Abstract
Among emerging pollutants, endocrine disruptors such as estradiol are of most concern. Conventional water treatment technologies are not capable of removing this compound from water. This study aims to assess a method that combines physicochemical and biological strategies to eliminate estradiol even when there are other compounds present in the water matrix. Na-montmorillonite, Ca-montmorillonite and zeolite were used to remove estradiol in a medium with sulfamethoxazole, triclosan, and nicotine using a Plackett–Burman experimental design; each treatment was followed by biological filtration with Daphnia magna. Results showed between 40 to 92% estradiol adsorption in clays; no other compounds present in the mixture were adsorbed. The most significant factors for estradiol adsorption were the presence of nicotine and triclosan which favored the adsorption, the use of Ca-montmorillonite, Zeolite, and time did not favor the adsorption of estradiol. After the physicochemical treatment, Daphnia magna was able to remove between 0–93% of the remaining estradiol. The combination of adsorption and biological filtration in optimal conditions allowed the removal of 98% of the initial estradiol concentration.
1. Introduction
Currently, there is a growing interest in the so-called emerging contaminants (ECs), compounds of different origins and chemical nature whose presence in the environment is not considered significant in terms of distribution and/or concentration [1]. ECs include drugs and personal care products, surfactants, flame retardants, industrial and food additives, steroids, hormones, bactericides, illicit drugs, compounds such as caffeine and nicotine, and disinfection by-products [2,3]. ECs enter the environment through anthropogenic contamination. For instance, human beings consume large amounts of pharmaceutical and personal care products generating waste that often ends up in wastewater. Different compounds have been detected in municipal and natural water systems which are poured into through residential or commercial discharges [4]. For example, pharmaceutical and personal hygiene products generally present in human and animal excretions enter the environment through domestic wastewater via discharge from toilets, domestic water, among other sources [5]. Pharmaceutical wastewater is usually recalcitrant with high chemical oxygen demand (COD), high biological toxicity, low biodegradability, intense color, and unpleasant odor. It contains high concentrations of solvents, catalysts, additives, and reagents, especially antibiotics [6]. Wastewaters containing these compounds are discharged directly into the environment or are treated in wastewater treatment plants where ECs are not effectively eliminated due to their low concentrations and complexity [1,7,8]. One of the characteristics of these pollutants is that they do not have to be persistent in the environment to cause negative effects since their high transformation and elimination rates can be offset by their continuous introduction into the environment [9]. Generally, they are characterized by the following properties: high chemical stability, low biodegradability, high solubility in water, and low adsorption coefficient [10]. The emerging pollutants that cause the most concern are antibiotics and endocrine disruptors. Antibiotics such as sulfamethoxazole can induce bacterial resistance, even at low concentrations, through continuous exposure [11]. Endocrine disruptors such as estradiol and triclosan interfere with the endocrine system and disrupt the physiological function of hormones by mimicking, blocking, or disrupting their function thus affecting the health of humans and animal species [12,13,14,15,16]. Nicotine, sulfamethoxazole, triclosan and estradiol have been found in superficial water bodies such as rivers, lakes, seas, and oceans [1,17,18,19,20,21,22] and even in drinking water. Hence pointing out that the current treatment methods are not effective to eliminate these compounds [17,18,19,20,21,22,23,24,25,26].
Recently, the ability of some techniques to remove ECs from water, such as advanced oxidation and adsorption has been discussed. For instance, estrogens removal rates vary between 100 to 10 in time periods that oscillate between 7 to 300 min with electrochemical advanced oxidation treatment [27]; however, these processes present a high cost and difficulty of implementation [1]. On the other hand, adsorption methods have the disadvantage that they only generate a phase transfer. Nevertheless, they are still a valid option for removing ECs from water due to their simplicity and low cost. Among the different sorbents used for this purpose, clay minerals, such as bentonite and zeolite, have shown effective results as adsorbent and ion exchange media for water and wastewater treatment applications, especially for removing heavy metals, organic pollutants, and nutrients [28,29,30]. Bentonite and/or zeolite have been used to remove organic pollutants such as dyes, hormones, pharmaceuticals, caffeine, and other ECs [31,32,33,34,35,36]; the rate of adsorption in these materials depends on factors such as temperature and pH, as well as the chemical nature of the retained compound. The adsorption of estradiol in clay minerals is an exothermic and spontaneous process; levels of pH higher to 10 decrease the adsorption capability [37]. In contrast, nicotine adsorption is a spontaneous process, endothermic or exothermic depending on the adsorbent; nicotine is an electron donor due to the aliphatic nitrogen of the pyrrolidine ring. Moreover, pH determines the adsorption mechanism such as hydrogen bonds, π–π interaction, cation-π bonding, Van der Waals forces, inner-sphere complex formation, and electrostatic interactions [38].
