All-Purpose Nano- and Microcontainers: A Review of the New Engineering Possibilities
Abstract
1. Introduction
2. Materials and Methods
2.1. Inorganic Containers
2.2. Organic Containers
3. Discussion
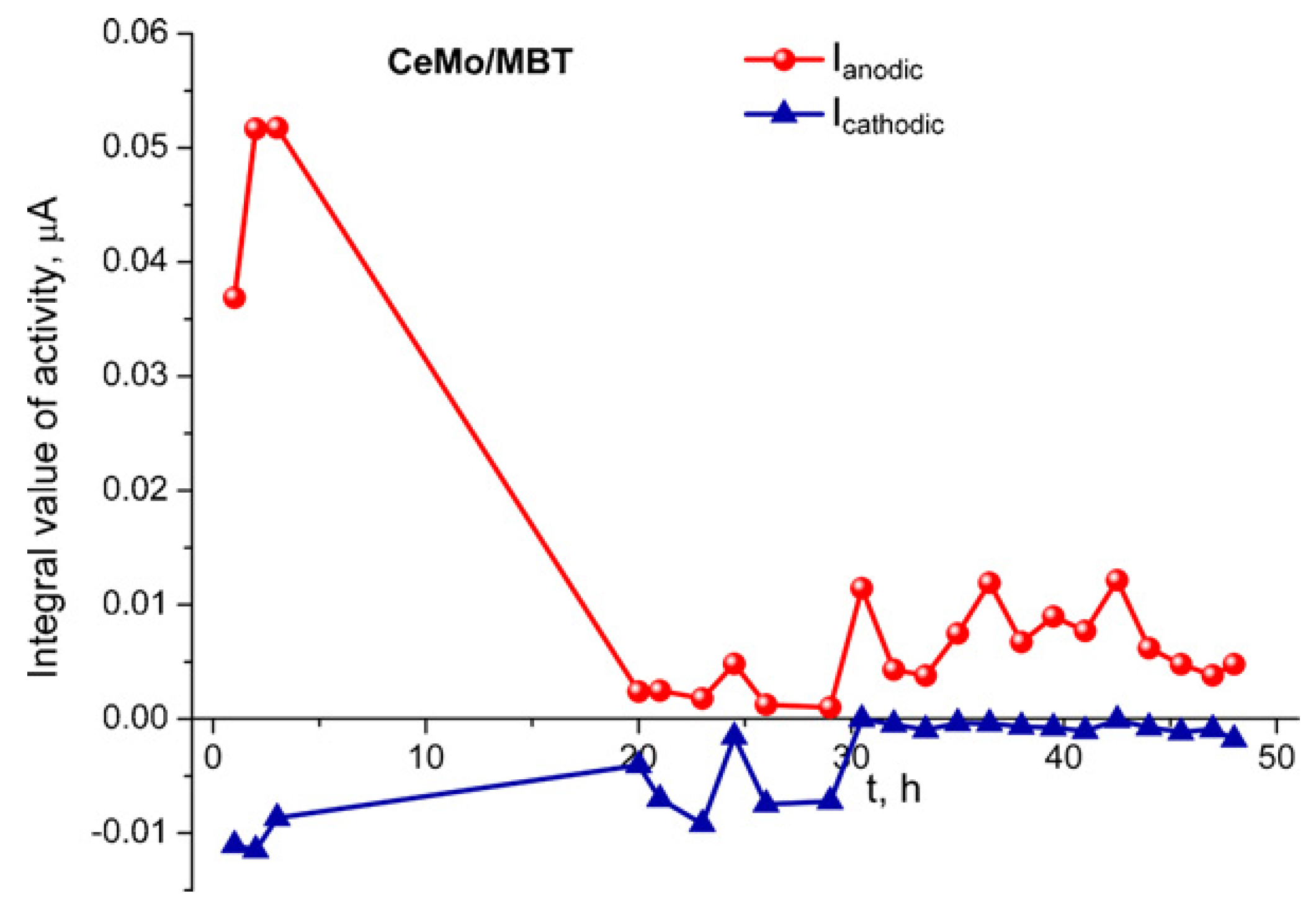
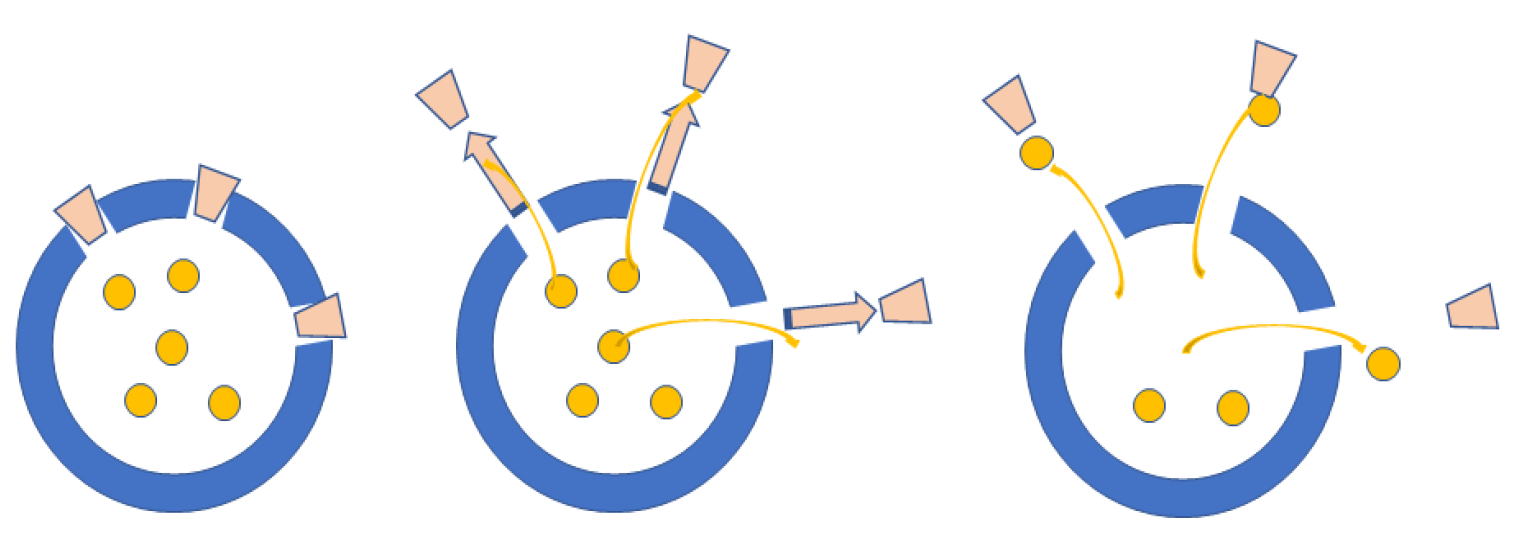
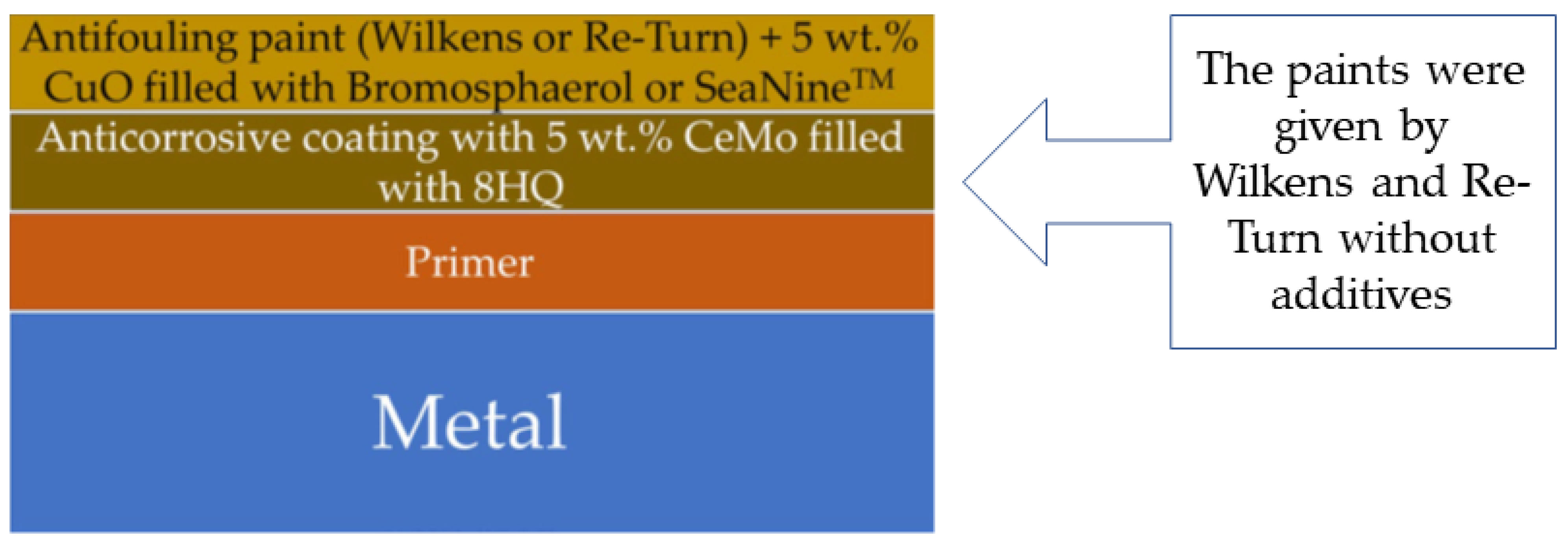
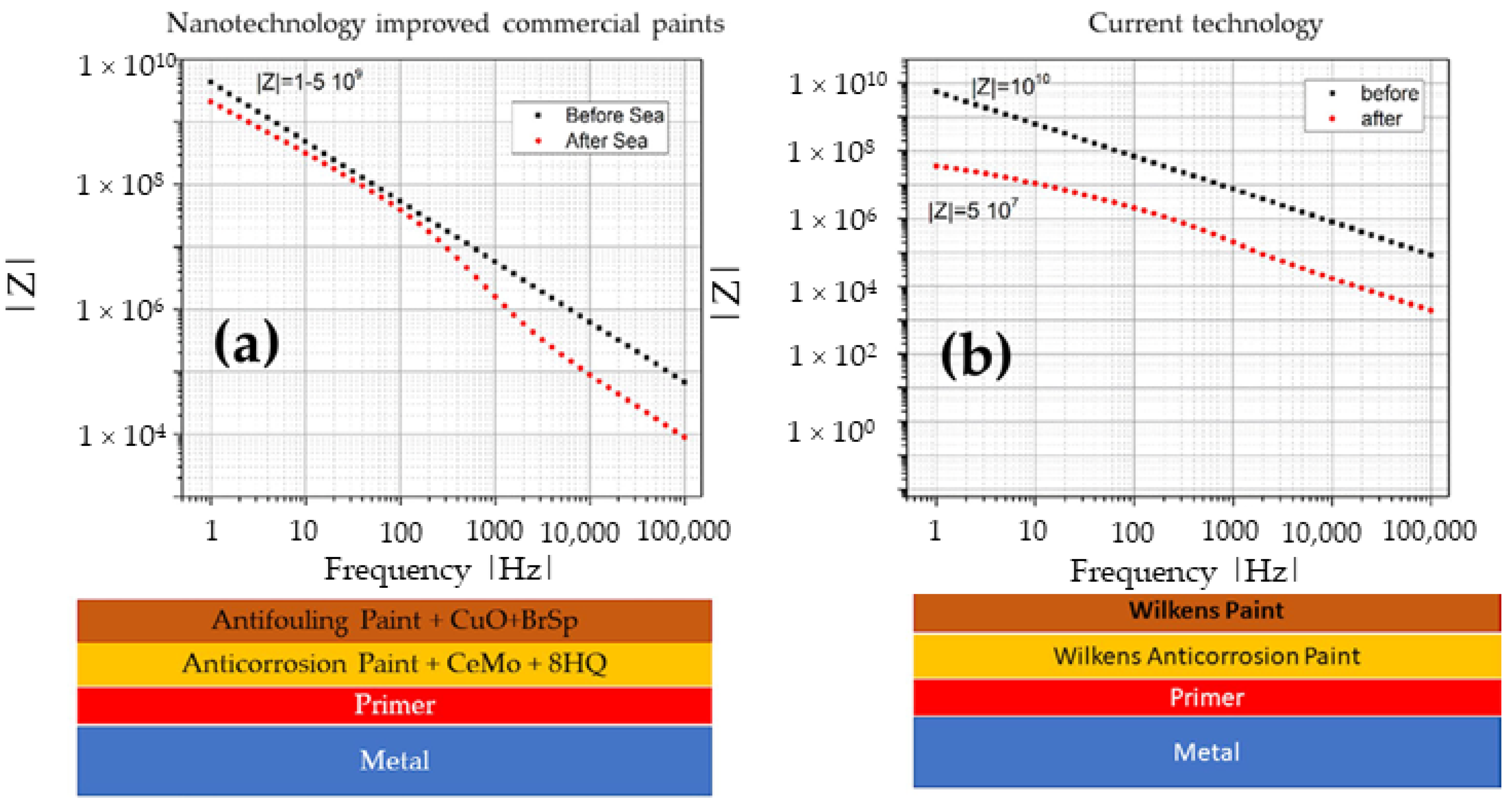
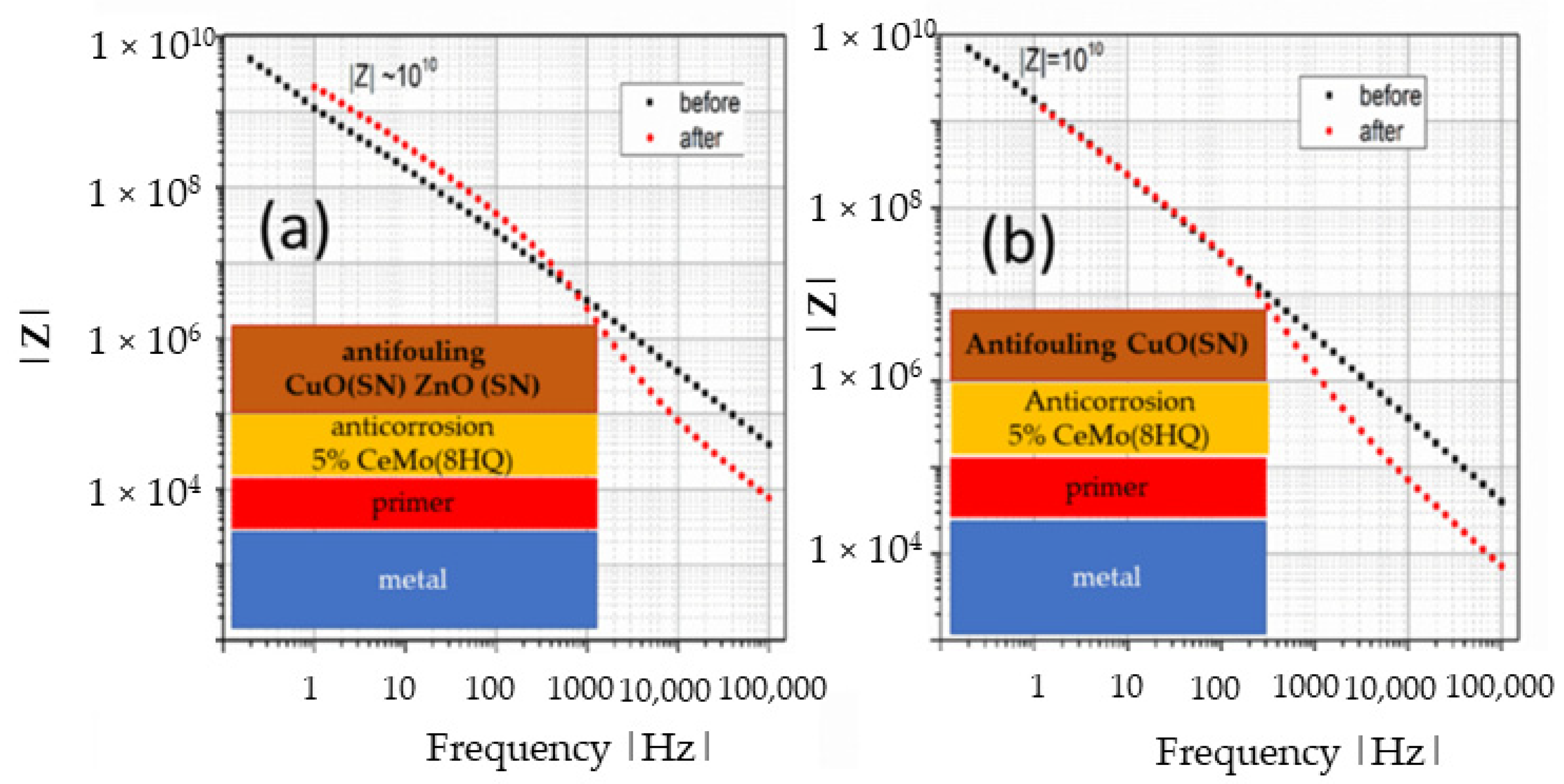
3.1. Corrosion
3.2. Antifouling
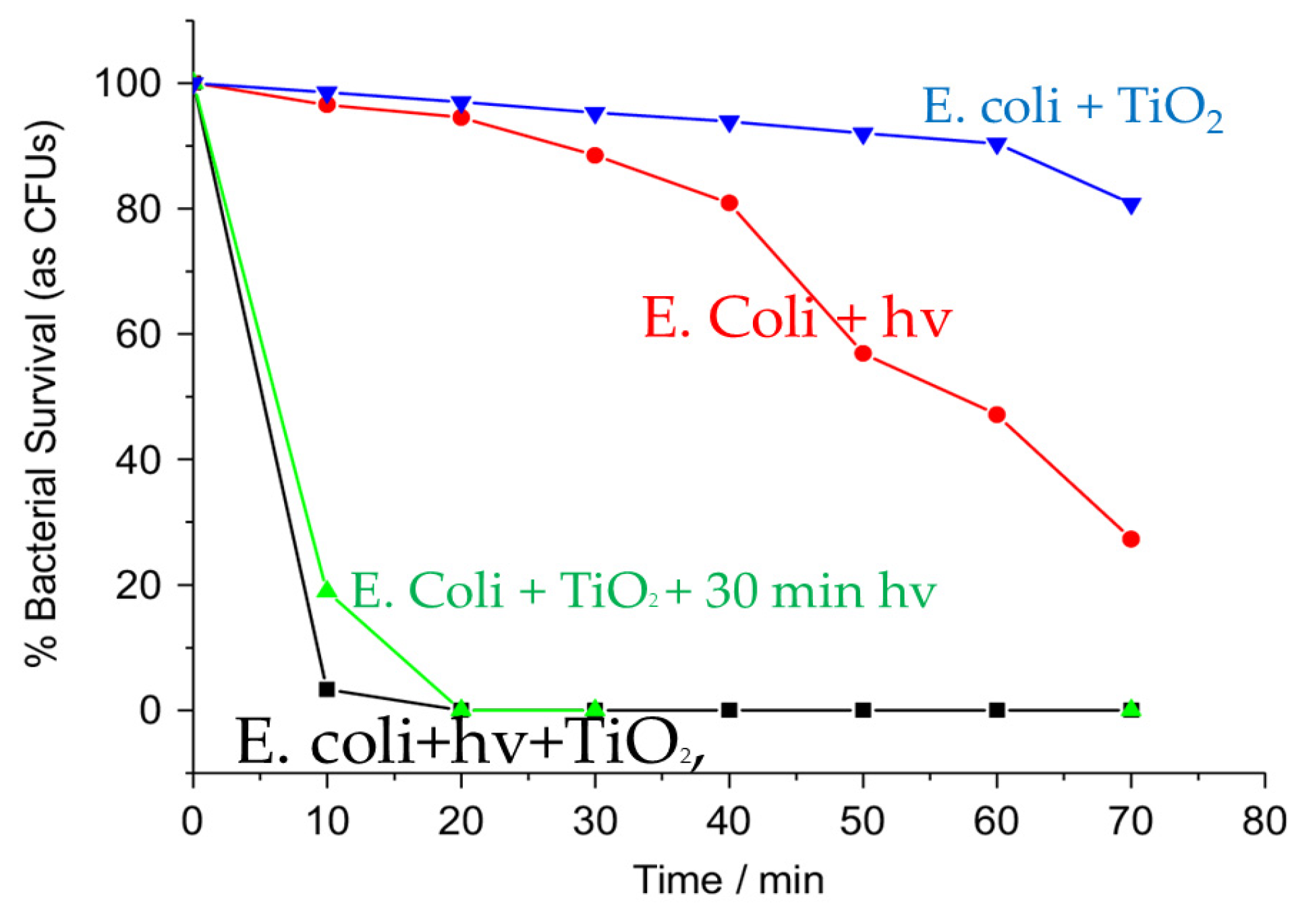
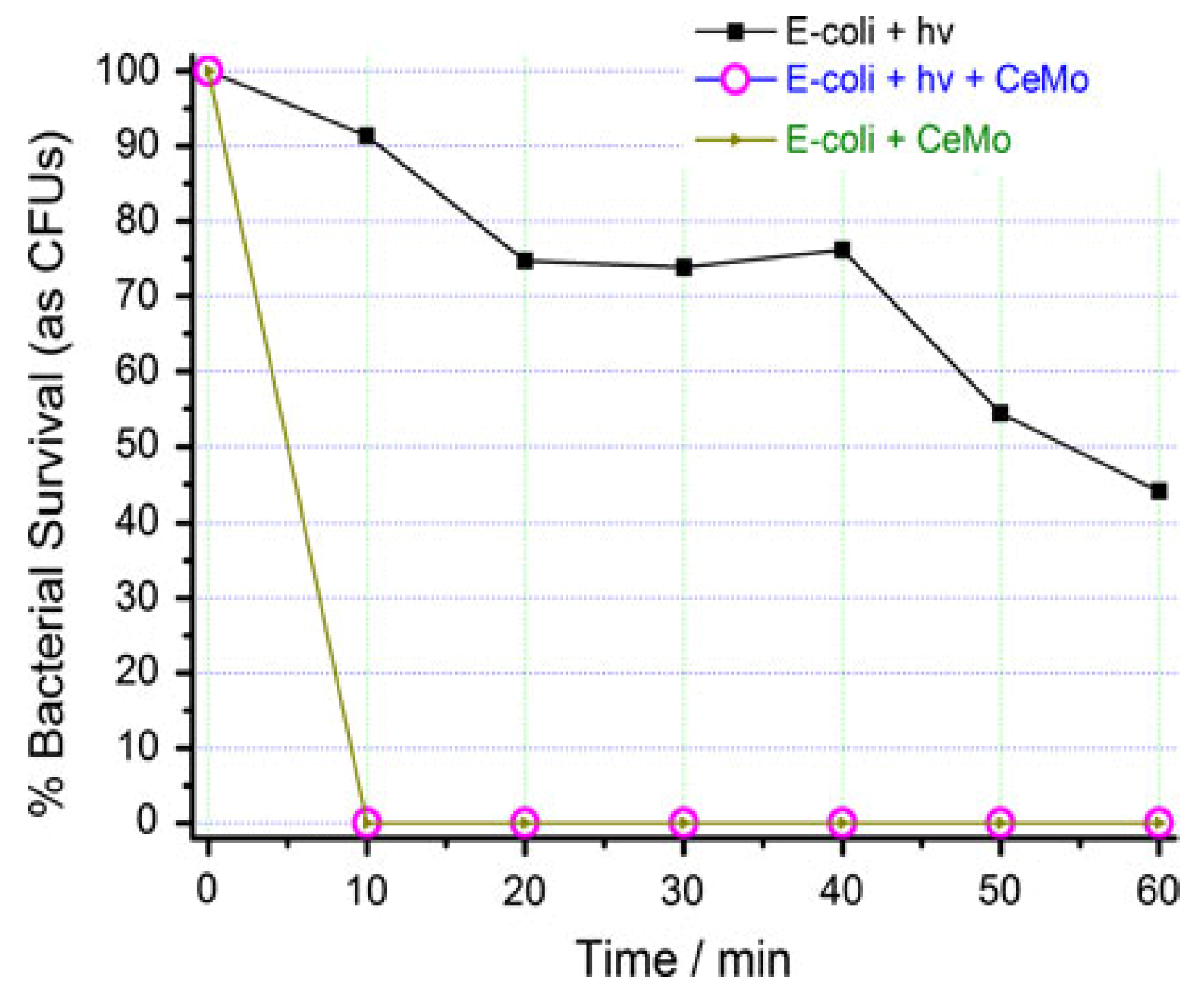
3.3. Antibacterial
3.4. Energy
Nanocontainers Encapsulating PCMs
3.5. Biomaterials
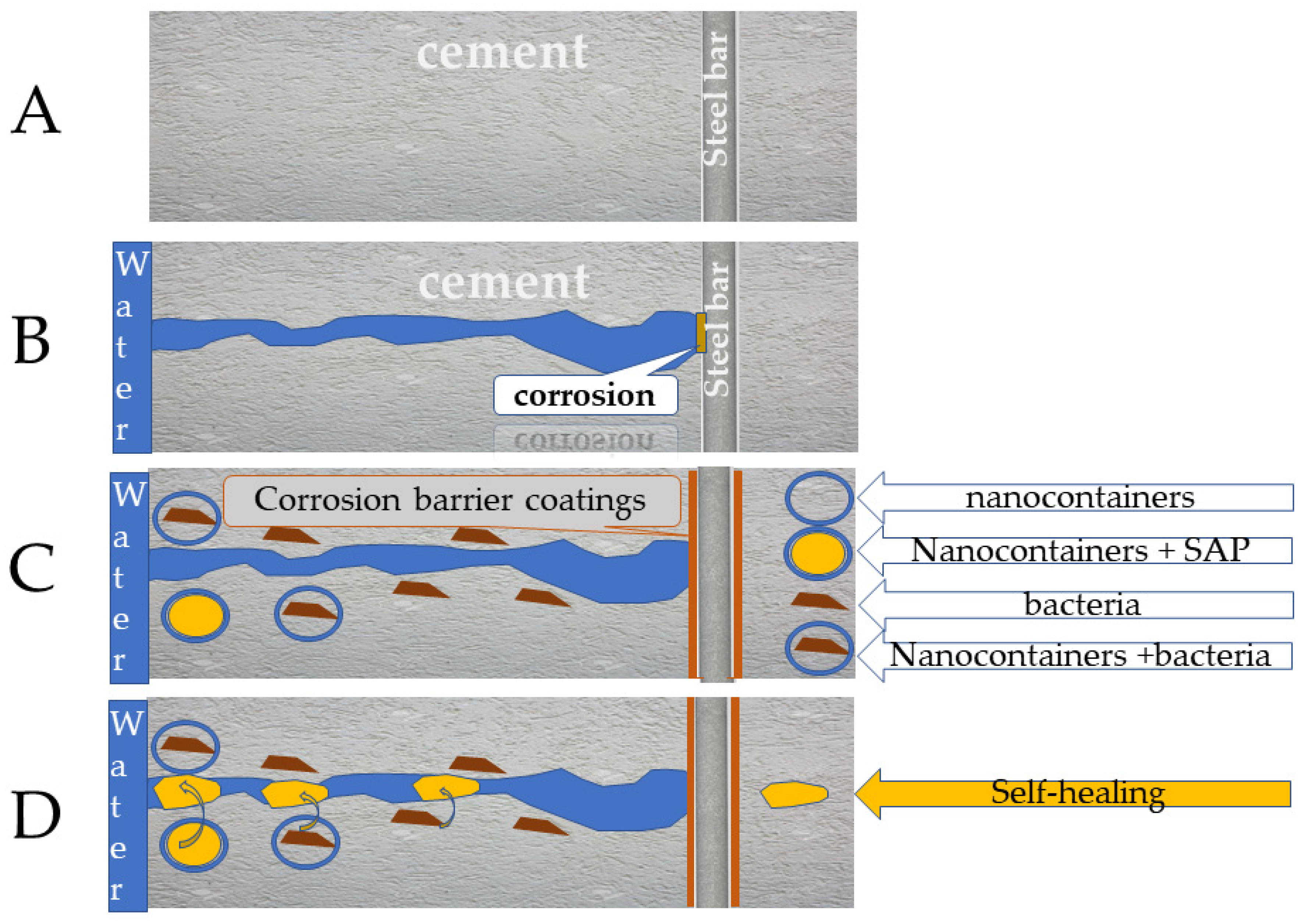
3.6. Cement
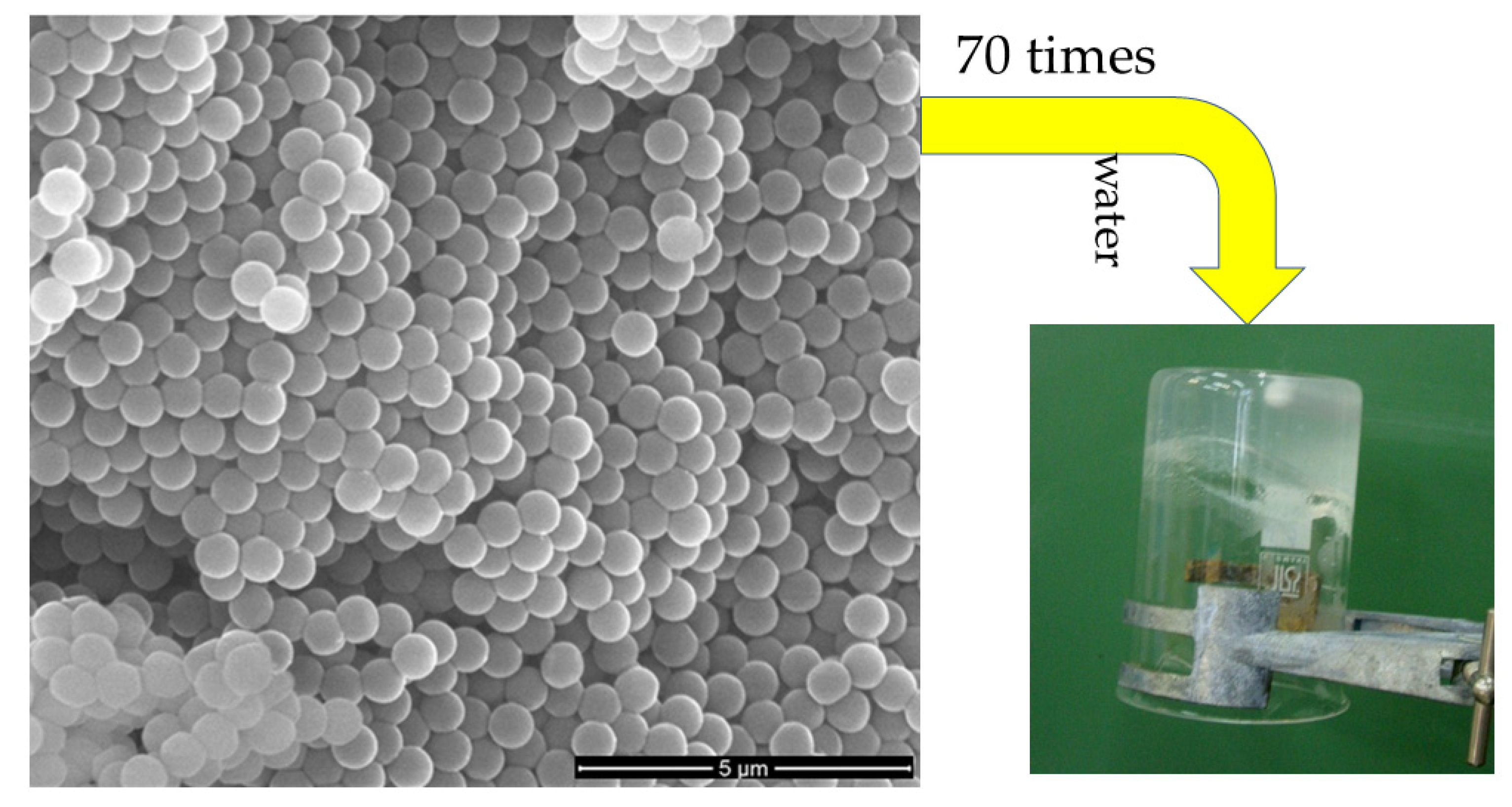
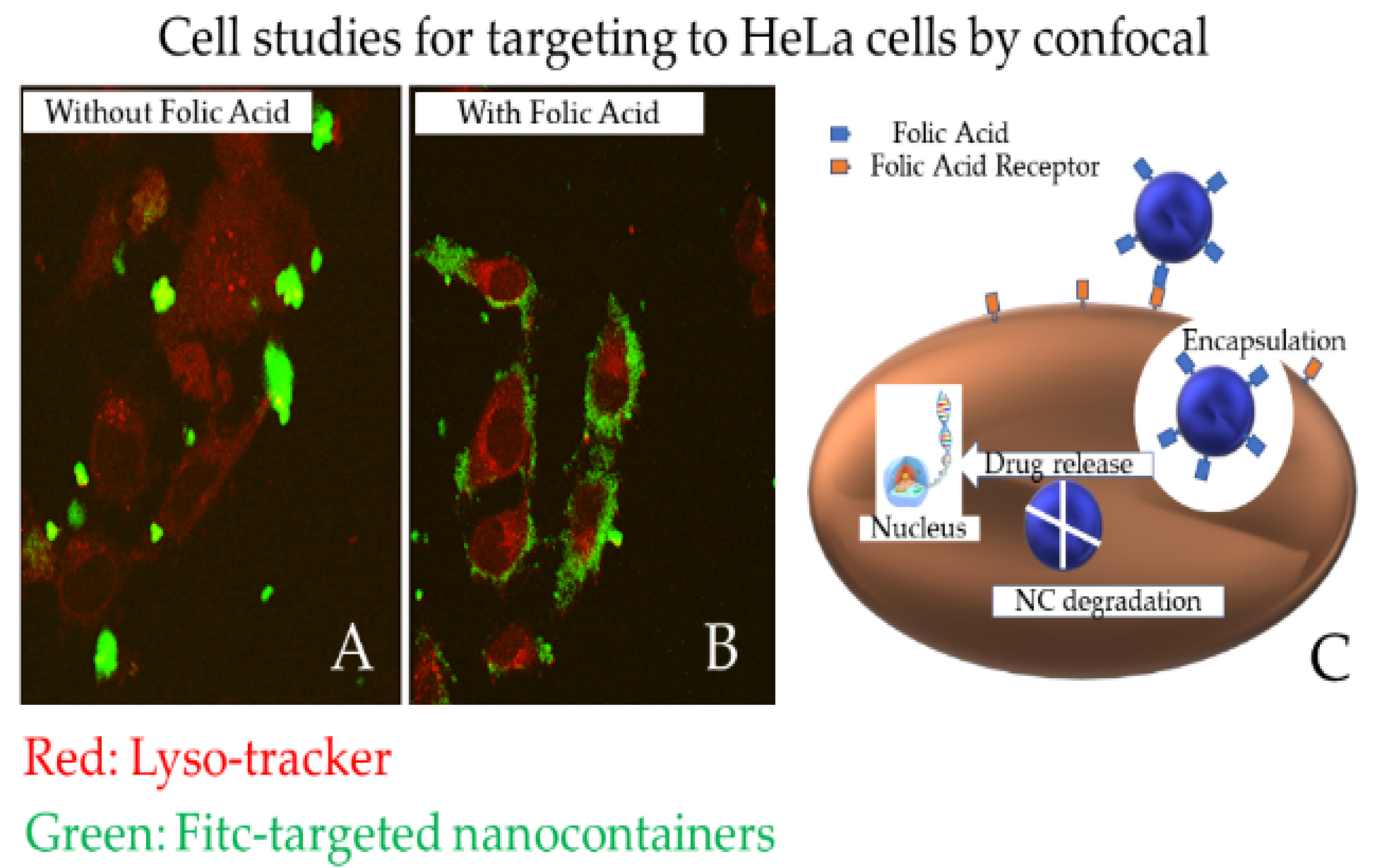
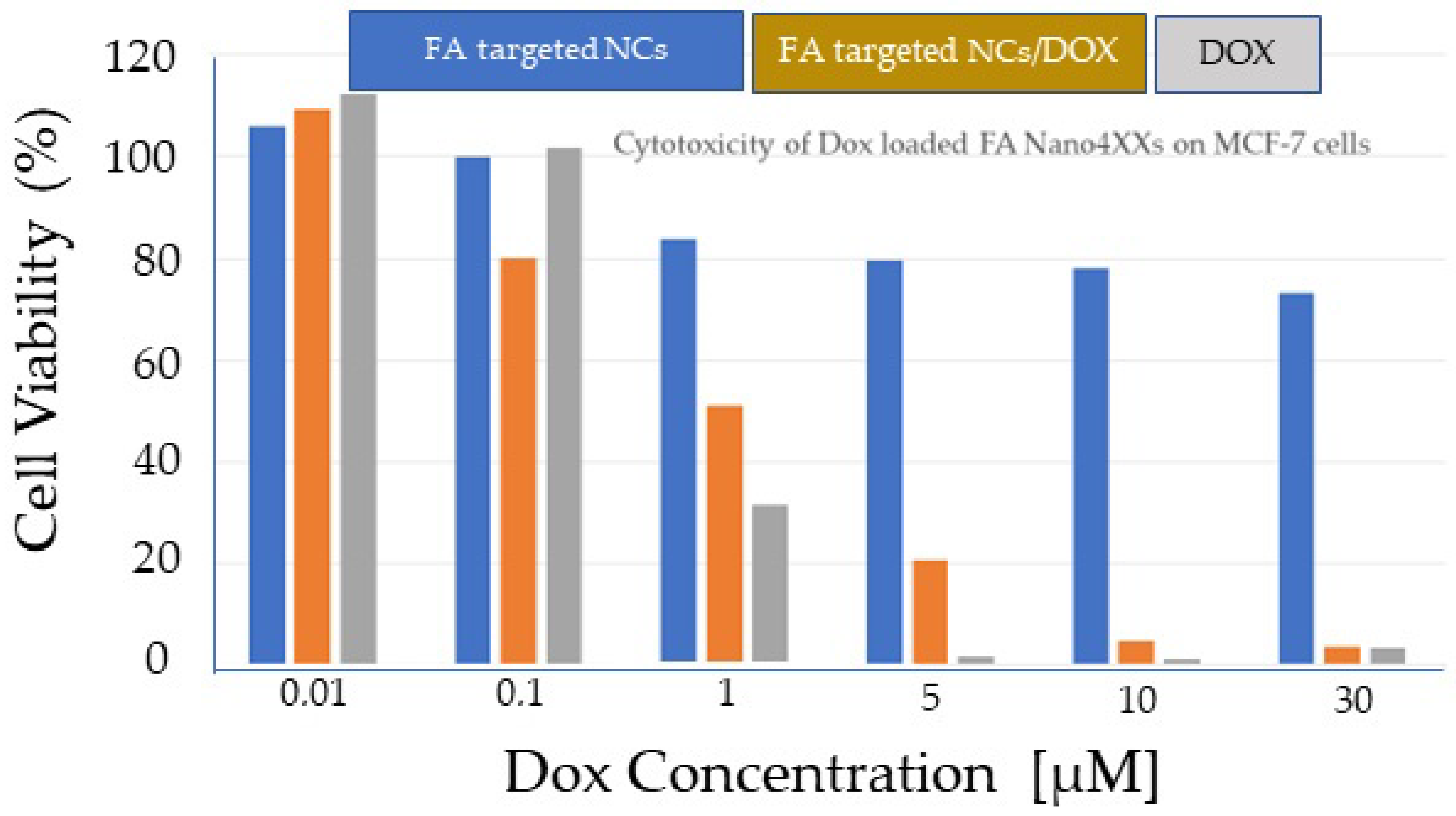
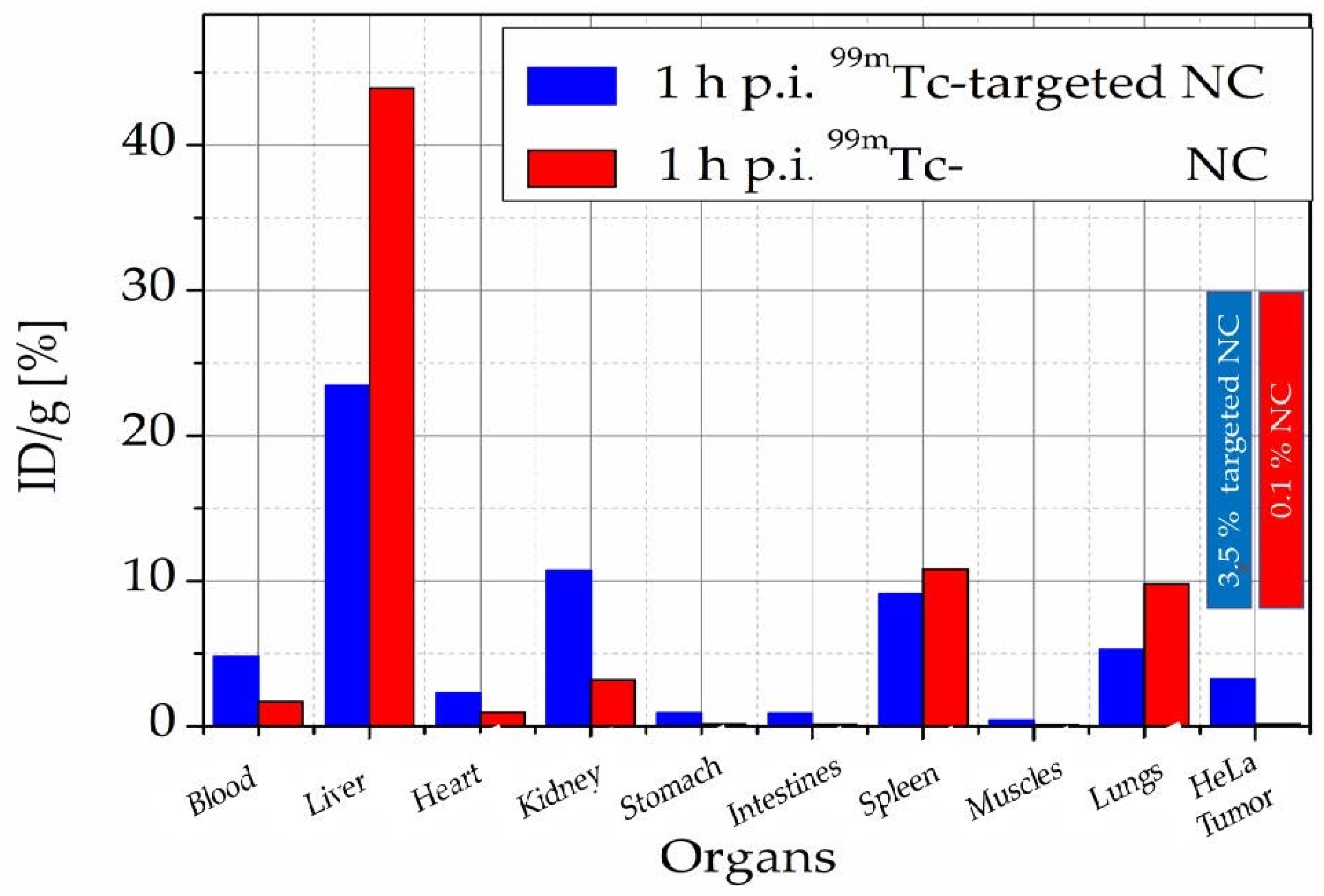
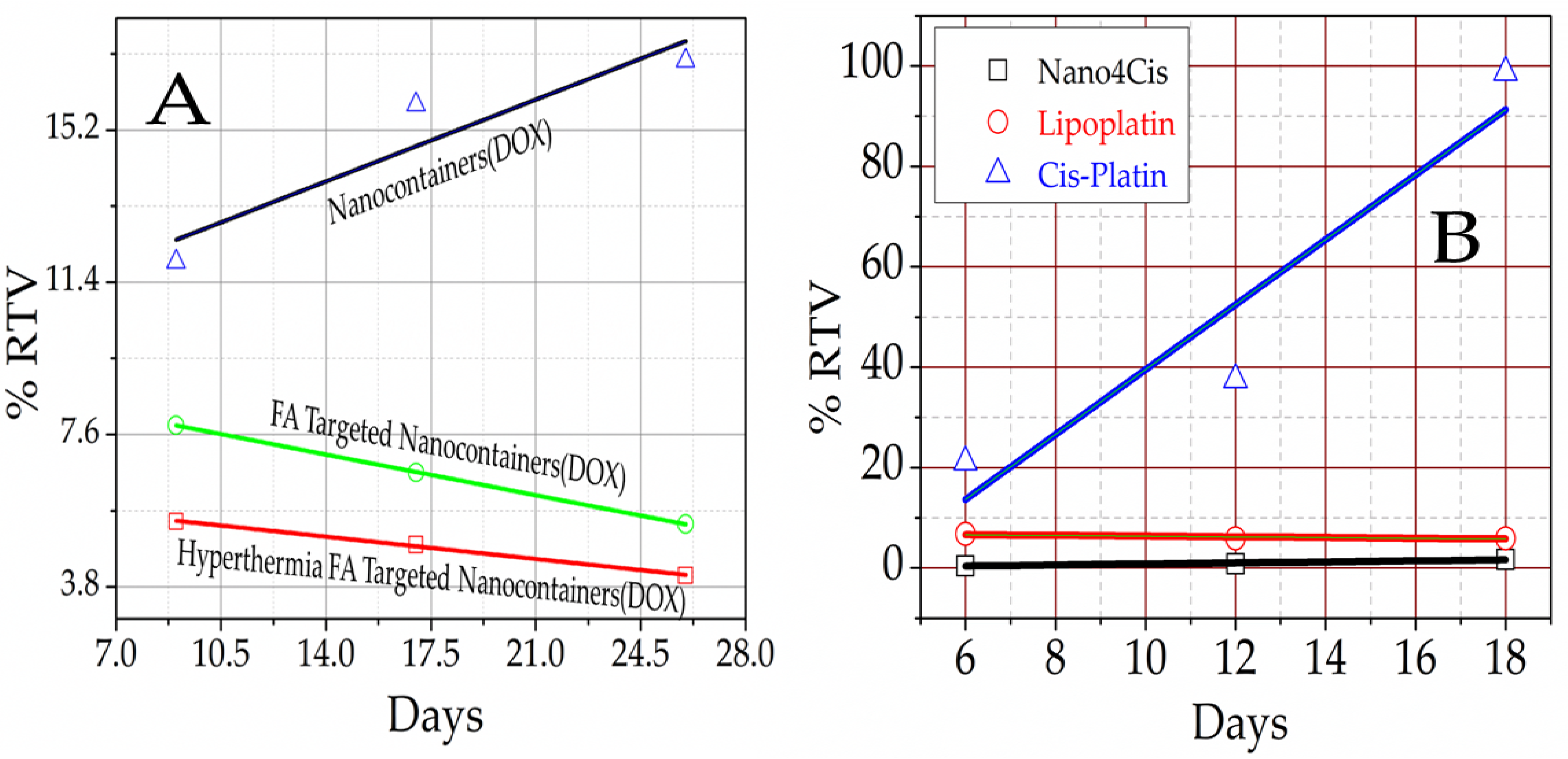
3.7. Nanomedicine
4. Conclusions and Perspectives
5. Patents
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tapeinos, C.; Efthimiadou, E.K.; Boukos, N.; Kordas, G. Sustained release profile of quatro stimuli nanocontainers as a multi sensitive vehicle exploiting cancer characteristics. Colloids Surf. B Biointerfaces 2016, 148, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kordas, G. Corrosion Barrier Coatings: Progress and Perspectives of the Chemical Route. Corros. Mater. Degrad. 2022, 3, 376–413. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Papadokostaki, K.G.; Favvas, E.P.; Efthimiadou, E.K.; Karellas, S. Preparation and investigation of distinct and shape stable paraffin/SiO2 composite PCM nanospheres. Energy Convers. Manag. 2018, 168, 382–394. [Google Scholar] [CrossRef]
- Kordas, G. Nanocontainers Against Biofouling and Corrosion Degradation of Materials: A Short Review With Prospects. Front. Nanotechnol. 2022, 4, 1–13. [Google Scholar] [CrossRef]
