Synthesis of CuAl-, CoAl-, and CuCoAl-Catalysts from Layered Hydroxides for Furfural Hydrogenation
Abstract
1. Introduction
2. Materials and Methods
2.1. CuAl-, CoAl- and CuCoAl-LDHs Synthesis
2.2. Physicochemical Characterization of the Samples
2.3. Catalyst Testing
3. Results and Discussion
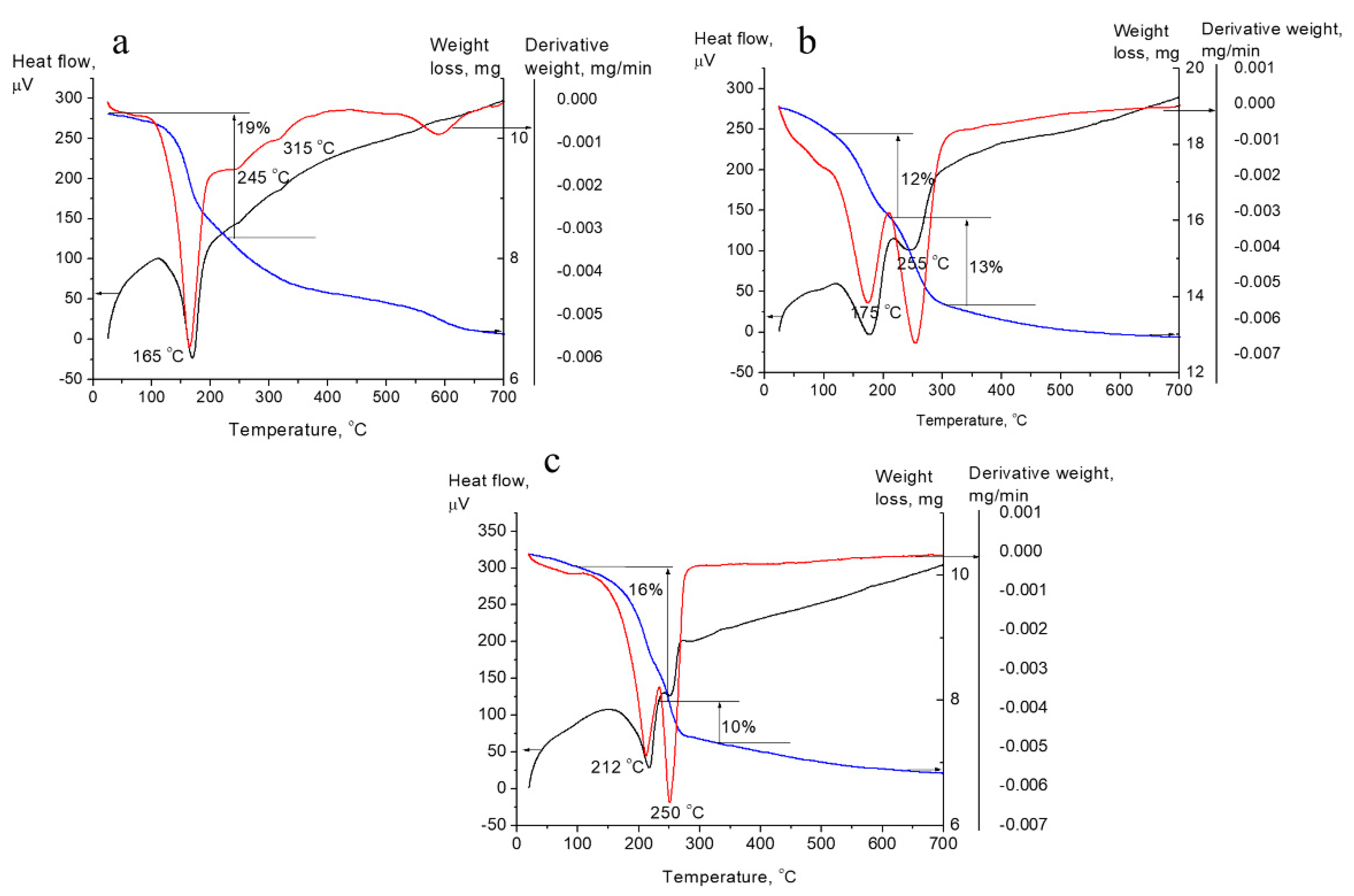
3.1. Effect of the Samples Composition on Their Structural Characteristics and Oxide Phase Formation
3.2. Study of the Surface Morphology of the Samples and Their Textural Characteristics
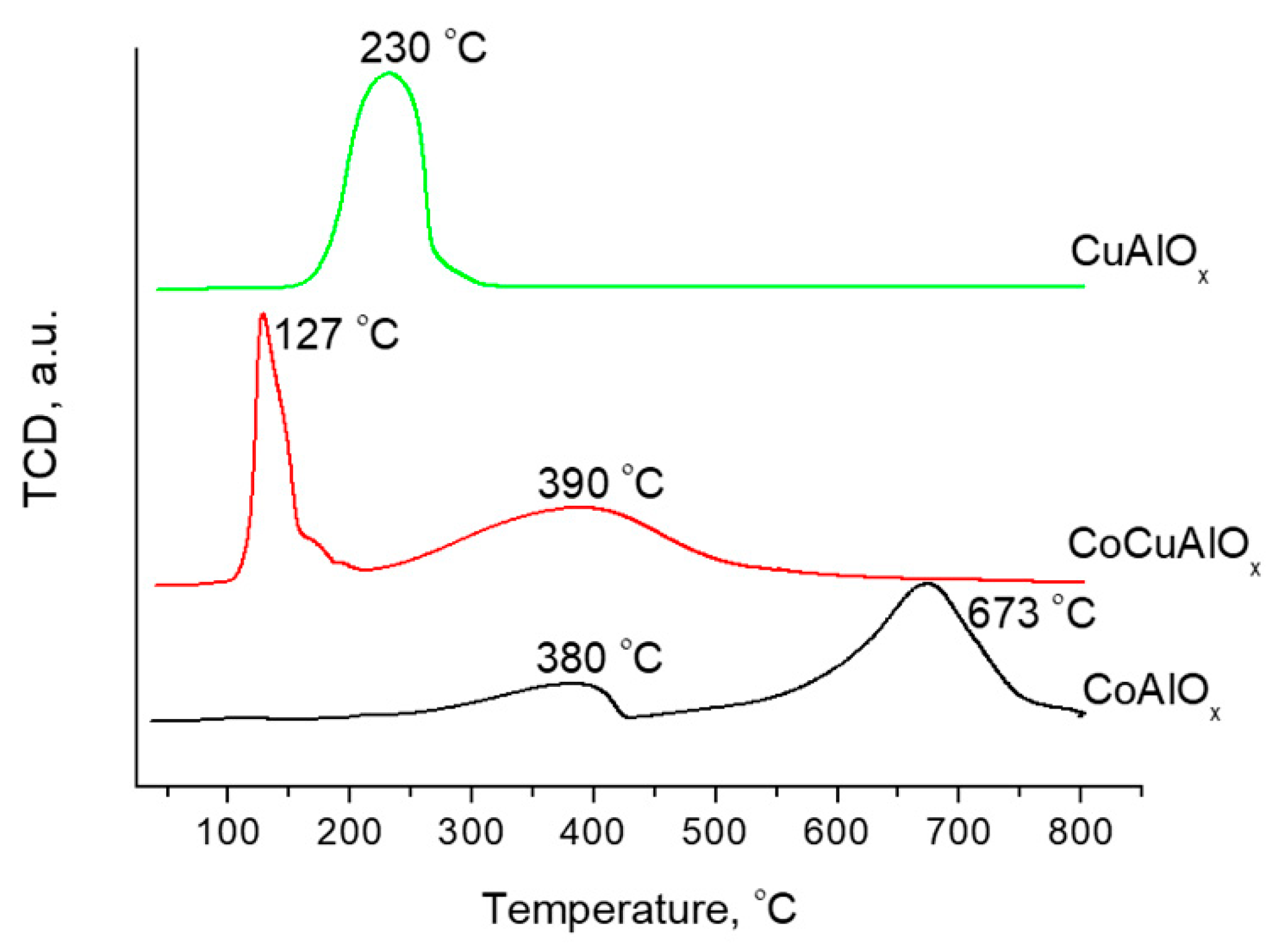
3.3. Study of Metal Reduction from Mixed Oxides by H2-TPR
3.4. Catalytic Properties of the Samples in FAL Hydrogenation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Balsamo, N.; Mendieta, S.; Oliva, M.; Eimer, G.; Crivello, M. Synthesis and characterization of metal mixed oxides from layered double hydroxides. Procedia Mater. Sci. 2012, 1, 506–513. [Google Scholar] [CrossRef][Green Version]
- Xu, M.; Wei, M. Layered double hydroxide-based catalysts: Recent advances in preparation, structure, and applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- He, S.; An, Z.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxide-based catalysts: Nanostructure design and catalytic performance. Chem. Commun. 2013, 49, 5912–5920. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent advances in catalytic hydrogenation of furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Vargas-Hernández, D.; Rubio-Caballero, J.M.; Santamaría-González, J.; Moreno-Tost, R.; Mérida-Robles, J.M.; Pérez-Cruz, M.A.; Jiménez-López, A.; Hernández-Huesca, R.; Maireles-Torres, P. Furfuryl alcohol from furfural hydrogenation over copper supported on SBA-15 silica catalysts. J. Mol. Catal. A Chem. 2014, 383–384, 106–113. [Google Scholar] [CrossRef]
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Catal. B 2016, 180, 580–585. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts. J. Catal. 2011, 277, 1–13. [Google Scholar] [CrossRef]
