to those obtained by firing in a traditional kiln, regardless of the preparation method used.
The study results from the use of scanning electron microscopy allow us to observe that the particle size obtained is much smaller and narrower when the pigments are prepared by coprecipitation than when they are prepared by traditional synthesis, which increases their specific surface area and, therefore, their reactivity, allowing for a higher intensity of color, as shown in the colorimetry results.
Furthermore, scanning electron microscopy results show that microwave firing always produces narrower particle size distributions (similar morphology and size) and smaller particle sizes (up to nanometric sizes) than when pigment is fired in a traditional kiln. This is due to the rapid heating speed, the homogeneity of the temperature and the short dwelling time in the microwave, which prevents unwanted aggregates or crystalline growth from occurring.
On the other hand, colorimetry analysis allows us to observe how the previous microscopic properties are transferred to real pieces and allows us to corroborate that the most intense glazes are those that were colored using the pigments that formed the greatest amount of CoAl2O4 spinel and that also had smaller particle sizes.
With all the above, we can highlight that coprecipitation offers great advantages over the traditional method, such as greater development of the crystalline phase and a smaller particle size, which translates into better reactivity and greater color intensity and allows avoiding the use of expensive and often polluting mineralizing agents. It is also highly recommended for the development of pigments for inkjet inks, which require nanometric-sized pigments for their preparation in order not to clog the nozzles used in digital-head printers for the application of the inks [16].
It should also be noted that microwave firing provides great benefits in the synthesis of ceramic pigments, since it requires a very short time to reach the maximum temperature and cool (short firing cycles), which prevents crystalline growth, speeds up production processes and reduces energy consumption to a large degree. On the other hand, firing in a traditional kiln requires higher temperatures and longer residence times at the maximum firing temperature to favor the process of pigment formation via the solid-state route, and even then, it obtains worse results than using microwaves. The reduction in the operating temperature from the traditional 1200 °C to 970 °C when using the microwave technology represents a reduction in overall thermal losses.
Therefore, we could finally conclude that the optimal conditions for the synthesis of the ceramic pigment with a spinel structure (CoAl2O4) would be the preparation of the precursors by coprecipitation and calcination in a microwave kiln for 40 min (970 °C).
Energetic Savings Estimation
After the tests were carried out in the laboratory and under the conditions described in the experimental part, we can affirm that the traditional kiln requires an energetic consumption of 3.5 KW/h, with a firing cycle of 3 h, and the consumption is 10.5 KW. On the other hand, the microwave kiln requires a consumption of 0.8 KW/h, with a firing cycle of 0.5 h, and consumes a total of 0.4 KW.
The cooling time is also significantly reduced, since the microwave does not have the thermal inertia that is found in the traditional kiln, so the total firing time is much shorter.
As a summary of these considerations, this study represents that the same ceramic pigment fired on a microwave has 96.2% energy savings in electrical consumption and 90% savings in total firing time compared to its manufacture in a traditional kiln.
These data are estimated for a laboratory muffle. In industrial conditions, the savings would be greater.
5. Conclusions
The results shown in this study allow us to consider the possibility of using this method for the industrial production of ceramic pigments in a faster way and with less energy consumption than the traditional methods used until now. In all cases, microwave treatment improves the pigment crystallization and allows much smaller particle sizes to be obtained without the formation of agglomerates, which allows for greater color performance and favors its use in the manufacture of ceramic inks for digital decoration, reducing the problems derived from grinding that currently occur.
Author Contributions
Investigation, G.P.-R.; Project administration, I.N.-D.; Supervision, I.N.-D.; Writing—review and editing, G.P.-R. and I.N.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the Scientific Instrumentation Central Service from Jaume I University of Castellon (Spain) and the Characterization Laboratory of TORRECID for helping with XRD and SEM characterization of samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nebot-Díaz, I.; Dal Corso, P. (Eds.) Digital Ceramic Decoration, an Introduction; ATC: Castellón, Spain, 2017. [Google Scholar]
- Escribano-López, P.; Carda-Castelló, J.B.; Cordoncillo-Cordoncillo, E. (Eds.) Esmaltes y Pigmentos Cerámicos; Faenza Editrice Ibérica: Castellón, Spain, 2001. [Google Scholar]
- Enríquez, E.; Reinosa, J.J.; Fuertes, V.; Fernández, J.F. Advances and challenges of ceramic pigments for inkjet printing. Cer. Int. 2022, 48, 31080–31101. [Google Scholar] [CrossRef]