Biologically-based filtration using aquatic invertebrates such as Daphnia magna, which live in biological-based wastewater treatment plants, has shown promising results to remove ECs [39], consequently becoming an optimal complement to adsorption processes. Besides, one of the main advantages of D. magna is their ability to reduce the biochemical oxygen demand in wastewater [40]. D. magna under direct solar radiation also could remove 80% of ECs combining biodegradation, photodegradation, adsorption, and adsorption processes [41]. Nonetheless, many of the evaluated studies do not consider how the presence of other contaminants affect the ECs removal performance and adsorption efficiency; for instance, studies used individual pollutants such as 17β-Estradiol, dyes, and phenol as model compounds for the adsorption studies [31,33,34,35,36].
The aim of this study is to investigate the removal of estradiol from wastewater using a combination of adsorption with Na-montmorillonite, Ca-montmorillonite, Zeolite, and biologically-based filtration with Daphnia magna. We employ an experimental design to assess the influence of adsorbent type and other EC’s presence in estradiol’s removal rate; nicotine, triclosan, and sulfamethoxazole were used for this purpose, as they are commonly found in water bodies along with estradiol.
2. Materials and Methods
2.1. Chemicals
Synthetic waters spiked with emerging contaminants were prepared for this study using Sigma Aldrich technical grade (99% purity) nicotine, triclosan, sulfamethoxazole, and estradiol. Table 1 reports the structures and properties of these four molecules [32,42,43,44]. For the mobile phase, high-performance liquid chromatography (HPLC) grade methanol was purchased from Merck, and Type 1 water (from a Milli-Q system, Merck Millipore) was used.

Table 1.
Emerging contaminants targeted in the study.
2.2. Bentonites and Zeolites
Ecuadorian natural Na-montmorillonite, Ca-montmorillonite and zeolites processed by the company Minmetec Ecuador Cia. Ltda. were used. The zeolite is a clinoptilolite, (Ca)3(Si30Al6)O72·H2O, belonging to the heulandite group. Na-montmorillonite, (Na1+)0.33(Al,Mg)2(Si4O10)(OH)2·n(H2O), is a smectite clay consisting mainly of montmorillonite, magnesium silicate, hydrated aluminum, and sodium. Ca-montmorillonite, (Ca2+)0.33(Al,Mg)2(Si4O10)(OH)2·n(H2O), is a smectite clay, consisting mainly of montmorillonite. Physicochemical characteristics are shown in Table 2 and chemical structure is presented in Figure 1.

Table 2.
Zeolite and montmorillonite composition and characteristics.
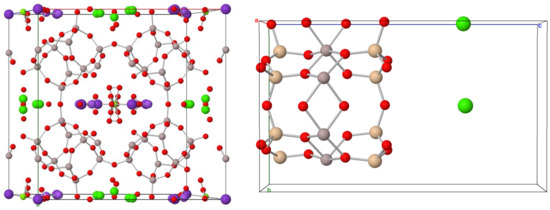
Figure 1.
In the left Zeolite Clinoptilolite and in the right montmorillonite taken from mindat.org. Red ball: Oxygen, Light brown: Silica, Gray: Aluminum, Purple: Sodium or Potassium, Green: Sodium or Calcium.
2.3. Daphnia Magna Culture
The cultivation of D. magna was conducted according to Organization of Economic Co-operation and Development (OECD) 1981 standardized protocols. The photoperiod was set to a 14 h light:10 h dark cycle and the temperature was set at 20 ± 1 °C. Cultures were maintained in 100 mL of ASTM hard synthetic water and fed every three days with spirulina algae.
2.4. Experimental Design
2.4.1. Zeolites and Bentonites Adsorption
Preliminary adsorption tests were performed to determine whether the clays could adsorb estradiol. Once the effectiveness of the materials to adsorb estradiol was verified, a Plackett–Burman experimental design was conducted; eight variables were selected to take into account: the concentration of estradiol, nicotine, sulfamethoxazole and triclosan, the mass of zeolite, Na-montmorillonite, Ca-montmorillonite, and the exposure time. For the assays, a stock solution of 100 of each of four compounds was prepared in a hydroalcoholic solution. Then, 15 mixtures of emerging contaminants were prepared with the concentrations shown in Table 3 and following the scheme depicted in Table 4. The aqueous mixtures were prepared with tap water up to a volume of 500 mL.

Table 3.
Variables and levels of the applied experimental design.

Table 4.
Variable levels for each trial as applied in the Plackett–Burman design.
All the experiments were performed in fluid bed batch reactors. The concentration measurements of the four studied ECs were made by HPLC-DAD to assess the efficiency of the suggested removal method before and after the use of the clay and the D. magna treatments. In the end of the experiment, samples were centrifuged and filtered before the HPLC analysis (Section 2.5). The existing difference of the same sample, expressed as a percentage, was used as the response variable of the experimental design. The software used for the data analysis was MINITAB 17.