- Sato, S.; Igarashi, J.; Yamada, Y. Stable vapor-phase conversion of tetrahydrofurfuryl alcohol into 3, 4-2H-dihydropyran. Appl. Catal. A 2013, 453, 213–218. [Google Scholar] [CrossRef]
- Meng, X.; Yang, Y.; Chen, L.; Xu, M.; Zhang, X.; Wei, M. A Control over hydrogenation selectivity of furfural via tuning exposed facet of Ni catalysts. ACS Catal. 2019, 9, 4226–4235. [Google Scholar] [CrossRef]
- Shi, D.; Yang, Q.; Peterson, C.; Lamic-Humblot, A.-F.; Girardon, J.-S.; Griboval-Constant, A.; Stievano, L.; Sougrati, M.T.; Briois, V.; Bagot, P.A.J.; et al. Bimetallic Fe-Ni/SiO2 catalysts for furfural hydrogenation: Identification of the interplay between Fe and Ni during deposition-precipitation and thermal treatments. Catal. Today 2019, 334, 162–172. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Bertero, N.M.; Garetto, T.F.; Marchi, A.J. Selective liquid-phase hydrogenation of furfural to furfuryl alcohol over Cu-based catalysts. Catal. Today 2013, 213, 87–92. [Google Scholar] [CrossRef]
- Manikandan, M.; Venugopal, A.K.; Nagpure, A.S.; Chilukuri, S.; Raja, T. Promotional effect of Fe on the performance of supported Cu catalyst for ambient pressure hydrogenation of furfural. RSC Adv. 2016, 6, 3888–3898. [Google Scholar] [CrossRef]
- Seemala, B.; Cai, C.M.; Kumar, R.; Wyman, C.E.; Christopher, P. Effects of Cu–Ni bimetallic catalyst composition and support on activity, selectivity, and stability for furfural conversion to 2-methyfuran. ACS Sustain. Chem. Eng. 2018, 6, 2152–2161. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Li, S.; Gao, L.; Xiao, G. Metal-organic frameworks derived bimetallic Cu-Co catalyst for efficient and selective hydrogenation of biomass-derived furfural to furfuryl alcohol. Mol. Catal. 2017, 436, 128–137. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Du, H.; Sun, K.; Zhang, Z.; Zhang, L.; Li, Q.; Zhang, S.; Liu, Q.; Hu, X. Importance of magnesium in Cu-based catalysts for selective conversion of biomass-derived furan compounds to diols. ACS Sustain. Chem. Eng. 2020, 8, 5217–5228. [Google Scholar] [CrossRef]