- Kordas, G.; Efthimiadou, E.K. Self-Healing Coatings for Corrosion Protection of Metals. Sol-Gel Handb. 2015, 3, 1371–1384. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Efthimiadou, E.K.; Kordas, G. A new approach to fabricate bioactive silica binary and ternary hybrid microspheres. Mater. Sci. Eng. C 2015, 53, 76–82. [Google Scholar] [CrossRef]
- Tapeinos, C.; Kartsonakis, I.; Liatsi, P.; Daniilidis, I.; Kordas, G. Synthesis and characterization of magnetic nanocontainers. J. Am. Ceram. Soc. 2008, 91, 1052–1056. [Google Scholar] [CrossRef]
- Kordas, G. Protection of HDG Steel Using ORMOSIL Coatings Enhanced with CeO (5-ATDT)-Ceramic Nanocontainers. Appl. Sci. Eng. Prog. 2022, 16, 6329. [Google Scholar] [CrossRef]
- Li, D.; Wang, F.; Yu, X.; Wang, J.; Liu, Q.; Yang, P.; He, Y.; Wang, Y.; Zhang, M. Anticorrosion organic coating with layered double hydroxide loaded with corrosion inhibitor of tungstate. Prog. Org. Coat. 2011, 71, 302–309. [Google Scholar] [CrossRef]
- Shchukina, E.; Shchukin, D.G. Nanocontainer-Based Active Systems: From Self-Healing Coatings to Thermal Energy Storage. Langmuir 2019, 35, 8603–8611. [Google Scholar] [CrossRef]
- Mekeridis, E.D.; Kartsonakis, I.A.; Pappas, G.S.; Kordas, G.C. Release studies of corrosion inhibitors from cerium titanium oxide nanocontainers. J. Nanoparticle Res. 2011, 13, 541–554. [Google Scholar] [CrossRef]
- Kordas, G. ORMOSIL Coatings Enriched with CeO2 (5-ATDT)-Ceramic Nanocontainers for Enhanced Protection of HDG Steel Used in Concrete. Materials 2022, 15, 3913. [Google Scholar] [CrossRef] [PubMed]
- Mekeridis, E.D.; Kartsonakis, I.A.; Kordas, G.C. Multilayer organic-inorganic coating incorporating TiO 2 nanocontainers loaded with inhibitors for corrosion protection of AA2024-T3. Prog. Org. Coat. 2012, 73, 142–148. [Google Scholar] [CrossRef]