2.4.2. Daphnia magna Adsorption
Approximately 50 D. magna individuals were kept in an aliquot of 100 mL of water samples for 8 days; all assays were performed in three replicates. The experimental setup was kept at room temperature, 20 °C at 12 h of light and 12 h of darkness. D. magna were fed with 1 mL of a mixture of 12 mg of yeast and 30 mg of spirulina every 2 days. After the adsorption experiments, aliquots of 100 mL of water samples were centrifuged (Eppendorf Centrifuge 5702R) at 4000 rpm for 15 min. The supernatant was filtered using 0.45 μm PVDF filters. Then, the estradiol concentration was determined by liquid chromatography to calculate the removal percentage achieved.
2.5. HPLC Analysis
RP-HPLC analyses were performed using means of an HPLC-DAD instrument (Thermo Scientific UltiMate 3000, USA). An isocratic elution program was used with a mixture of 60:40 methanol:water v/v as mobile phase, and a C18 column as stationary phase. The flow rate was 0.6 mLs−1,while the column was thermostated at 25 °C. The injection volume was 5 μL for all standards and samples. Compound elution was detected at 220 nm. Under these conditions, estradiol retention time was 6.62 min. The LOD and LOQ were respectively: estradiol: 0.540 μgL−1 and 1.042 μgL−1; nicotine: 11.55 μgL−1 and 22.29 μgL−1; triclosan: 0.1645 μgL−1 and 0.603 μgL−1; sulfamethoxazole 4.89 μgL−1, and 9. 44 μgL−1.
2.6. Isoterm
Adsorption isotherms are used to evaluate the equilibrium of particles in a system with a liquid and a solid phase, at a constant temperature. For the development of this study, Langmuir and Freundlich models were used to calculate the adsorption isotherms of estradiol on the different clays used [48,49]. The Langmuir isotherm was developed from the assumption that adsorbate molecules form a monolayer on the surface of the adsorbent. Considering that the adsorbed molecules do not interact with each other, it is assumed that the adsorption of adsorbate at a specific site is independent of what happens with neighboring sites. The Langmuir isotherm is represented by the Equation (1) where Ce is the adsorbate concentration at equilibrium, qe is the amount of adsorbate per unit mass of adsorbent at equilibrium, qm is the maximum amount of adsorbate adsorbed per unit mass of adsorbent for the formation of the complete monolayer on the surface of the adsorbent, KL is the Langmuir constant related to the adsorption energy [50,51]. The Freundlich isotherm, Equation (2), is applied when adsorption processes occur on heterogeneous surfaces, thus being a model that can explain both monolayer and multilayer adsorption. The expression resulting from this isotherm defines the heterogeneity of the surface and how the active sites and their energies are exponentially distributed [52,53]. KF is the Freundlich constant that is related to the adsorption capacity; n represents the heterogeneity factor and 1/n is related to the adsorption intensity.
The parameters of the isotherms were obtained from measuring the estradiol concentration in the supernatant of a suspension of 50 mL of estradiol solutions at different concentrations, in contact with 100 mg of each of the clays. It was left overnight at a constant temperature of 30 °C. The concentration of the estradiol standards used were: 0.5, 1.0, 2.5, 3, 3.5, 4, 4.5, 5, 10, 15, and 20 . Absorbance was measured using a UV-Vis Spectrophotometer (Thermo Scientific, Evolution 60) at a wavelength of 280 nm. Measurements were made in duplicate.
3. Results and Discussion
The results of the set of experiments are presented in Table 5. In the adsorption tests with clays, the removal of estradiol was quantified, the removal percentage was from 40 to 92% (0.1 –0.92 , respectively). Nicotine, sulfamethoxazole, and triclosan were not quantified in this study. Other studies show that these compounds are poorly or not removed with natural clays and a pre-treatment of clay is necessary to achieve the adsorption [54,55,56]. Considering the soil adsorption coefficient (Koc) which measures the amount of chemical substance adsorbed onto soil per amount of water, estradiol is prone to be adsorbed in clays.

Table 5.
Amount and percentage of estradiol removal obtained in each applied treatment.
The experiments with natural clays showed the lowest removal with trials 1, 8, 9, 10, and 12 which achieved a removal of estradiol of 0.10, 0.09, 0.13, 0.11, and 0.14 respectively. All these assays were performed with nicotine concentration in the lowest level (0.25 ). On the other hand, the experiments with natural clays where greater estradiol removal was achieved were trials number 2, 3, 5, 7, and 11, corresponding to 0.92, 0.76, 0.71, 0.85, and 0.69 , respectively. In these cases, all the experiments contain the maximum amount of nicotine.
The results of the analysis of the experimental design in clays are presented in Figure 2. The significant effects are shown in a red square, then, the significant factors in the experimental design with a confidence level of 95% are nicotine and triclosan as a positive influence in the estradiol adsorption, and Na-montmorillonite, Zeolite, and time with negative influence in the estradiol adsorption. In the Pareto chart, the five factors are presented where nicotine is the most important factor. The level of significance of each factor can be seen in Table 6.