- Prakruthi, H.R.; Chandrashekara, B.M.; Jai Prakash, B.S.; Bhat, Y.S. Hydrogenation efficiency of highly porous Cu-Al oxides derived from dealuminated LDH in the conversion of furfural to furfuryl alcohol. J. Ind. Eng. Chem. 2018, 62, 96–105. [Google Scholar] [CrossRef]
- Fu, X.; Ren, X.; Shen, J.; Jiang, Y.; Wang, Y.; Orooji, Y.; Xu, W.; Liang, J. Synergistic catalytic hydrogenation of furfural to 1,2-pentanediol and 1,5-pentanediol with LDO derived from CuMgAl hydrotalcite. Mol. Catal. 2021, 499, 111298. [Google Scholar] [CrossRef]
- Rives, V.; Dubey, A.; Kannan, S. Synthesis, characterization and catalytic hydroxylation of phenol over CuCoAl ternary hydrotalcites. Phys. Chem. Chem. Phys. 2001, 3, 4826–4836. [Google Scholar] [CrossRef]
- Sun, K.; Gao, X.; Bai, Y.; Tan, M.; Yanga, G.; Tan, Y. Synergetic catalysis of bimetallic copper–cobalt nanosheets for direct synthesis of ethanol and higher alcohols from syngas. Catal. Sci. Technol. 2018, 8, 3936–3947. [Google Scholar] [CrossRef]
- Sulmonetti, T.; Hu, B.; Lee, S.; Agrawal, P.K.; Jones, C.W. Reduced Cu-Co-Al mixed metal oxides for the ring-opening of furfuryl alcohol to produce renewable diols. ACS Sustain. Chem. Eng. 2017, 5, 8959–8969. [Google Scholar] [CrossRef]
- Pan, K.; Yu, F.; Liu, Z.; Zhou, X.; Sun, R.; Li, W.; Zhao, H.; Liu, M.; Guo, X.; Dai, B. Enhanced low-temperature CO-SCR denitration performance and mechanism of two-dimensional CuCoAl layered double oxide. J. Environ. Chem. Eng. 2022, 10, 108030. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Sharma, A.; Srinivasan, K. CoCuAl layered double hydroxides—Efficient solid catalysts for the preparation of industrially important fatty epoxides. Catal. Sci. Technol. 2015, 5, 1187–1197. [Google Scholar] [CrossRef]