- Kordas, G.C.; Balaskas, A.C.; Kartsonakis, I.A.; Efthimiadou, E.K. A Raman study of 8-Hydroxyquinoline release from loaded TiO2 nanocontainer. Int. J. Struct. Integr. 2013, 4, 121–126. [Google Scholar] [CrossRef]
- Pappas, G.S.; Liatsi, P.; Kartsonakis, I.A.; Danilidis, I.; Kordas, G. Synthesis and characterization of new SiO2-CaO hollow nanospheres by sol-gel method: Bioactivity of the new system. J. Non-Cryst. Solids 2008, 354, 755–760. [Google Scholar] [CrossRef]
- Kanellopoulou, I.; Karaxi, E.K.; Karatza, A.; Kartsonakis, I.A.; Charitidis, C. Hybrid superabsorbent polymer networks (SAPs) encapsulated with SiO2 for structural applications. MATEC Web Conf. 2018, 188, 01025. [Google Scholar] [CrossRef][Green Version]
- Karatzas, A.; Bilalis, P.; Kartsonakis, I.A.; Kordas, G.C. Reversible spherical organic water microtraps. J. Non-Cryst. Solids 2012, 358, 443–445. [Google Scholar] [CrossRef]
- Krzak, M.; Tabor, Z.; Nowak, P.; Warszyński, P.; Karatzas, A.; Kartsonakis, I.A.; Kordas, G.C.; Warszy, P.; Karatzas, A.; Kartsonakis, I.A.; et al. Water diffusion in polymer coatings containing water-trapping particles. Part 2. Experimental verification of the mathematical model. Prog. Org. Coat. 2012, 75, 207–214. [Google Scholar] [CrossRef]
- Kordas, G. Quadrupole Stimuli-Responsive Targeted Polymeric Nanocontainers for Cancer Therapy: Artificial Intelligence in Drug Delivery Systems. Nanoeng. Biomater. 2022, 1, 505–522. [Google Scholar] [CrossRef]
- Kordas, G. Nanotechnology in Cancer Treatment as a Trojan Horse: From the Bench to Preclinical Studies; Sarat Kumar Swain, M.J., Ed.; Elsevier Inc.: London, UK, 2019; ISBN 9780128167717. [Google Scholar]
- Rollett, A.; Reiter, T.; Nogueira, P.; Cardinale, M.; Loureiro, A.; Gomes, A.; Cavaco-Paulo, A.; Moreira, A.; Carmo, A.M.; Guebitz, G.M. Folic acid-functionalized human serum albumin nanocapsules for targeted drug delivery to chronically activated macrophages. Int. J. Pharm. 2012, 427, 460–466. [Google Scholar] [CrossRef]