- Lyubenova, T.S.; Carda, J.B.; Ocaña, M. Synthesis by pyrolysis of aerosols and ceramic application of Cr-doped CaYAlO4 red-orange pigments. J. Eur. Ceram. Soc. 2009, 29, 2193–2198. [Google Scholar] [CrossRef]
- El Hadri, M.; Ahamdane, H.; El Idrissi Raghni, M.A. Sol gel synthesis of forsterite, M-doped forsterite (M=Ni,Co) solid solutions and their use as ceramic pigments. J. Eur. Ceram. Soc. 2015, 35, 765–777. [Google Scholar] [CrossRef]
- Benchikhi, M.; Hattaf, R.; El Ouatib, R. Sol-Gel-Assisted Molten-Salt Synthesis of Co2SiO4 Pigments for Ceramic Tiles Application. Silicon 2023, 15, 2003–2010. [Google Scholar] [CrossRef]
- Betancur-Granados, N.; Restrepo-Baena, O.J. Flame spray pyrolysis synthesis of ceramic nanopigments CoCr2O4: The effect of key variables. J. Eur. Ceram. Soc. 2017, 37, 5051–5056. [Google Scholar] [CrossRef]
- Chavarriaga, E.; Lopera, A.; Bergmann, C.; Alarcón, J. Effect of the substitution of Co2+ by Mg2+ on the color of the CoCr2O4 ceramic pigment synthesized by solution combustion. Bol. Soc. Esp. Cer. Y Vidr. 2020, 59, 176–184. [Google Scholar] [CrossRef]
- Rives, V.; Pérez-Bernal, M.E.; Ruano-Casero, R.J.; Nebot-Diaz, I. Development of a black pigment from non-stoichiometric hydrotalcites. J. Eur. Cer. Soc. 2012, 32, 975–987. [Google Scholar] [CrossRef]
- Oset, M.; Moya, A.; Paulo-Redondo, G.; Nebot-Díaz, I. Nanoparticle black ceramic pigment obtained by hydrotalcite-like compound microwave treatment. Chemengineerig 2022, 6, 54. [Google Scholar] [CrossRef]
- Miguel, E.; Carda, J.B.; Nebot-Díaz, I. Development of Red Ceramic Pigments with Perovskite Structure Prepared through a Traditional Route. Eng 2023, 4, 159–173. [Google Scholar] [CrossRef]
- Monrós, G.; Badenes, J.; García, A.; Tena, M. El Color de la Cerámica: Nuevos Mecanismos en Pigmentos para los Nuevos Procesados de la Industria Cerámica, 1st ed.; Jaume I University: Castellón, Spain, 2003. [Google Scholar]
- Nebot-Diaz, I. Estudio y Caracterización de Compuestos tipo Espinela Mediante Rutas de Síntesis no Convencionales. Ph.D. Thesis, University Jaume I, Castellón de la Plana, Spain, January 2002. [Google Scholar]
- Calbo, J.; Tena, M.A.; Monrós, G.; Llusar, M.; Galindo, R.; Badenes, J.A. Flux agent effect on nickel ferrite black pigment. Am. Ceram. Soc. Bull. 2005, 84, 10–14. [Google Scholar]
- Escardino, A.; Mestre, S.; Barba, A.; Beltrán, V.; Blasco, A. Synthesis mechanism of an iron-chromium ceramic pigment. J. Am. Ceram. Soc. 2000, 83, 29–32. [Google Scholar] [CrossRef]
- Kim, J.; Son, B.; Yoon, D.; Hwang, K.; Noh, H.; Cho, W.; Kim, U. Characterization of blue CoAl2O4 nanopigment synthesized by ultrasonic hydrothermal method. Ceram. Int. 2012, 38, 5707–5712. [Google Scholar] [CrossRef]
- Chang, Y.; Feng, T.; Wu, C.; Chen, Y.; Ke, K.; Liu, Y.; Wang, H.; Dong, S. Controlled synthesis of blue spherical CoAl2O4 pigment powder in pickering emulsion assisted by hydrothermal process. Adv. Powder Technol. 2018, 29, 1222–1229. [Google Scholar] [CrossRef]
- Jafari, M.; Hassazadeh-Tabrizi, S.A. Preparation of CoAl2O4 nanoblue pigment via polyacrylamide gel method. Powder Technol. 2014, 266, 236–239. [Google Scholar] [CrossRef]
- Yu, F.; Yang, J.; Ma, J.; Du, J.; Zhou, Y. Preparation of nanosized CoAl2O4 powders by sol-gel and sol-gel hydrothermal methods. J. Alloys Compd. 2009, 468, 443–446. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, Q.; Wang, Y.; Wang, X.; Zhou, J. Ultrafine CoAl2O4 ceramic pigment prepared by Pechini-sacrificial agent method. Mater. Lett. 2016, 173, 64–67. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Guo, J. Synthesis and characterization of nanocrystalline CoAl2O4 spinel powder by low temperature combustion. J. Eur. Ceram. Soc. 2003, 23, 2289–2295. [Google Scholar] [CrossRef]
- Gilabert, J.; Palacios, M.D.; Sanz, V.; Mestre, S. Effects of composition and furnace temperature on (Ni,Co)(Cr,Al)2O4 pigments synthesized by solution combustion route. Int. J. Appl. Ceram. Technol. 2017, 15, 179–190. [Google Scholar] [CrossRef]
- Hu, G.; Deng, X.; Cao, Y.; Peng, Z. Synthesis of spherical CoAl2O4 pigment particles with high reflectivity by polymeric-aerosol pyrolysis. Rare Met. 2007, 26, 236–241. [Google Scholar] [CrossRef]
- García-Baños, B.; Sánchez, J.R.; Godes, J.L.; Leonelli, C.; Catalá-Civera, J.M. Evaluation of microwave synthesis of ceramic pigments base done in situ dielectric characterization. Materials 2023, 16, 2976. [Google Scholar] [CrossRef]
- Ramos, P.A.; Albuquerque, D.M.; Pereira, J.C. Numerical simulation and optimization of the ceramic pigments production process using microwave. Chem. Eng. Process.-Process Intensif. 2021, 169, 108567. [Google Scholar] [CrossRef]
- Veronesi, P.; Leonelli, C.; Bondioli, F. Energy efficiency in the microwave-assisted solid-state synthesis of cobalt aluminate pigment. Technologies 2017, 5, 42. [Google Scholar] [CrossRef]
- Obata, S.; Kato, M.; Yokohama, H.; Iwata, Y.; Kikumoto, M.; Sakurada, O. Synthesis of nano CoAl2O4 pigment for inkjet printing to decorate porcelain. J. Ceram. Soc. Jpn. 2011, 119, 208–213. [Google Scholar] [CrossRef]
- Prado-Gonjal, J.; Morán, E. Síntesis asistida por microondas de sólidos inorgánicos. An. Quím. 2011, 107, 129–136. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).