- Akil, J.; Ciotonea, C.; Siffert, S.; Royer, S.; Pirault-Roy, L.; Cousin, R.; Poupin, C. NO reduction by CO under oxidative conditions over CoCuAl mixed oxides derived from hydrotalcite-like compounds: Effect of water. Catal. Today 2022, 384–386, 97–105. [Google Scholar] [CrossRef]
- Miata, S. The syntheses of hydrotalcite-like compounds and their structures and physicochemical properties. I: The systems Mg2+-Al3+-NO3−, Mg2+-Al3+-Cl−, Ni2+-Al3+-Cl−, Zn2+-Al3+-Cl−. Clays Clay Miner. 1975, 23, 363–375. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Rives, V. Comment on “Direct observation of a metastable solid phase of Mg/Al/CO3-layered double hydroxide by means of high-temperature in situ powder XRD and DTA/TG”. Inorg. Chem. 1999, 38, 406–407. [Google Scholar] [CrossRef]
- Ribet, S.; Tichit, D.; Coq, B.; Ducourant, B.; Morato, F. Synthesis and activation of Co–Mg–Al layered double hydroxides. J. Solid State Chem. 1999, 142, 382–392. [Google Scholar] [CrossRef]


| Sample | d003, Å | c, Å | a, Å | Lc, Å | La, Å |
|---|---|---|---|---|---|
| CuAl | 7.56 | 22.673 | 3.095 | 351 | 199 |
| CuCoAl | 7.60 | 22.802 | 3.081 | 81 | 195 |
| CoAl | 7.59 | 22.779 | 3.068 | 189 | 267 |
| Sample | M2+/Al, at. % | Co/Cu, at. % | Ssp, m2 g−1 |
|---|---|---|---|
| CuAl | 1.4–2.5 | - | - |
| CuAlOx | 1.9–2.3 | - | 51 |
| CuAl-Red | 2.1–3.1 | - | 26 |
| CuCoAl | 3.1–3.8 | 3.1–3.6 | - |
| CuCoAlOx | 3.1–5.9 | 2.0–3.3 | 65 |
| CuCoAl-Red | 5.3–7.5 | 3.6–3.9 | 37 |
| CoAl | 1.8–2.7 | - | - |
| CoAlOx | 1.7–2.2 | - | 85 |
| CoAl-Red | 1.7–1.8 | - | 60 |
| Catalyst a | Solvent in FAL Hydrogenation b | Reaction Time, h | FAL Conversion, mol.% c | Yield of FOL, mol.% c | Selectivity to FOL, mol.% c |
|---|---|---|---|---|---|
| CuAlOx d | H2O | 1 | 1 | <1 | 44 |
| CoAlOx e | H2O | 1 | 89 | 87 | 98 |
| CoAlOx e | H2O | 5 | >99 | 96 | 96 |
| CuCoAlOx f | H2O | 1 | 32 | 32 | >99 |
| CuCoAlOx f | H2O | 5 | 81 | 81 | >99 |
| CuAlOx d | EtOH | 1 | 3 | <1 | <1 |
| CoAlOx e | EtOH | 1 | 6 | 4 | 67 |