- Montemor, M.F.; Snihirova, D.V.; Taryba, M.G.; Lamaka, S.V.; Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C.; Tedim, J.; Kuznetsova, A.; Zheludkevich, M.L.; et al. Evaluation of self-healing ability in protective coatings modified with combinations of layered double hydroxides and cerium molibdate nanocontainers filled with corrosion inhibitors. Electrochim. Acta 2012, 60, 31–40. [Google Scholar] [CrossRef]
- Poornima Vijayan, P.; Al-Maadeed, M.A.S.A. TiO2 nanotubes and mesoporous silica as containers in self-healing epoxy coatings. Sci. Rep. 2016, 6, 38812. [Google Scholar] [CrossRef] [PubMed]
- Kordas, G. Nanocontainers-Based Anti-Biofouling Coatings—A Pilot Study. Supramol. Chem. Corros. Biofouling Prot. 2021, 383–392. [Google Scholar] [CrossRef]
- Kordas, G. Nanocontainers (CeO2): Synthesis, Characterization, Properties, and Anti-corrosive Application. In Sustainable Corrosion Inhibitors II: Synthesis, Design, and Practical Applications; ACS Symposium Series; Hussain, C.M., Verma, C., Aslam, J., Eds.; American Chemical Society: Washington, DC, USA, 2021; Chapter 8; pp. 177–185. [Google Scholar] [CrossRef]
- Kordas, G. Nanotechnology to improve the biofouling and corrosion performance of marine paints: From lab experiments to real tests in sea. Int. J. Phys. Res. Appl. 2019, 2, 033–037. [Google Scholar] [CrossRef]
- Kartsonakis, I.; Daniilidis, I.; Kordas, G. Encapsulation of the corrosion inhibitor 8-hydroxyquinoline into ceria nanocontainers. J. Sol-Gel Sci. Technol. 2008, 48, 24–31. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Athanasopoulou, E.; Snihirova, D.; Martins, B.; Koklioti, M.A.; Montemor, M.F.; Kordas, G.; Charitidis, C.A. Multifunctional epoxy coatings combining a mixture of traps and inhibitor loaded nanocontainers for corrosion protection of AA2024-T3. Corros. Sci. 2014, 85, 147–159. [Google Scholar] [CrossRef]