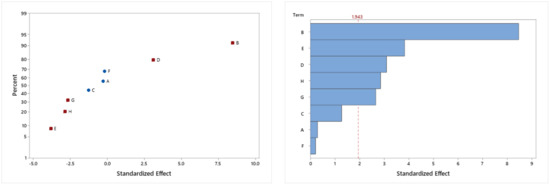
Figure 2.
Standardized effect in a normal graph and a Pareto chart of the factors in estradiol removal. A: estradiol, B: nicotine, C: sulfamethoxazole, D: triclosan, E: Na-montmorillonite, F: Ca-montmorillonite, G: Zeolite, and H: Time.

Table 6.
ANOVA of the experimental design performed to assess estradiol removal with clays.
The model obtained is statistically significant (p(0.02) < 0.05, Table 6) and this is presented in Equation (3). The effects of each factor are presented in Figure 3. Factors that reduced the adsorption of estradiol were sulfamethoxazole, Ca-montmorillonite, zeolite, and long exposure. In contrast, the nicotine and triclosan presence increases the adsorption of estradiol.
% Estradiol removal = 62.4 − 0.5∗Estradiol + 13.1∗Nicotine − 2∗Sulfamethoxazole + 4.8∗Triclosan − 5.9∗Na-montmorillonite − 0.3∗Ca-montmorillonite − 4.1∗Zeolite − 4.4∗Time
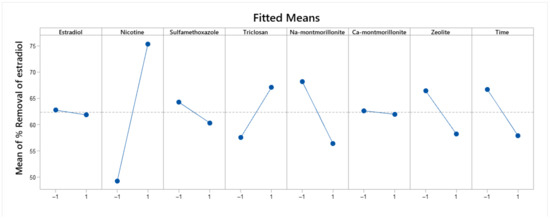
Figure 3.
Fitted means effect of each factor involved in estradiol removal.
The fitted means effects graph (Figure 3) reveals that the initial concentration of estradiol does not make a difference in terms of the final percentage of estradiol removal. Although a lower concentration slightly improves the removal efficiency, it is not statistically significant. It was expected that a higher concentration drives removal, given that the adsorption kinetics for this compound corresponds to a second-order reaction [57]. This result is explained by the short range and low concentrations used in this study (1 to 0.25 ), which were selected to be similar to those amounts found in the environment [1]. Furthermore, shorter elapsed time favors adsorption; this result is consistent with a pseudo second order kinetics, meaning that the adsorption occurs early while the concentration is high enough but when concentration decreases, adsorption decreases too, and equilibrium is achieved. Similar results were found in other studies [58,59].
The results show that nicotine and triclosan are statistically significant to promote the estradiol adsorption; possibly, they stimulate the estradiol removal due to the formation of a complex between the three compounds. Estradiol can act as a two hydrogen bond donor and two hydrogen bond acceptor. Triclosan is a hydrogen bond donor and two hydrogen bond acceptor. Nicotine is an acceptor of two hydrogen bonds [42]. These characteristics for forming bonds favor the formation of complexes between the three compounds. Nicotine and triclosan, which are located in the end of estradiol, form hydrogen bonds with their OH groups [60,61,62]. The formation of the complex does not affect its binding with the adsorbent, since Van der Waals forces are the main interaction with the adsorbent. Van der Waals bonds increase as the length of the nonpolar part of the complex increases [63,64]. In turn, this ability to form a complex can facilitate multilayer adsorption.
Figure 4 shows the adsorption isotherms of estradiol on the three clays used in this study. Langmuir’s model was used to describe the adsorption equilibrium, hypothesizing the existence of a monolayer adsorption, and the linearized form of the Langmuir model was used to obtain the parameters of the model. The Freundlich model was calculated to describe the adsorption effect of the multilayer, for which the linearized form of the model was used. The Freundlich model parameters were optimized using the algorithm Generalized Reduced Gradient (GRG) Nonlinear. Parameters of the Langmuir and Freundlich models are presented in Table 7. Although the R2 values of the linearized Langmuir models are higher than the optimized Freundlich models, a strong leverage effect can be observed in the upper part of the curve, so the Langmuir model overestimates the effect of the monolayer. On the contrary, the optimized Freundlich model has a greater similarity with the curve described by the experimental data.
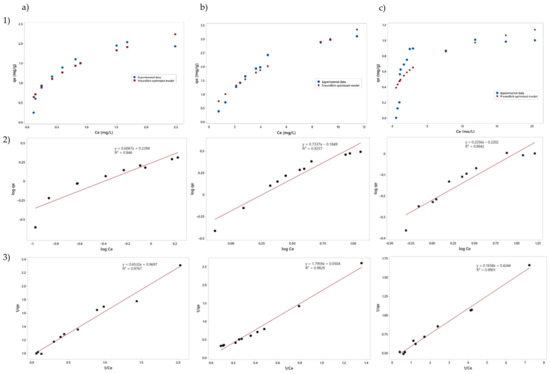
Figure 4.