| CoAlOx e | EtOH | 5 | 97 | 94 | 97 |
| CuCoAlOx f | EtOH | 1 | 19 | 16 | 84 |
| CuCoAlOx f | EtOH | 5 | >99 | 98 | 98 |
| Entry | Pretreatment Conditions | FAL Conversion, mol.% a | Yield of FOL, mol.% a | Selectivity to FOL, mol.% a |
|---|---|---|---|---|
| 1 | Calcination at 550 °C in air, reduction at 800 °C in H2 | 32 | 32 | >99 |
| 2 | Calcination at 550 °C in air, reduction at 500 °C in H2 | 27 | 26 | 96 |
| 3 | Calcination at 550 °C in air, reduction at 500 °C in H2 | 37 b | 31 b | 84 b |
| 4 | Reduction at 500 °C in H2, without calcination | 21 | 20 | 95 |
| 5 | Reduction at 800 °C in H2, without calcination | 17 | 17 | >99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, L.N.; Mironenko, R.M.; Kobzar, E.O.; Leont’eva, N.N.; Gulyaeva, T.I.; Vasilevich, A.V.; Serkova, A.N.; Salanov, A.N.; Lavrenov, A.V. Synthesis of CuAl-, CoAl-, and CuCoAl-Catalysts from Layered Hydroxides for Furfural Hydrogenation. Eng 2022, 3, 400-411. https://doi.org/10.3390/eng3040029
Stepanova LN, Mironenko RM, Kobzar EO, Leont’eva NN, Gulyaeva TI, Vasilevich AV, Serkova AN, Salanov AN, Lavrenov AV. Synthesis of CuAl-, CoAl-, and CuCoAl-Catalysts from Layered Hydroxides for Furfural Hydrogenation. Eng. 2022; 3(4):400-411. https://doi.org/10.3390/eng3040029
Chicago/Turabian StyleStepanova, Liudmila N., Roman M. Mironenko, Elena O. Kobzar, Natalia N. Leont’eva, Tatiana I. Gulyaeva, Anastasia V. Vasilevich, Aleksandra N. Serkova, Aleksei N. Salanov, and Aleksandr V. Lavrenov. 2022. "Synthesis of CuAl-, CoAl-, and CuCoAl-Catalysts from Layered Hydroxides for Furfural Hydrogenation" Eng 3, no. 4: 400-411. https://doi.org/10.3390/eng3040029
APA StyleStepanova, L. N., Mironenko, R. M., Kobzar, E. O., Leont’eva, N. N., Gulyaeva, T. I., Vasilevich, A. V., Serkova, A. N., Salanov, A. N., & Lavrenov, A. V. (2022). Synthesis of CuAl-, CoAl-, and CuCoAl-Catalysts from Layered Hydroxides for Furfural Hydrogenation. Eng, 3(4), 400-411. https://doi.org/10.3390/eng3040029