- Kordas, G. CuO (Bromosphaerol) and CeMo (8 Hydroxyquinoline) microcontainers incorporated into commercial marine paints. J. Am. Ceram. Soc. 2020, 103, 2340–2350. [Google Scholar] [CrossRef]
- Aldred, N.; Clare, A.S. The adhesive strategies of cyprids and development of barnacle-resistant marine coatings. Biofouling 2008, 24, 351–363. [Google Scholar] [CrossRef]
- Guezennec, J.; Herry, J.M.; Kouzayha, A.; Bachere, E.; Mittelman, M.W.; Bellon Fontaine, M.N. Exopolysaccharides from unusual marine environments inhibit early stages of biofouling. Int. Biodeterior. Biodegrad. 2012, 66, 1–7. [Google Scholar] [CrossRef]
- Qian, P.; Lau, S.C.K.; Dahms, H.; Dobretsov, S.; Harder, T. Invited Review Marine Biofilms as Mediators of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. The term fouling generally refers to an undesirable process in which relate to the initial attachment of fouling organisms. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Bidwell, J.R.; Cherry, D.S.; Farris, J.L.; Petrille, J.C.; Lyons, L.A. Effects of intermittent halogenation on settlement, survival and growth of the zebra mussel, Dreissena polymorpha. Hydrobiologia 1999, 394, 53–62. [Google Scholar] [CrossRef]
- Burgess, J.G.; Boyd, K.G.; Armstrong, E.; Jiang, Z.; Yan, L.; Berggren, M.; May, U.; Pisacane, T.; Granmo, Å.; Adams, D.R. The Development of a Marine Natural Product-based Antifouling Paint. Biofouling 2003, 19, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Rittschof, D.A.N. Natural product antifoulants: One perspective on the challenges related to coatings development Natural Product Antifoulants: One Perspective on the Challenges Related to Coatings Development. Biofouling 2009, 15, 37–41. [Google Scholar]