Adsorption isotherm for (a) Na-montmorillonite, (b) Ca-montmorillonite, and (c) Zeolite; (1) Experimental data, (2) the Freundlich adsorption isotherms, and (3) the Langmuir adsorption isotherms. qe: number of milligrams of adsorbate that is adsorbed per gram of adsorbent; Ce: concentration of adsorbate in solution when equilibrium has been reached.

Table 7.
Langmuir and Freundlich isotherm models parameters for clays.
The results show that natural bentonites montmorillonites and zeolites effectively removed estradiol despite the presence of other contaminants. Nevertheless, the combination of the clays did not represent an improvement in the removal system. The clays for this study did not receive any previous treatment since the intention is to evaluate their adsorbent capacity under natural conditions for their use in wastewater treatments. The value of the constant KL of the Langmuir model shows that Ca-montmorillonite has a higher binding force for estradiol than the other two clays. In addition, for this reason, the value of qmax is the highest of all. In the case of the Freundlich model, Ca-montmorillonite has a value of n, lower than the other two clays, so it should have a higher intensity of adsorption, saturating more slowly. When comparing the results obtained from the clay isotherms and the removal results from the experimental design, the interaction between different molecules changes their ability to be adsorbed. In this specific case, estradiol would form a complex with triclosan and nicotine, and its preference for Ca-montmorillonite changes to the point that Ca-montmorillonite does not have a significant effect on the experimental design. This could be due to the tendency of the complex to be adsorbed, not in a monolayer, but in a multilayer, and the amount of Ca-montmorillonite is sufficient for this purpose. According to Figure 2, a graph of standardized effects, it can be observed that all clays are aligned with the non-significant variables, thus it can be considered that the effect of the three clays on estradiol removal was non-significant. Consequently, nicotine and triclosan would be the variables influencing estradiol removal in this system.
Regarding the use of the Daphnia magna as a natural filter, the estradiol removal ranges between 0–93%. The trials where low estradiol concentration was removed were number 2 and 8 (Table 5) corresponding to 0 in both cases; their common characteristic is the presence of triclosan concentration (1 ). This result is explained since triclosan can bioaccumulate via the food chain causing adverse effects depending on the concentration [63], so it might interfere with the ability of D. magna to metabolize or adsorb estradiol. The maximum removal of estradiol was obtained in trials 3, 6, and 11 corresponding to 0.22, 0.35, and 0.27 . In all these trials, nicotine was in the maximum concentration, whereas triclosan was in the minimum concentration. The most feasible route for D. magna to remove these pollutants is biosorption and, secondly, ingestion; in both cases, the compounds/metabolites are subsequently eliminated from the D. magna through their excretions, growth, breeding, and desorption [65,66,67]. The formation of the complex not only improved the adsorption of zeolites or montmorillonites, but also favored Daphnia magna, since their mortality does not occur at the estradiol and nicotine concentrations studied.
Overall, the best conditions for estradiol removal were those in trials 3, 4, 11, 13, and 14 where 0.93, 0.98, 0.96, 0.48, and 0.48 were eliminated, respectively. Due to the kinetics of the system, the tests with the least amount of estradiol also showed the lowest removal. The environmental concentration of estradiol in surface waters is in the range of nanograms per liter, the results of this study show the feasible use of the proposed combination of physicochemical and biological treatments for removing estradiol of water. However, more studies should be done to evaluate the interaction with other ECs.
Author Contributions