- Hay, M.E. Marine chemical ecology: What ’s known and what ’s next? J. Exp. Mar. Biol. Ecol. 2009, 15, 119–127. [Google Scholar] [CrossRef]
- Chapman, J.; Hellio, C.; Sullivan, T.; Brown, R.; Russell, S.; Kiterringham, E.; Le Nor, L.; Regan, F. Bioinspired synthetic macroalgae: Examples from nature for antifouling applications. Int. Biodeterior. Biodegrad. 2014, 86, 6–13. [Google Scholar] [CrossRef]
- Pawlik, J.R. The Development of a Marine Natural Product-based Antifouling Paint. Oceanogr. Mar. Biol. Rev. 1992, 30, 273–335. [Google Scholar]
- Grandgirard, J.; Poinsot, D.; Krespi, L.; Nénon, J.P.; Cortesero, A.M. Chemical ecology of marine microbial defence. J. Chem. Ecol. 2002, 103, 1971–1985. [Google Scholar] [CrossRef]
- Hellio, C.; Berge, J.P.; Beaupoil, C.; Le Gal, Y.; Bourgougnon, N. Screening of marine algal extracts for anti-settlement activities against microalgae and macroalgae. Biofouling 2002, 18, 205–215. [Google Scholar] [CrossRef]
- Kordas, G. Novel Antifouling and Self-Healing Eco-Friendly Coatings for Marine Applications Enhancing the Performance of Commercial Marine Paints. In Engineering Failure Analysis; Thanapalan, K., Ed.; IntechOpen: London, UK, 2020; pp. 1–9. [Google Scholar]
- Krishna Mohan, M.V.; Bhanuprakash, T.V.K.; Mukherjee, A. Al2O3 and CuO nano particulate-based paints for marine applications. Eng. Res. Express 2022, 4, 035056. [Google Scholar] [CrossRef]
- Qing, Y.; Long, C.; An, K.; Liu, C. Natural rosin-grafted nanoparticles for extremely-robust and eco-friendly antifouling coating with controllable liquid transport. Compos. Part B Eng. 2022, 236, 109797. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Z.; Chen, Z.; Zhang, K.; Lu, G.; Zhang, G.; Yang, B. The synthesis and characterizations of monodisperse cross-linked polymer microspheres with carboxyl on the surface. Polymer 2002, 43, 4147–4152. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Kontogiani, P.; Pappas, G.S.; Kordas, G. Photocatalytic action of cerium molybdate and iron-titanium oxide hollow nanospheres on Escherichia coli. J. Nanoparticle Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Liatsi, P.; Danilidis, I.; Bouzarelou, D.; Kordas, G. Synthesis, characterization and antibacterial action of hollow titania spheres. J. Phys. Chem. Solids 2008, 69, 214–221. [Google Scholar] [CrossRef]
- Eiden, S.; Maret, G. Preparation and characterization of hollow spheres of rutile. J. Colloid Interface Sci. 2002, 250, 281–284. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Song, C.; Lin, Y.; Hu, Z. Preparation and characterization of TiO2 hollow spheres. Mater. Lett. 2006, 60, 77–80. [Google Scholar] [CrossRef]
- Song, C.; Wang, D.; Gu, G.; Lin, Y.; Yang, J.; Chen, L.; Fu, X.; Hu, Z. Preparation and characterization of silver/TiO2 composite hollow spheres. J. Colloid Interface Sci. 2004, 272, 340–344. [Google Scholar] [CrossRef]
- Shiho, H.; Kawahashi, N. Iron compounds as coatings on polystyrene latex and as hollow spheres. J. Colloid Interface Sci. 2000, 226, 91–97. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Liu, Q.; Sun, Y.; Song, D.; Yang, W.; Wang, J.; Liu, L. One-step synthesis of SnO2 hollow microspheres and its gas sensing properties. Mater. Lett. 2014, 136, 286–288. [Google Scholar] [CrossRef]
- Cai, J.; Wu, X.; Zheng, F.; Li, S.; Wu, Y.; Lin, Y.; Lin, L.; Liu, B.; Chen, Q.; Lin, L. Influence of TiO2 hollow sphere size on its photo-reduction activity for toxic Cr(VI) removal. J. Colloid Interface Sci. 2017, 490, 37–45. [Google Scholar] [CrossRef]
- Jackson, G.J.; Merker, R.I.; Bandler, R. Bacteriological Analytical Manual; U.S. Food & Drug Administration Center for Food Safety & Applied Nutrition Bacteriological: Silver Spring, MD, USA, 2001.