Conceptualization, P.T., V.P.-V. and A.A.; methodology, A.P.-G. and V.P.-V.; software, P.T.; validation, E.J.-N. and I.C.-A.; investigation, A.P.-G. and V.P.-V.; writing—original draft preparation, V.P.-V., A.P.-G., E.J.-N. and I.C.-A.; writing—review and editing, M.C., V.P.-V., A.P.-G., I.C.-A., E.J.-N. and J.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CEDIA, grant number CEPRA-XIV-2020-09.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank the Corporación Ecuatoriana para el Desarrollo de la Investigación y Academia—CEDIA for their contribution in innovation, through the CEPRA projects, especially the project CEPRA-XIV-2020-09 “Determinación del impacto y ocurrencia de Contaminantes Emergentes en ríos de la Costa Ecuatoriana y propuestas de tratamiento para su remoción”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vélez, V.P.P.; Esquivel-Hernández, G.; Cipriani-Avila, I.; Mora-Abril, E.; Cisneros, J.F.; Alvarado, A.; Abril-Ulloa, V. Emerging contaminants in trans-American waters. Ambient. Agua Interdiscip. J. Appl. Sci. 2019, 14, 1–26. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Cipriani-Avila, I.; Jara-Negrete, E.; Acosta-López, S.; Acosta, B.; Pérez-González, A.; Molinero, J.; Pinos-Vélez, V. Emerging contaminants in the northeast Andean foothills of Amazonia: The case of study of the city of Tena, Napo, Ecuador. Bull. Environ. Contam. Toxicol. 2021, 1–9. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2011, 83, 4614–4648. [Google Scholar] [CrossRef] [PubMed]
- Abu Hasan, H.; Abdullah, S.R.S.; Alattabi, A.W.; Nash, D.A.H.; Anuar, N.; Rahman, N.A.; Titah, H.S. Removal of ibuprofen, ketoprofen, COD and nitrogen compounds from pharmaceutical wastewater using aerobic suspension-sequencing batch reactor (ASSBR). Sep. Purif. Technol. 2016, 157, 215–221. [Google Scholar] [CrossRef]
- Bell, K.Y.; Wells, M.J.; Traexler, K.A.; Pellegrin, M.-L.; Morse, A.; Bandy, J. Emerging pollutants. Water Environ. Res. 2011, 83, 1906–1984. [Google Scholar] [CrossRef]
- Huang, B.; Wang, H.-C.; Cui, D.; Zhang, B.; Chen, Z.-B.; Wang, A.-J. Treatment of pharmaceutical wastewater containing β-lactams antibiotics by a pilot-scale anaerobic membrane bioreactor (AnMBR). Chem. Eng. J. 2018, 341, 238–247. [Google Scholar] [CrossRef]
- Gil, M.; Soto, A.; Usma, J.; Gutierrez, O. Contaminantes emergentes en aguas, efectos y posibles tratamientos. Prod. Limpia 2012, 7, 52–73. [Google Scholar]
- Meffe, R.; de Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Becerril, J. Contaminantes emergentes en el agua. Rev. Digit. Univ. 2009, 10, 1067–6079. [Google Scholar]
- Zwiener, C. Occurrence and analysis of pharmaceuticals and their transformation products in drinking water treatment. Anal. Bioanal. Chem. 2006, 387, 1159–1162. [Google Scholar] [CrossRef]
- Hernandez, F.; Sancho, J.V.; Ibañez, M.; Guerrero, C. Antibiotic residue determination in environmental waters by LC-MS. TrAC Trends Anal. Chem. 2007, 26, 466–485. [Google Scholar] [CrossRef]
- Aufartová, J.; Mahugo-Santana, C.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Nováková, L.; Solich, P. Determination of steroid hormones in biological and environmental samples using green microextraction techniques: An overview. Anal. Chim. Acta 2011, 704, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Arriaga, E.B.; Cortés-Muñoz, J.E.; González-Herrera, A.; Calderón-Mólgora, C.G.; Rivera-Huerta, M.D.L.; Ramírez-Camperos, E.; Montellano-Palacios, L.; Gelover-Santiago, S.L.; Pérez-Castrejón, S.; Cardoso-Vigueros, L.; et al. Assessment of full-scale biological nutrient removal systems upgraded with physico-chemical processes for the removal of emerging pollutants present in wastewaters from Mexico. Sci. Total Environ. 2016, 571, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Fast liquid chromatography—Quadrupole-linear ion trap mass spectrometry for the analysis of pharmaceuticals and hormones in water resources. J. Chromatogr. A 2010, 1217, 4212–4222. [Google Scholar] [CrossRef]
- Niemuth, N.J.; Klaper, R.D. Emerging wastewater contaminant metformin causes intersex and reduced fecundity in fish. Chemosphere 2015, 135, 38–45. [Google Scholar] [CrossRef]
- Siddique, S.; Kubwabo, C.; Harris, S. A review of the role of emerging environmental contaminants in the development of breast cancer in women. Emerg. Contam. 2016, 2, 204–219. [Google Scholar] [CrossRef]
- Campanha, M.B.; Awan, A.; de Sousa, D.N.R.; Grosseli, G.M.; Mozeto, A.A.; Fadini, P.S. A 3-year study on occurrence of emerging contaminants in an urban stream of São Paulo State of Southeast Brazil. Environ. Sci. Pollut. Res. 2015, 22, 7936–7947. [Google Scholar] [CrossRef]
- Fossi, M.C.; Baini, M.; Panti, C.; Galli, M.; Jiménez, B.; Muñoz-Arnanz, J.; Marsili, L.; Finoia, M.G.; Ramírez-Macías, D. Are whale sharks exposed to persistent organic pollutants and plastic pollution in the Gulf of California (Mexico)? First ecotoxicological investigation using skin biopsies. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 199, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Combi, T.; Pintado-Herrera, M.G.; Lara-Martin, P.A.; Miserocchi, S.; Langone, L.; Guerra, R. Distribution and fate of legacy and emerging contaminants along the Adriatic Sea: A comparative study. Environ. Pollut. 2016, 218, 1055–1064. [Google Scholar] [CrossRef]
- Roberts, J.; Kumar, A.; Du, J.; Hepplewhite, C.; Ellis, D.J.; Christy, A.G.; Beavis, S.G. Pharmaceuticals and personal care products (PPCPs) in Australia’s largest inland sewage treatment plant, and its contribution to a major Australian river during high and low flow. Sci. Total Environ. 2016, 541, 1625–1637. [Google Scholar] [CrossRef]
- Voloshenko-Rossin, A.; Gasser, G.; Cohen, K.; Gun, J.; Cumbal, L.; Parra-Morales, W.; Sarabia, F.; Ojeda, F.; Lev, O. Emerging pollutants in the Esmeraldas watershed in Ecuador: Discharge and attenuation of emerging organic pollutants along the San Pedro—Guayllabamba—Esmeraldas rivers. Environ. Sci. Process. Impacts 2014, 17, 41–53. [Google Scholar] [CrossRef]
- Bai, X.; Lutz, A.; Carroll, R.; Keteles, K.; Dahlin, K.; Murphy, M.; Nguyen, D. Occurrence, distribution, and seasonality of emerging contaminants in urban watersheds. Chemosphere 2018, 200, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Tejada, C.; Quiñones, E.; Peña, M. Contaminantes emergentes en aguas: Metabolitos de fármacos. Una Revision. Rev. Fac. de Cienc. Básicas 2014, 10, 80–101. [Google Scholar] [CrossRef]
- García, C.; Gortáres, P.; Drogui, P. Contaminantes emergentes: Efectos y tratamientos de remoción. Rev. Química Viva 2011, 10, 96–105. [Google Scholar]
- Batt, A.L.; Furlong, E.T.; Mash, H.E.; Glassmeyer, S.T.; Kolpin, D.W. The importance of quality control in validating concentrations of contaminants of emerging concern in source and treated drinking water samples. Sci. Total Environ. 2017, 579, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.H.; Santos, G.D.O.S.; Ferreira, L.F.R.; Américo-Pinheiro, J.H.P.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Environmental aspects of hormones estriol, 17β-estradiol and 17α-ethinylestradiol: Electrochemical processes as next-generation technologies for their removal in water matrices. Chemosphere 2021, 267, 128888. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, R.; Abustan, I.; Ibrahim, A.N.M. Wastewater treatment using bentonite, the combinations of bentonite-zeolite, bentonite-alum, and bentonite-limestone as adsorbent and coagulant. Int. J. Environ. Sci. 2013, 4, 379. [Google Scholar]
- Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Using zeolitic adsorbents to cleanup special wastewater streams: A review. Microporous Mesoporous Mater. 2015, 214, 224–241. [Google Scholar] [CrossRef]
- Yuna, Z. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Mirzaei, N.; Hadi, M.; Gholami, M.; Fard, R.F.; Aminabad, M.S. Sorption of acid dye by surfactant modificated natural zeolites. J. Taiwan Inst. Chem. Eng. 2016, 59, 186–194. [Google Scholar] [CrossRef]
- Rossner, A.; Snyder, S.; Knappe, D.R. Removal of emerging contaminants of concern by alternative adsorbents. Water Res. 2009, 43, 3787–3796. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Z.; Dai, H.; Lu, X.; Peng, L.; Tan, X.; Shi, L.; Fahim, R. Preparation and application of modified zeolites as adsorbents in wastewater treatment. Water Sci. Technol. 2018, 2017, 621–635. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Toor, M.; Jin, B.; Dai, S.; Vimonses, V. Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J. Ind. Eng. Chem. 2015, 21, 653–661. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y.; Zhang, F.; Chen, X.; Zhao, Y.; Hu, B.; Zhang, X. Adsorption of 17β-estradiol onto humic-mineral complexes and effects of temperature, pH, and bisphenol A on the adsorption process. Environ. Pollut. 2019, 254, 112924. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Pashalidis, I.; Orfanos, A.G.; Manariotis, I.D.; Tatarchuk, T.; Sellaoui, L.; Bonilla-Petriciolet, A.; Mittal, A.; Núñez-Delgado, A. Removal of caffeine, nicotine and amoxicillin from (waste)waters by various adsorbents. A review. J. Environ. Manag. 2020, 261, 110236. [Google Scholar] [CrossRef]
- Matamoros, V.; Sala, L.; Salvado, V. Evaluation of a biologically-based filtration water reclamation plant for removing emerging contaminants: A pilot plant study. Bioresour. Technol. 2012, 104, 243–249. [Google Scholar] [CrossRef]
- Shiny, K. Biotreatment of wastewater using aquatic invertebrates, Daphnia magna and Paramecium caudatum. Bioresour. Technol. 2005, 96, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.M. Preparación y Actividad Catalítica de Sistemas Cromo-Arcilla y Níquel-Arcilla. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2012. Available online: http://purl.org/dc/dcmitype/Text, (accessed on 15 May 2021).
- National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 10 March 2021).