- Kartsonakis, I.A.; Liatsi, P.; Daniilidis, I.; Kordas, G. Synthesis, characterization, and antibacterial action of hollow ceria nanospheres with/without a conductive polymer coating. J. Am. Ceram. Soc. 2008, 91, 372–378. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Duan, X. Nanoparticle enhanced paraffin and tailing ceramic composite phase change material for thermal energy storage. Sustain. Energy Fuels 2020, 4, 4547–4557. [Google Scholar] [CrossRef]
- Roget, F.; Favotto, C.; Rogez, J. Study of the KNO3-LiNO3 and KNO3-NaNO3-LiNO3 eutectics as phase change materials for thermal storage in a low-temperature solar power plant. Sol. Energy 2013, 95, 155–169. [Google Scholar] [CrossRef]
- Wickramaratne, C.; Dhau, J.S.; Kamal, R.; Myers, P.; Goswami, D.Y.; Stefanakos, E. Macro-encapsulation and characterization of chloride based inorganic Phase change materials for high temperature thermal energy storage systems. Appl. Energy 2018, 221, 587–596. [Google Scholar] [CrossRef]
- Hench, L.L.; Best, S.M. Chapter I. 2.4 Ceramics, Glasses, and Glass-Ceramics: Basic Principles Types of Bioceramics: Tissue, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Hench, L.L. Sol-gel materials for bioceramic. Curr. Opin. Solid State Mater. Sci. 1997, 2, 604–610. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.S.; Bilalis, P.; Kordas, G.C. Synthesis and characterization of SiO2-CaO-P2O5 hollow nanospheres for biomedical applications. Mater. Lett. 2012, 67, 273–276. [Google Scholar] [CrossRef]
- Kanellopoulou, I.; Karaxi, E.K.; Karatza, A.; Kartsonakis, I.A.; Charitidis, C.A. Effect of submicron admixtures on mechanical and self-healing properties of cement-based composites. Fatigue Fract. Eng. Mater. Struct. 2019, 42, 1494–1509. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Bilalis, P.; Chatzipavlidis, A.; Tziveleka, L.A.; Boukos, N.; Kordas, G. Nanodesigned magnetic polymer containers for dual stimuli actuated drug controlled release and magnetic hyperthermia mediation. J. Mater. Chem. 2012, 22, 13451–13454. [Google Scholar] [CrossRef]
- Kordas, G.; Efthimiadou, E. Comparison of therapeutic efficacy of quadrupole stimuli-targeted nanocontainers loaded with Doxorubicin (Nano4Dox platform) and cisplatin (Nano4Cis platform) to Doxil and Lipoplatin, respectively. Ann. Clin. Pharmacol. Toxicol. 2018, 1, 1–5. [Google Scholar]
- Sahoo, B.; Devi, K.S.P.; Banerjee, R.; Maiti, T.K.; Pramanik, P.; Dhara, D. Thermal and pH responsive polymer-tethered multifunctional magnetic nanoparticles for targeted delivery of anticancer drug. ACS Appl. Mater. Interfaces 2013, 5, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.K.; Fragogeorgi, E.; Palamaris, L.; Karampelas, T.; Lelovas, P.; Loudos, G.; Tamvakopoulos, C.; Kostomitsopoulos, N.; Kordas, G. Versatile quarto stimuli nanostructure based on Trojan Horse approach for cancer therapy: Synthesis, characterization, in vitro and in vivo studies. Mater. Sci. Eng. C 2017, 79, 605–612. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Charitidis, C.A.; Kordas, G.C. Synthesis and characterization of ceramic hollow nanocomposites and nanotraps. Nanocomposites Mater. Manuf. Eng. 2013, 2, 1–31. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Tapeinos, C.; Tziveleka, L.A.; Boukos, N.; Kordas, G. PH- and thermo-responsive microcontainers as potential drug delivery systems: Morphological characteristic, release and cytotoxicity studies. Mater. Sci. Eng. C 2014, 37, 271–277. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Tapeinos, C.; Chatzipavlidis, A.; Boukos, N.; Fragogeorgi, E.; Palamaris, L.; Loudos, G.; Kordas, G. Dynamic in vivo imaging of dual-triggered microspheres for sustained release applications: Synthesis, characterization and cytotoxicity study. Int. J. Pharm. 2014, 461, 54–63. [Google Scholar] [CrossRef]
- Lelovas, P.; Efthimiadou, E.K.; Mantziaras, G.; Siskos, N.; Kordas, G.; Kostomitsopoulos, N. In vivo toxicity study of quatro stimuli nanocontainers in pregnant rats: Gestation, parturition and offspring evaluation. Regul. Toxicol. Pharmacol. 2018, 98, 161–167. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Xu, M.; Wu, C.; Miyoshi, H.; Liu, Y. Investigation of folate-conjugated fluorescent silica nanoparticles for targeting delivery to folate receptor-positive tumors and their internalization mechanism. Int. J. Nanomed. 2011, 6, 2023–2032. [Google Scholar] [CrossRef]
- Roger, E.; Kalscheuer, S.; Kirtane, A.; Guru, B.R.; Grill, A.E.; Whittum-Hudson, J.; Panyam, J. Folic acid functionalized nanoparticles for enhanced oral drug delivery. Mol. Pharm. 2012, 9, 2103–2110. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Lelovas, P.; Fragogeorgi, E.; Boukos, N.; Balafas, V.; Loudos, G.; Kostomitsopoulos, N.; Theodosiou, M.; Tziveleka, A.L.; Kordas, G. Folic acid mediated endocytosis enhanced by modified multi stimuli nanocontainers for cancer targeting and treatment: Synthesis, characterization, in-vitro and in-vivo evaluation of therapeutic efficacy. J. Drug Deliv. Sci. Technol. 2020, 55, 101481. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Oflmmunologicalmethods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadou, E.; Tziveleka, L.-A.; Bilalis, P.; Kordas, G. Novel PLA modification of organic microcontainers based on ring opening polymerization: Synthesis, characterization, biocompatibility and drug loading/release properties. Int. J. Pharm. 2012, 428, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kordas, G. Adjustable Quarto Stimuli (T, pH, Redox, Hyperthermia) Targeted Nanocontainers (Nano4Dox and Nano4Cis) for Cancer Therapy Based on Trojan Horse Approach. Arch. Pharm. Pharmacol. Res. 2018, 1, 1–7. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordas, G. All-Purpose Nano- and Microcontainers: A Review of the New Engineering Possibilities. Eng 2022, 3, 554-572. https://doi.org/10.3390/eng3040039
Kordas G. All-Purpose Nano- and Microcontainers: A Review of the New Engineering Possibilities. Eng. 2022; 3(4):554-572. https://doi.org/10.3390/eng3040039
Chicago/Turabian StyleKordas, George. 2022. "All-Purpose Nano- and Microcontainers: A Review of the New Engineering Possibilities" Eng 3, no. 4: 554-572. https://doi.org/10.3390/eng3040039
APA StyleKordas, G. (2022). All-Purpose Nano- and Microcontainers: A Review of the New Engineering Possibilities. Eng, 3(4), 554-572. https://doi.org/10.3390/eng3040039