- Barron, L.; Havel, J.; Purcell, M.; Szpak, M.; Kelleher, B.; Paull, B. Predicting sorption of pharmaceuticals and personal care products onto soil and digested sludge using artificial neural networks. Analyst 2009, 134, 663–670. [Google Scholar] [CrossRef]
- Karnjanapiboonwong, A.; Morse, A.N.; Maul, J.D.; Anderson, T. Sorption of estrogens, triclosan, and caffeine in a sandy loam and a silt loam soil. J. Soils Sediments 2010, 10, 1300–1307. [Google Scholar] [CrossRef]
- Minmetec. Ficha Técnica Agrolite; Minmetec: Cuenca, Ecuador, 2020. [Google Scholar]
- Minmetec. Ficha Técnica Bentonita Sódica; Minmetec: Cuenca, Ecuador, 2019. [Google Scholar]
- Minmetec. Ficha Técnica Bentonita Cálcica; Minmetec: Cuenca, Ecuador, 2021. [Google Scholar]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Toor, M.; Jin, B. Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem. Eng. J. 2012, 187, 79–88. [Google Scholar] [CrossRef]
- Gallouze, H.; Akretche, D.-E.; Daniel, C.; Coelhoso, I.; Crespo, J.G. Removal of synthetic estrogen from water by adsorption on modified bentonites. Environ. Eng. Sci. 2021, 38, 4–14. [Google Scholar] [CrossRef]
- Yang, C.-H. Statistical mechanical study on the Freundlich isotherm equation. J. Colloid Interface Sci. 1998, 208, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Boparai, H.K.; Joseph, M.; O’Carroll, D. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- De Rezende, J.C.T.; Ramos, V.H.S.; Silva, A.S.; Santos, E.; Oliveira, H.A.; de Jesus, E. Assessment of sulfamethoxazole adsorption capacity on Pirangi clay from the state of Sergipe, Brazil, modified by heating and addition of organic cation. Cerâmica 2019, 65, 626–634. [Google Scholar] [CrossRef]
- Styszko, K.; Nosek, K.; Motak, M.; Bester, K. Preliminary selection of clay minerals for the removal of pharmaceuticals, bisphenol A and triclosan in acidic and neutral aqueous solutions. Comptes Rendus Chim. 2015, 18, 1134–1142. [Google Scholar] [CrossRef]
- Behera, S.K.; Oh, S.-Y.; Park, H.-S. Sorption of triclosan onto activated carbon, kaolinite and montmorillonite: Effects of pH, ionic strength, and humic acid. J. Hazard. Mater. 2010, 179, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Parsegian, V.A. Van der Waals Forces: A Handbook for Biologists, Chemists, Engineers, and Physicists; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Liu, J.; Carr, S.A. Removal of estrogenic compounds from aqueous solutions using zeolites. Water Environ. Res. 2013, 85, 2157–2163. [Google Scholar] [CrossRef]
- Singhal, J.; Singh, R. Studies on the adsorption of nicotine on kaolinites. Soil Sci. Plant Nutr. 1976, 22, 35–41. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Leszczynski, J. Unrevealing the nature of hydrogen bonds: π-electron delocalization shapes H-bond features. Intramolecular and intermolecular resonance-assisted hydrogen bonds. In Hydrogen Bonding—New Insights; Grabowski, S.J., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 487–512. ISBN 9781402048531. [Google Scholar]
- Shikii, K.; Seki, H.; Sakamoto, S.; Sei, Y.; Utsumi, H.; Yamaguchi, K. Intermolecular hydrogen bonding of steroid compounds: PFG NMR diffusion study, cold-spray ionization (CSI)-MS and X-ray analysis. Chem. Pharm. Bull. 2005, 53, 792–795. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leermakers, F.; Eriksson, J.C.; Lyklema, H. Chapter 4—Association colloids and their equilibrium modelling. In Fundamentals of Interface and Colloid Science; Lyklema, J., Ed.; Soft Colloids; Elsevier: Amsterdam, The Netherlands, 2005; Volume 5, pp. 4.1–4.123. [Google Scholar]
- Thanhmingliana, T.; Lalhriatpuia, C.; Tiwari, D.; Lee, S.-M. Efficient removal of 17β-estradiol using hybrid clay materials: Batch and column studies. Environ. Eng. Res. 2016, 21, 203–210. [Google Scholar] [CrossRef]
- Peng, Y.; Luo, Y.; Nie, X.-P.; Liao, W.; Yang, Y.-F.; Ying, G.-G. Toxic effects of Triclosan on the detoxification system and breeding of Daphnia magna. Ecotoxicology 2013, 22, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Sobek, A.; Yuan, B.; Breitholtz, M. Bioaccumulation potential of CPs in aquatic organisms: Uptake and depuration in Daphnia magna. Environ. Sci. Technol. 2019, 53, 9533–9541. [Google Scholar] [CrossRef]
- Dai, Z.; Xia, X.; Guo, J.; Jiang, X. Bioaccumulation and uptake routes of perfluoroalkyl acids in Daphnia magna. Chemosphere 2013, 90, 1589–1596. [Google Scholar] [CrossRef]
- Baldwin, W.S.; Milam, D.L.; Leblanc, G.A. Physiological and biochemical perturbations in Daphnia magna following exposure to the model environmental estrogen diethylstilbestrol. Environ. Toxicol. Chem. 1995, 14, 945–952. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).